Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Serum 25(OH)D
2.3. Grip Strength
2.4. Timed Up-and-Go Test
2.5. Potential Confounders
2.6. Effect Modifier
2.7. Statistical Analysis
Sensitivity Analysis
3. Results
3.1. Season-Specific 25(OH)D and GS Decline
3.2. Season-Specific 25(OH)D and Decline in TUG
3.3. Results for Sensitivity Analysis
3.3.1. Pre-Defined 25OHD Categories and GS Decline
3.3.2. Pre-Defined 25(OH)D Categories and Decline in TUG
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zitterman, A.; Gummert, J.F. Nonclassical vitamin D actions. Nutrients 2010, 2, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.; Griffith, L.E.; Strifler, L.; Henderson, M.; Patterson, C.; Heckman, G.; Llewellyn, D.J.; Raina, P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology 2012, 79, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Manson, J.E.; Pilz, S.; März, W.; Michaëlsson, K.; Lundqvist, A.; Jassal, S.K.; Barrett-Connor, E.; Zhang, C.; et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A D-Lightful health perspective. Nutr. Rev. 2008, 66, S182–S194. [Google Scholar] [CrossRef] [PubMed]
- Dror, Y.; Giveon, S.M.; Hoshen, M.; Feldhamer, I.; Balicer, R.D.; Feldman, B.S. Vitamin D levels for preventing acute coronary syndrome and mortality: Evidence of a nonlinear association. J. Clin. Endocrinol. Metab. 2013, 98, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord. 2012, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016, 8, E319. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Smith, Z.K.; Jenkinson, C.; Smith, D.J.; Hernandez, I.; Morgan, S.A.; Crabtree, N.J.; Gittoes, N.J.; Keevil, B.G.; Stewart, P.M.; Hewison, M. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE 2017, 12, e0170665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; DeLuca, H.F. Is the vitamin D receptor found in muscle? Endocrinology 2010, 152, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Elamin, K.B.; Abu Elnour, N.O.; Elamin, M.B.; Alkatib, A.A.; Fatourechi, M.M.; Almandoz, J.P.; Mullan, R.J.; Lane, M.A.; Liu, H.; et al. Clinical review: The effect of vitamin D on falls: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Moretti, A.; de Sire, A.; Calafiore, D.; Gimigliano, F. Effectiveness of calcifediol in improving muscle function in post-menopausal women: A prospective cohort study. Adv. Ther. 2017, 34, 744–752. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.K.; Kiely, M. Vitamin D and muscle strength throughout the life course: A review of epidemiological and intervention studies. J. Hum. Nutr. Diet. 2015, 28, 636–645. [Google Scholar] [CrossRef] [PubMed]
- The Scientific Advisory Committee on Nutrition (SACN)—GOV.UK. Available online: https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition (accessed on 6 September 2016).
