Novel Genetic Variants Associated with Child Refractory Esophageal Stricture with Food Allergy by Exome Sequencing
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Exome Capture and Illumina Sequencing
2.3. Read Mapping and Variant Calling
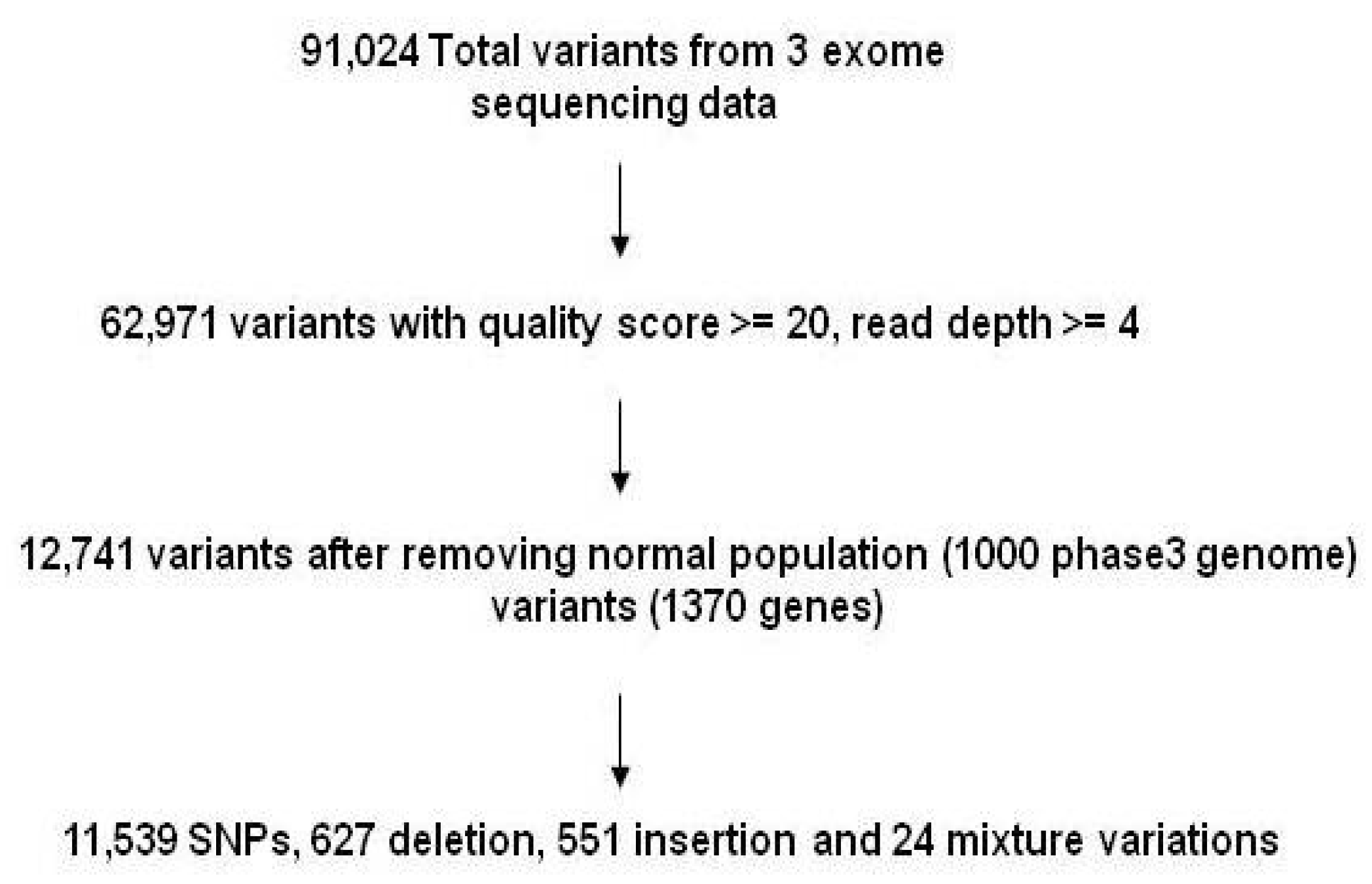
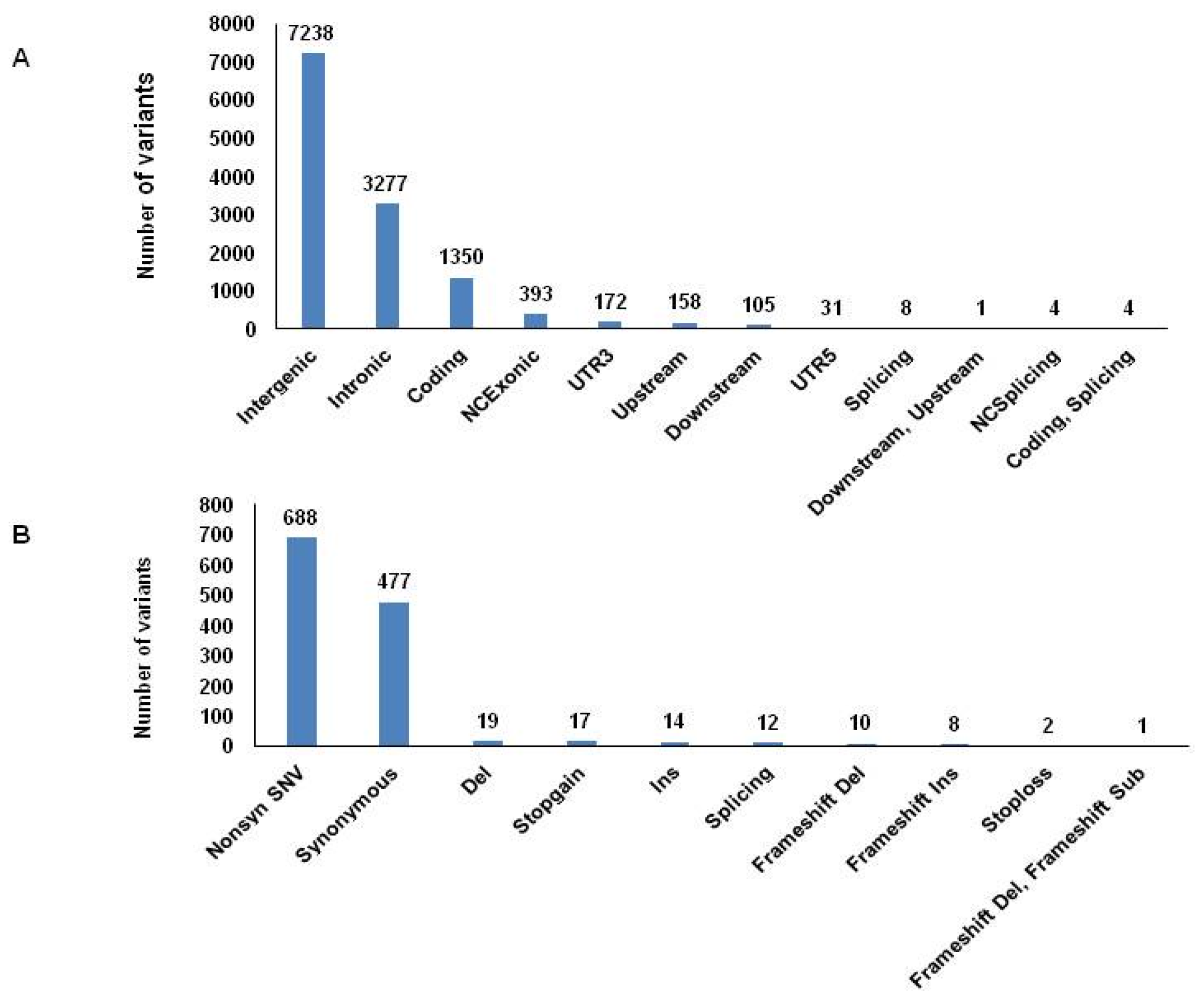
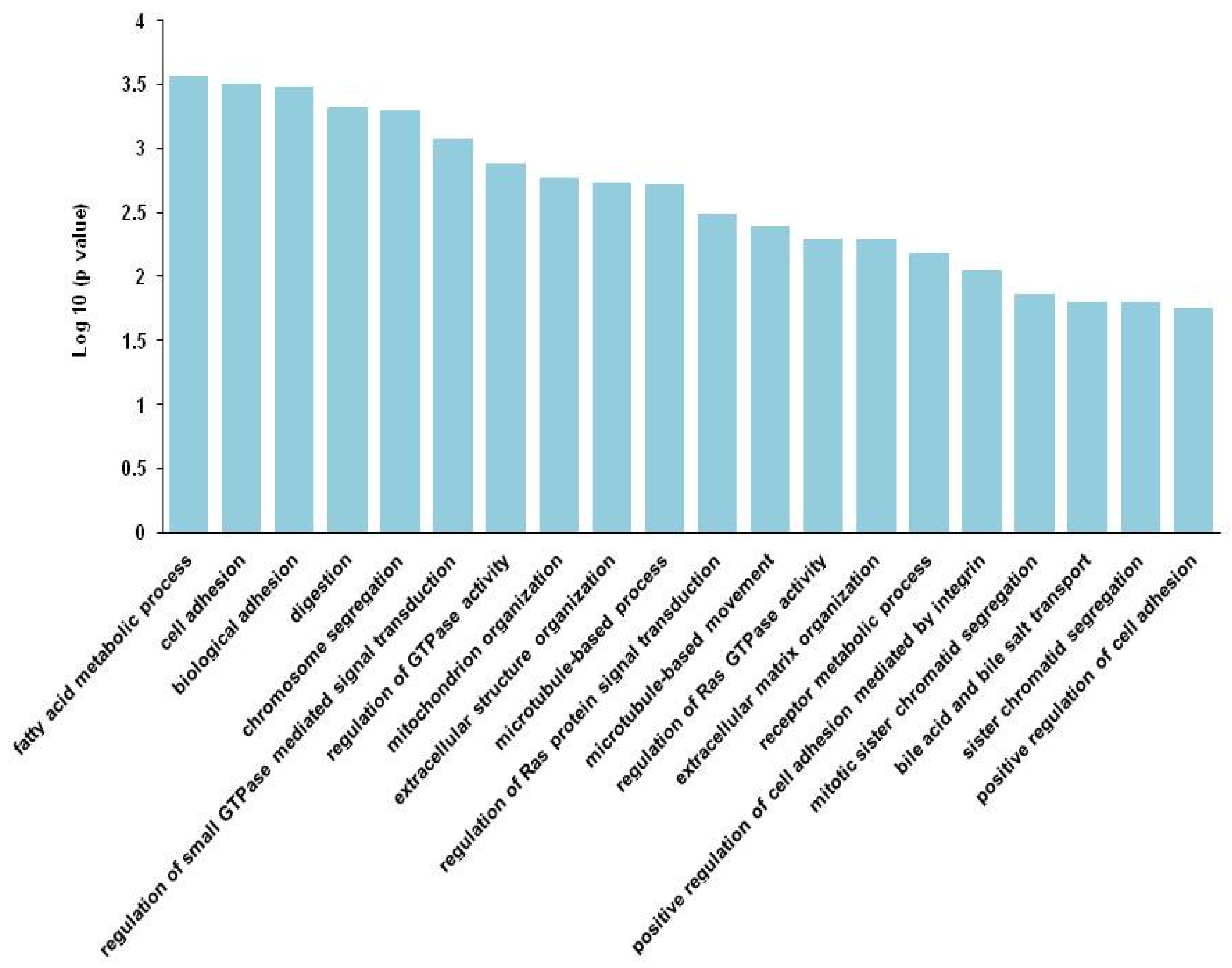