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.R.; Kirkwood, T.B.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Saxby, B.K.; Wesnes, K.A.; Collerton, D.; et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: The Newcastle 85+ Study. Eur. J. Neurol. 2015, 22, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Aspray, T.; Hill, T.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Kirkwood, T.B.; Mathers, J.C.; Jagger, C. 25-hydroxyvitamin D and increased all-cause mortality in very old women: The Newcastle 85+ study. J. Intern. Med. 2015, 277, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; van Schoor, N.M.; de Jongh, R.T.; Visser, M.; Deeg, D.J.; Lips, P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J. Clin. Endocrinol. Metab. 2013, 98, E1483–E1490. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.T.; von Mühlen, D.; Barrett-Connor, E.L. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos. Int. 2009, 20, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Davis, C.C.; Chaves, P.H.; Hirsch, C.H.; Robbins, J.A.; Arnold, A.M.; Newman, A.B.; Kritchevsky, S.B. Serum 25-hydroxyvitamin D and physical function in older adults: The Cardiovascular Health Study All Stars. J. Am. Geriatr. Soc. 2011, 59, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Neiberg, R.H.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Cawthon, P.M.; Shea, M.K.; Schwartz, G.G.; et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: The Health, Aging, and Body Composition Study. Am. J. Epidemiol. 2012, 176, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Hausman, D.B.; Johnson, M.A.; Nicklas, B.J.; Miller, M.E.; Neiberg, R.H.; Marsh, A.P.; Newman, A.B.; Blair, S.N.; et al. Change in 25-hydroxyvitamin D and physical performance in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Granic, A.; Davies, K.; Kirkwood, T.B.L.; Jagger, C.; Sayer, A.A. Prevalence and incidence of sarcopenia in the very old: Findings from the Newcastle 85+ study. J. Cachexia Sarcopenia Muscle 2016. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Prado, C.; Hooper, L.; Munro, A.; Collerton, J.; Davies, K.; Kingston, A.; Mathers, J.C.; Kirkwood, T.B.; Jagger, C. Serum osmolarity and haematocrit do not modify the association between the impedance index (Ht2/Z) and total body water in the very old: The Newcastle 85+ Study. Arch. Gerontol. Geriatr. 2015, 60, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kempen, G.I.; Ranchor, A.V.; van Sonderen, E.; van Jaarsveld, C.H.; Sanderman, R. Risk and protective factors of different functional trajectories in older persons: Are these the same? J. Gerontol. B Psychol. Sci. Soc. Sci. 2006, 61, P95–P101. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.R.; Granic, A.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; Siervo, M.; Mathers, J.C.; Adamson, A.J.; Francis, R.M.; Pearce, S.H.; et al. Serum 25-hydroxyvitamin D concentration and its determinants in the very old: The Newcastle 85+ Study. Osteoporos. Int. 2016, 27, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Collerton, J.; Barrass, K.; Bond, J.; Eccles, M.; Jagger, C.; James, O.; Martin-Ruiz, C.; Robinson, L.; von Zglinicki, T.; Kirkwood, T. The Newcastle 85+ study: Biological, clinical and psychological factors associated with healthy ageing: Study protocol. BMC Geriatr. 2007, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Collerton, J.; Davies, K.; Jagger, C.; Kingston, A.; Bond, J.; Eccles, M.P.; Robinson, L.A.; Martin-Ruiz, C.; von Zglinicki, T.; James, O.F.; et al. Health and disease in 85 year olds: Baseline findings from the Newcastle 85+ cohort study. BMJ 2009, 399, b4904. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Kingston, A.; Robinson, L.; Hughes, J.; Hunt, J.M.; Barker, S.A.; Edwards, J.; Collerton, J.; Jagger, C.; Kirkwood, T.B. Improving retention of very old participants in longitudinal research: Experiences from the Newcastle 85+ study. PLoS ONE 2014, 9, e108370. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.; Jagger, C.; Kingston, A.; Collerton, J.; Catt, M.; Davies, K.; Dunn, M.; Hilkens, C.; Keavney, B.; Pearce, S.H.; et al. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ Study. Mech. Ageing Dev. 2011, 132, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jacobs, E.J.; McCullough; Rodriguez, C.; Thun, M.J.; Calle, E.E.; Flanders, W.D. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: Insights from simulation based on serum 25-hydroxyvitamin D. Am. J. Epidemiol. 2009, 170, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Haidar, S.G.; Kumar, D.; Bassi, R.S.; Deshmukh, S.C. Average versus maximum grip strength: Which is more consistent? J. Hand Surg. Br. 2004, 29B, 82–84. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Granic, A.; Davies, K.; Jagger, C.; Kirkwood, T.B.; Syddall, H.E.; Sayer, A.A. Grip strength decline and its determinants in the very old: Longitudinal findings from the Newcastle 85+ Study. PLoS ONE 2016, 11, e0163183. [Google Scholar]
- Sternäng, O.; Reynolds, C.A.; Finkel, D.; Ernsth-Bravell, M.; Pedersen, N.L.; Dahl Aslan, A.K. Factors associated with grip strength decline in older adults. Age Aging 2015, 44, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Batterham, M.; Langford, K.; Lateo, J.; Brock, E.; Walton, K.; Lyons-Wall, P.; Eisenhauer, K.; Green, N.; McLean, C. Lean body mass associated with upper body strength in healthy older adults while higher body fat limits lower extremity performance and endurance. Nutrients 2015, 7, 7126–7142. [Google Scholar] [CrossRef] [PubMed]