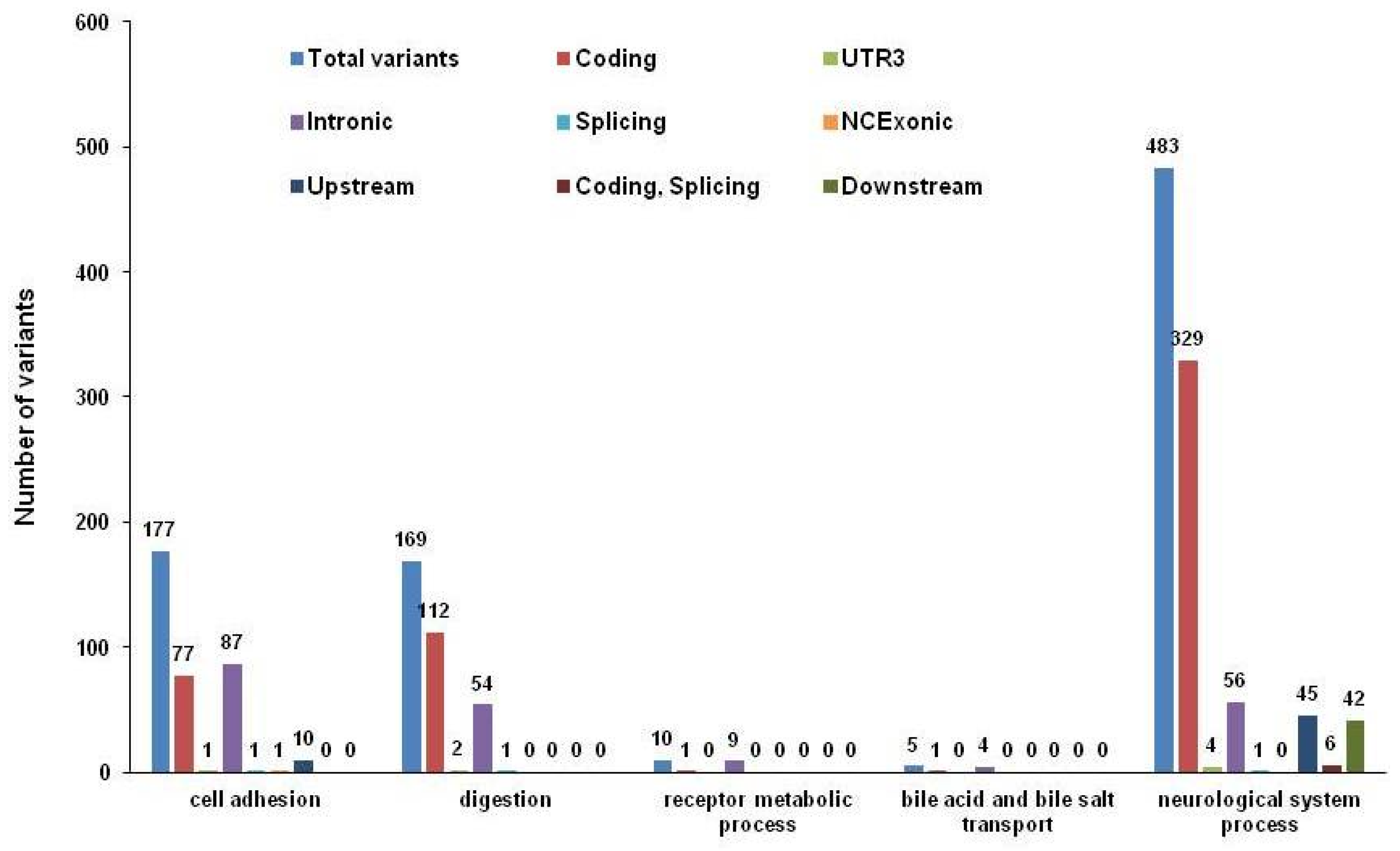
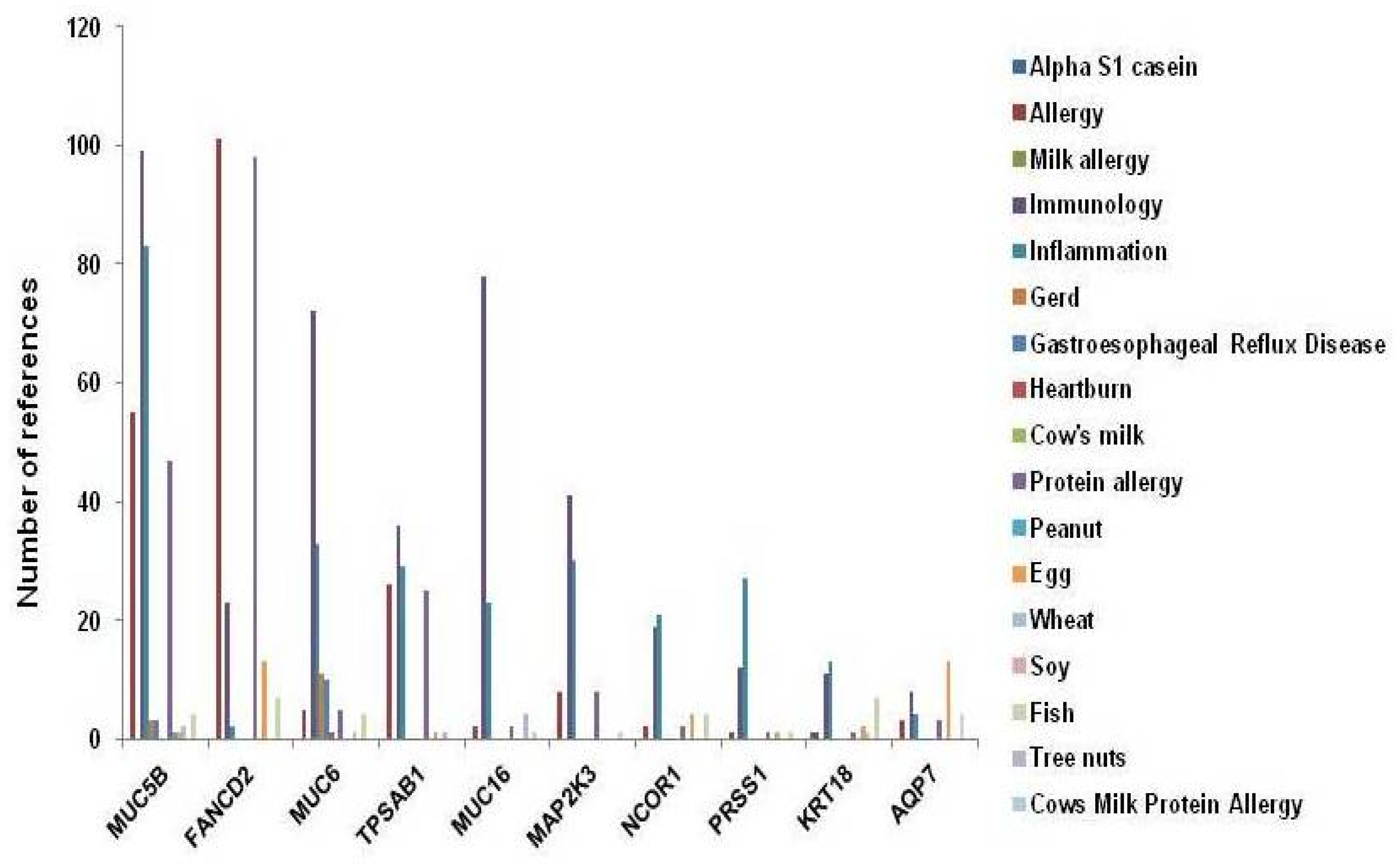
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ball, W.S.; Strife, J.L.; Rosenkrantz, J.; Towbin, R.B.; Noseworthy, J. Esophageal strictures in children. Treatment by balloon dilatation. Radiology 1984, 150, 263e4. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, J.; Quesnel, S.; Pierrot, S.; Couloigner, V. Endoscopic balloon dilatation of esophageal strictures in children. Int. J. Ped. Otorhinolaryngol. 2011, 75, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- El Hassani, A.; Michaud, L.; Chartier, A.; Penel-Capelle, D.; Sfeir, R.; Besson, R.; Turck, D.; Gottrand, F. Cow’s milk protein allergy after neonatal intestinal surgery. Arch. Pediatr. 2005, 12, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ida, S.; Kawahara, H.; Kawamoto, K.; Etani, Y.; Kubota, A. Importance of evaluating for cow’s milk allergy in pediatric surgical patients with functional bowel symptoms. J. Pediatr. Surg. 2011, 46, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Kaga, A.; Kanda, S.; Suzuki, Y.; Tanabu, M.; Sawa, N. Intestinal malrotation with suspected cow’s milk allergy: A case report. BMC Res. Notes 2012, 5, 481. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.G.; Hosack, D.A.; Dennis, G., Jr.; Lempicki, R.A.; Bright, T.J.; Cheadle, C.; Engel, J. PubMatrix: A tool for multiplex literature mining. BMC Bioinform. 2003, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Heruth, D.P.; Zhang, L.Q.; Ye, S.