- Oksuzyan, A.; Maier, H.; McGue, M.; Vaupel, J.W.; Christensen, K. Sex differences in the level and rate of change of physical function and grip strength in the Danish 1905-Cohort Study. J. Aging Health 2010, 22, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Kingston, A.; Davies, K.; Collerton, J.; Robinson, L.; Duncan, R.; Bond, J.; Kirkwood, T.B.; Jagger, C. The contribution of diseases to the male-female disability-survival paradox in the very old: Results from the Newcastle 85+ study. PLoS ONE 2014, 9, e88016. [Google Scholar] [CrossRef] [PubMed]
- Innerd, P.; Catt, M.; Collerton, J.; Davies, K.; Trenell, M.; Kirkwood, T.B.; Jagger, C. A comparison of subjective and objective measures of physical activity from the Newcastle 85+ study. Age Ageing 2015, 44, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Matheï, C.; Van Pottelbergh, G.; Vaes, B.; Adriaensen, W.; Gruson, D.; Degryse, J.M. No relation between vitamin D status and physical performance in the oldest old: Results from the Belfrail study. Age Ageing 2013, 42, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Verreault, R.; Semba, R.D.; Volpato, S.; Ferrucci, L.; Fried, L.P.; Guralnik, J.M. Low serum vitamin d does not predict new disability or loss of muscle strength in older women. J. Am. Geriatr. Soc. 2002, 50, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; de Jongh, R.T.; Heijboer, A.C.; Swart, K.M.; Brouwer-Brolsma, E.M.; Enneman, A.W.; de Groot, C.P.; van der Velde, N.; Dhonukshe-Rutten, R.A.; Lips, P.; et al. Vitamin D status is associated with physical performance: The results of three independent cohorts. Osteoporos. Int. 2013, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Karras, S.N.; Bischoff-Ferrari, H.A.; Annweiler, C.; Boucher, B.J.; Juzeniene, A.; Garland, C.F.; Holick, M.F. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects? Dermatoendocrinology 2016, 8, e1187349. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; de Jongh, R.T.; Heymans, M.W.; van Schoor, N.M.; Lips, P. Thresholds for serum 25(OH)D concentrations with respect to different outcomes. J. Clin. Endocrinol. Metab. 2015, 100, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.; Lips, P. Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, Y.; Tian, Q.; Papasian, C.J.; Hu, T.; Deng, H.W. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos. Int. 2016, 27, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, E.; Cheng, S.; Häkkinen, K.; Finni, T.; Walker, S.; Pesola, A.; Ahtiainen, J.; Stenroth, L.; Selänne, H.; Sipilä, S. Body composition in 18- to 88-year-old adults–comparison of multifrequency bioimpedance and dual-energy X-ray absorptiometry. Obesity (Silver Spring) 2014, 22, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.D. Accuracy of 25-hydroxyvitamin D assays: Confronting the issues. Curr. Drug Targets 2011, 12, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Perna, L.; Haug, U.; Schöttker, B.; Müller, H.; Raum, E.; Jansen, E.H.J.M.; Brenner, H. Public health implications of standardized 25-hydroxyvitamin D levels: A decrease in the prevalence of vitamin D deficiency among older women in Germany. Prev. Med. 2012, 55, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Mavrogianni, C.; Tsirigoti, L.; Hoeller, U.; Roos, F.F.; Bendik, I.; Eggersdorfer, M.; Celis-Morales, C.; et al. Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: The Food4Me study. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
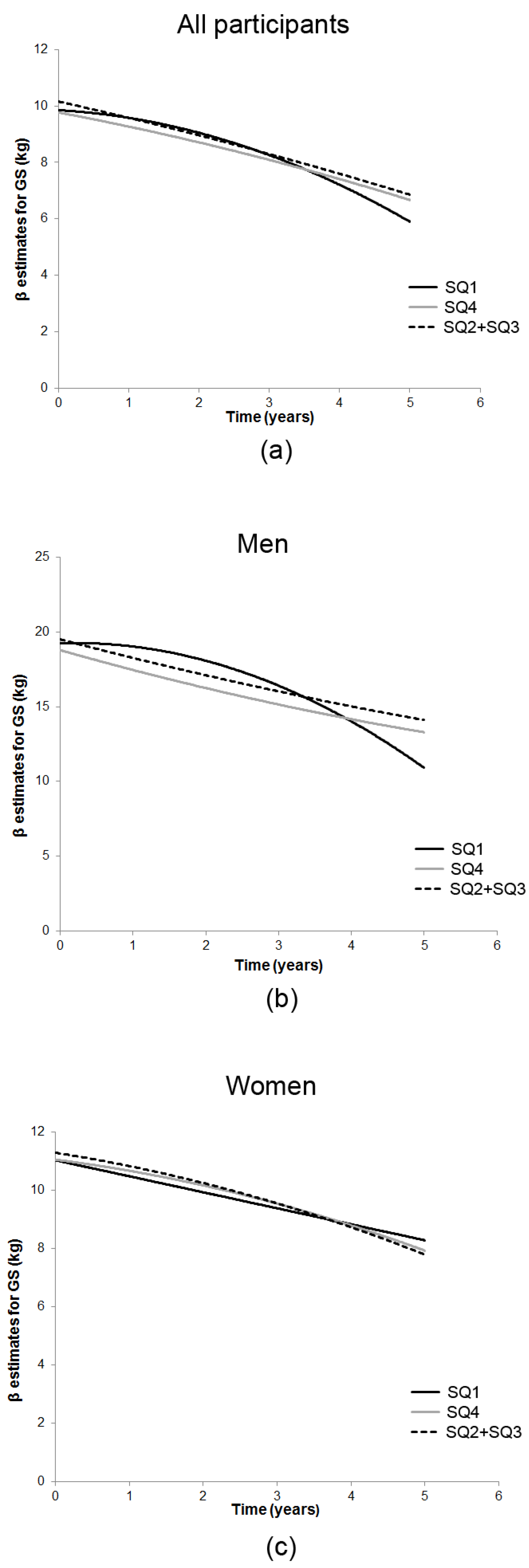
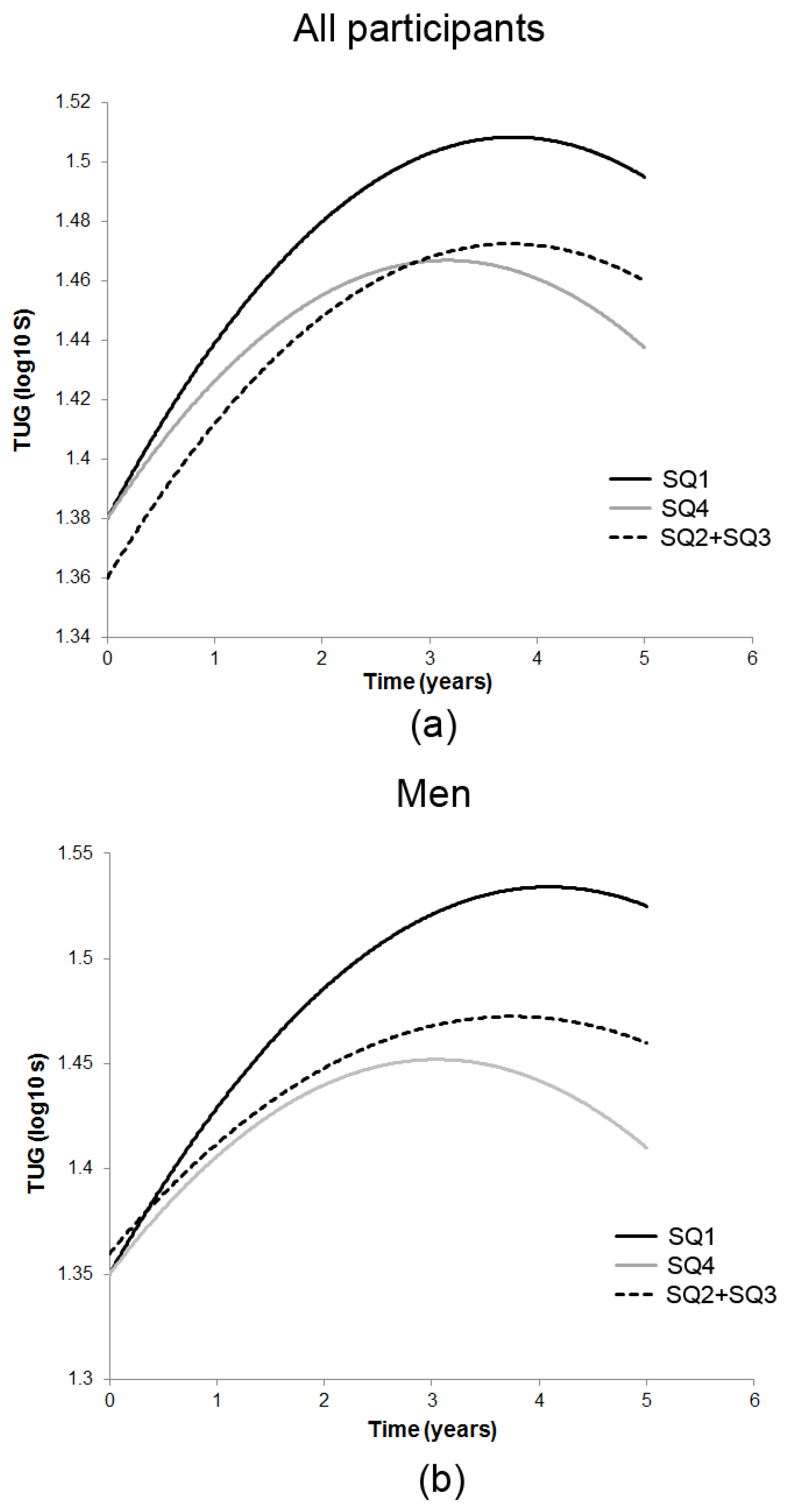
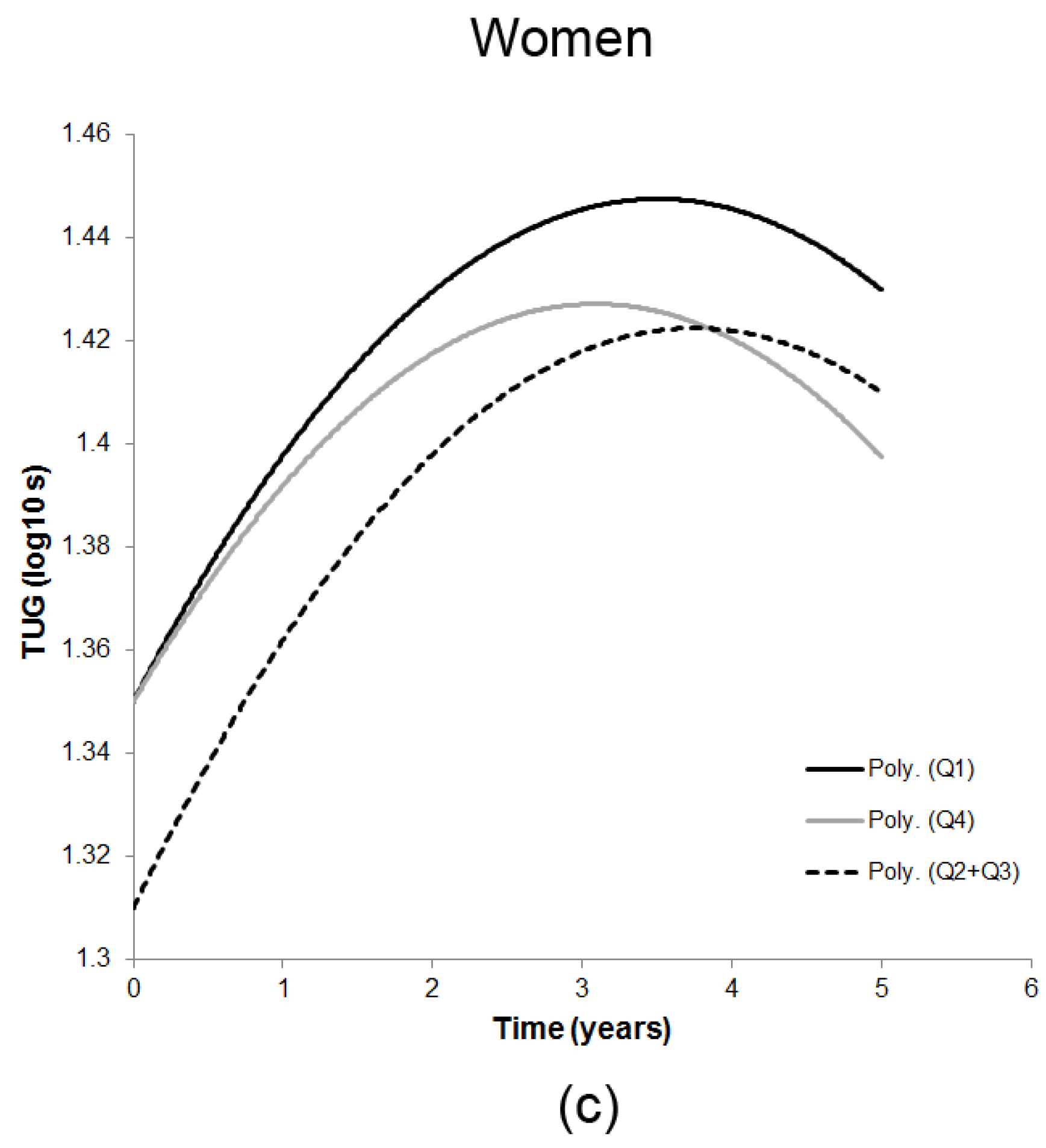
| Measure/Time of Assessment ‡ | n | SQ1 25(OH)D | SQ2 + SQ3 25(OH)D | SQ4 25(OH)D |
|---|---|---|---|---|
| Lowest | Middle | Highest | ||
| Grip strength, kg (SD) | ||||
| All participants | ||||
| Baseline | 754 | 16.83 (6.80) | 19.20 (8.13) | 15.92 (7.29) |
| 1.5-year follow-up | 582 | 16.35 (7.73) | 18.26 (7.86) | 15.21 (7.63) |
| 3-year follow-up | 434 | 16.26 (7.16) | 17.26 (7.46) | 15.26 (6.92) |
| 5-year follow-up | 286 | 13.66 (6.15) | 15.64 (7.38) | 14.58 (6.83) |
| Men | ||||
| Baseline | 301 | 23.12 (5.43) | 25.44 (7.14) | 23.67 (6.65) |
| 1.5-year follow-up | 224 | 23.43 (6.80) | 24.39 (7.12) | 23.01 (7.72) |
| 3-year follow-up | 163 | 22.79 (6.24) | 23.05 (6.85) | 22.91 (7.35) |
| 5-year follow-up | 104 | 17.41 (7.57) | 22.01 (6.48) | 22.32 (6.77) |
| Women | ||||
| Baseline | 453 | 12.92 (4.10) | 13.97 (4.33) | 12.68 (4.61) |
| 1.5-year follow-up | 358 | 12.29 (4.72) | 13.38 (4.13) | 12.21 (5.05) |
| 3-year follow-up | 271 | 12.63 (4.63) | 12.96 (4.34) | 12.45 (4.08) |
| 5-year follow-up | 182 | 12.63 (4.63) | 11.39 (4.21) | 11.76 (4.21) |
| Timed Up-and-Go Test, s (SD) | ||||
| All participants | ||||
| Baseline | 717 | 20.93 (17.39) | 16.75 (13.32) | 19.76 (13.72) |
| 1.5-year follow-up | 529 | 22.48 (14.53) | 20.06 (15.53) | 22.14 (14.51) |
| 3-year follow-up | 389 | 24.99 (25.67) | 19.70 (14.08) | 22.08 (20.26) |
| 5-year follow-up | 266 | 24.37 (16.44) | 19.88 (10.55) | 19.71 (11.56) |
| Men | ||||
| Baseline | 287 | 18.99 (19.00) | 15.06 (11.65) | 15.76 (8.83) |
| 1.5-year follow-up | 210 | 22.23 (16.97) | 18.47 (13.78) | 16.57 (6.86) |
| 3-year follow-up | 149 | 22.50 (20.78) | 16.91 (8.23) | 22.58 (32.58) |
| 5-year follow-up | 94 | 19.71 (11.04) | 17.92 (9.77) | 15.83 (6.43) |
| Women | ||||
| Baseline | 430 | 22.15 (18.78) | 18.14 (14.43) | 21.54 (15.10) |
| 1.5-year follow-up | 319 | 22.63 (12.95) | 21.33 (16.74) | 24.56 (16.24) |
| 3-year follow-up | 240 | 26.37 (28.13) | 21.74 (16.87) | 21.86 (11.71) |
| 5-year follow-up | 172 | 26.15 (17.91) | 21.14 (10.89) | 21.41 (12.89) |
| Outcome | Effects/Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| β (SE) † | p | β (SE) † | p | β (SE) † | p | ||
| All participants | |||||||
| GS (kg) | Intercept | 19.10 (0.38) | <0.001 | 19.14 (0.39) | <0.001 | 10.16 (0.75) | <0.001 |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | −2.24 (0.65) | 0.001 | −2.48 (0.69) | <0.001 | −0.31 (0.45) | 0.49 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | −3.06 (0.65) | <0.001 | −3.37 (0.69) | <0.001 | −0.39 (0.45) | 0.39 | |
| GS decline ‡ | Time | −0.80 (0.04) | <0.001 | −0.74 (0.14) | <0.001 | −0.56 (0.14) | <0.001 |
| Time2 | −0.02 (0.03) | 0.43 | −0.02 (0.03) | 0.5 | |||
| Rate of decline | Slope § | ||||||
| 25(OH)D ×Time | |||||||
| Lowest × Time | 0.56 (0.26) | 0.03 | 0.42 (0.26) | 0.1 | |||
| Middle × Time (ref) | 0 | 0 | |||||
| Highest × Time | 0.17 (0.25) | 0.51 | 0.09 (0.26) | 0.73 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | −0.13 (0.05) | 0.02 | −0.11 (0.05) | 0.03 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | −0.001 (0.51) | 0.99 | −0.01 (0.05) | 0.85 | |||
| Men | |||||||
| GS (kg) | Intercept | 25.48 (0.51) | <0.001 | 25.50 (0.51) | <0.001 | 19.5 (1.46) | <0.001 |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | −2.02 (0.93) | 0.03 | −2.56 (0.96) | 0.008 | −0.25 (0.89) | 0.78 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | −2.12 (1.03) | 0.04 | −2.16 (1.06) | 0.04 | −0.89 (0.96) | 0.35 | |
| GS decline ‡ | Time | −1.10 (0.08) | <0.001 | −1.18 (0.24) | <0.001 | −1.28 (0.23) | <0.001 |
| Time2 | 0.02 (0.05) | 0.64 | 0.04 (0.05) | 0.4 | |||
| Rate of decline | Slope § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | 1.71 (0.46) | <0.001 | 1.41 (0.47) | 0.003 | |||
| Middle × Time (ref) | 0 | 0 | |||||
| Highest × Time | 0.02 (0.51) | 0.03 | −0.12 (0.52) | 0.82 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | −0.44 (0.09) | <0.001 | −0.40 (0.1) | <0.001 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | 0.01 (0.11) | 0.91 | 0.03 (0.1) | 0.77 | |||
| Women | |||||||
| GS (kg) | Intercept | 13.94 (0.29) | <0.001 | 13.88 (0.31) | <0.001 | 11.30 (0.75) | <0.001 |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | −1.08 (0.48) | 0.03 | −1.10 (0.52) | 0.04 | −0.26 (0.48) | 0.59 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | −1.14 (0.46) | 0.01 | −1.28 (0.50) | 0.01 | −0.24 (0.45) | 0.60 | |
| GS decline ‡ | Time | −0.59 (0.05) | <0.001 | −0.33 (0.16) | 0.05 | −0.40 (0.17) | 0.02 |
| Time2 | −0.07 (0.03) | 0.04 | −0.06 (0.03) | 0.07 | |||
| Rate of decline | Slope § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | −0.17 (0.30) | 0.56 | −0.15 (0.30) | 0.62 | |||
| Middle × Time | 0 | 0 | |||||
| Highest × Time | 0.05 (0.28) | 0.85 | 0.08 (0.28) | 0.79 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | 0.06 (0.06) | 0.35 | 0.06 (0.06) | 0.37 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | 0.01 (0.06) | 0.83 | −0.001 (0.06) | 0.98 | |||
| Restricted cohort | |||||||
| GS (kg) | Intercept | 19.6 (0.41) | <0.001 | 19.6 (0.43) | <0.001 | 10.23 (0.85) | <0.001 |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | −2.76 (0.68) | <0.001 | −2.95 (0.72) | <0.001 | −0.35 (0.48) | 0.47 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | −1.08 (0.88) | 0.22 | −1.25 (0.93) | 0.18 | −0.35 (0.59) | 0.55 | |
| GS decline ‡ | Time | −0.75 (0.15) | <0.001 | −0.54 (0.15) | 0.001 | ||
| Time2 | −0.02 (0.03) | 0.5 | −0.02 (0.03) | 0.52 | |||
| Rate of decline | Slope § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | 0.52 (0.27) | 0.05 | 0.36 (0.27) | 0.19 | |||
| Middle × Time (ref) | 0 | 0 | |||||
| Highest × Time | 0.07 (0.33) | 0.84 | 0.04 (0.33) | 0.91 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | −0.12 (0.05) | 0.02 | −0.11 (0.05) | 0.05 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | 0.003 (0.06) | 0.96 | 0.002 (0.06) | 0.97 |
| Outcome | Effects/Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| β (SE) † | p | β (SE) † | p | β (SE) † | p | ||
| All participants | |||||||
| TUG (log10 s) | Intercept | 1.17 (0.01) | 1.16 (0.01) | <0.001 | 1.56 (0.03) | <0.001 | |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | 0.09 (0.02) | <0.001 | 0.08 (0.02) | <0.001 | 0.02 (0.02) | 0.23 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | 0.06 (0.02) | 0.005 | 0.07 (0.02) | 0.003 | 0.02 (0.02) | 0.17 | |
| TUG decline ‡ | Time | 0.03 (0.002) | <0.001 | 0.06 (0.006) | <0.001 | 0.06 (0.01) | <0.001 |
| Time2 | −0.01 ((0.001) | <0.001 | −0.01 (0.001) | <0.001 | |||
| Rate of decline | Slopes § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | 0.01 (0.01) | 0.58 | 0.01 (0.01) | 0.51 | |||
| Middle × Time (ref) | 0 | 0 | |||||
| Highest × Time | −0.005 (0.01) | 0.65 | −0.01 (0.01) | 0.63 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | −0.0001 (0.002) | 0.97 | −0.001 (0.002) | 0.62 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | −0.001 (0.002) | 0.71 | −0.001 (0.002) | 0.77 | |||
| Men | |||||||
| TUG (log10 s) | Intercept | 1.13 (0.02) | <0.001 | 1.12 (0.02) | <0.001 | 1.57 (0.04) | <0.001 |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | 0.09 (0.03) | 0.002 | 0.09 (0.03) | 0.006 | −0.01 (0.03) | 0.69 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | 0.03 (0.03) | 0.78 | 0.03 (0.03) | 0.42 | −0.01 (0.03) | 0.68 | |
| TUG decline ‡ | Time | 0.04 (0.003) | <0.001 | 0.06 (0.01) | <0.001 | 0.06 (0.01) | <0.001 |
| Time2 | −0.01 (0.001) | 0.001 | −0.01 (0.001) | <0.001 | |||
| Rate of decline | Slopes § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | 0.02 (0.02) | 0.22 | −0.003 (0.004) | 0.41 | |||
| Middle × Time (ref) | 0 | 0 | |||||
| Highest × Time | 0.001 (0.002) | 0.96 | −0.003 (0.004) | 0.47 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | −0.002 (0.004) | 0.61 | −0.01 (0.001) | 0.41 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | −0.002 (0.004) | 0.57 | −0.003 (0.004) | 0.47 | |||
| Women | |||||||
| TUG (log10 s) | Intercept | 1.21 (0.02) | <0.001 | 1.19 (0.02) | <0.001 | 1.51 (0.03) | <0.001 |
| 25(OH)D quartiles | |||||||
| Lowest (SQ1) | 0.07 (0.03) | 0.007 | 0.07 (0.03) | 0.01 | 0.04 (0.02) | 0.04 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | 0.06 (0.03) | 0.03 | 0.07 (0.03) | 0.02 | 0.04 (0.02) | 0.03 | |
| TUG decline ‡ | Time | 0.03 (0.003) | <0.001 | 0.06 (0.01) | <0.001 | 0.06 (0.01) | <0.001 |
| Time2 | 0.006 (0.002) | <0.001 | −0.01 (0.002) | <0.001 | |||
| Rate of decline | Slope § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | −0.003 (0.02) | 0.86 | −0.004 (0.02) | 0.8 | |||
| Middle × Time | 0 | 0 | |||||
| Highest × Time | −0.01 (0.01) | 0.59 | −0.01 (0.01) | 0.45 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | 0.001 (0.003) | 0.7 | 0.00001 (0.003) | 0.99 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | −0.0004 (0.003) | 0.9 | 0.0001 (0.003) | 0.97 | |||
| Restricted cohort | |||||||
| TUG (log10 s) | Intercept | 1.16 (0.01) | <0.001 | 1.15 (0.01) | <0.001 | 1.55 (0.03) | <0.001 |
| 25(OH)D quartile | |||||||
| Lowest (SQ1) | 0.10 (0.02) | <0.001 | 0.10 (0.02) | <0.001 | 0.03 (0.02) | 0.07 | |
| Middle (ref) (SQ2 + SQ3) | 0 | 0 | 0 | ||||
| Highest (SQ4) | -0.02 (0.02) | 0.50 | −0.02 (0.03) | 0.56 | −0.01 (0.02) | 0.71 | |
| TUG decline ‡ | Time | 0.03 (0.002) | <0.001 | 0.06 (0.01) | <0.001 | 0.06 (0.01) | <0.001 |
| Time2 | −0.01 (0.001) | <0.001 | −0.01 (0.001) | <0.001 | |||
| Rate of decline | Slope § | ||||||
| 25(OH)D × Time | |||||||
| Lowest × Time | 0.006 (0.01) | 0.61 | 0.01 (0.01) | 0.52 | |||
| Middle × Time (ref) | 0 | 0 | |||||
| Highest × Time | 0.007 (0.01) | 0.61 | 0.002 (0.01) | 0.85 | |||
| 25(OH)D × Time2 | |||||||
| Lowest × Time2 | 0.0001 (0.002) | 0.98 | −0.001 (0.002) | 0.61 | |||
| Middle × Time2 | 0 | 0 | |||||
| Highest × Time2 | −0.003 (0.003) | 0.26 | −0.002 (0.003) | 0.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.L.; Mathers, J.C.; Sayer, A.A. Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study. Nutrients 2017, 9, 379. https://doi.org/10.3390/nu9040379
Granic A, Hill TR, Davies K, Jagger C, Adamson A, Siervo M, Kirkwood TBL, Mathers JC, Sayer AA. Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study. Nutrients. 2017; 9(4):379. https://doi.org/10.3390/nu9040379
Chicago/Turabian StyleGranic, Antoneta, Tom R. Hill, Karen Davies, Carol Jagger, Ashley Adamson, Mario Siervo, Thomas B. L. Kirkwood, John C. Mathers, and Avan A. Sayer. 2017. "Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study" Nutrients 9, no. 4: 379. https://doi.org/10.3390/nu9040379





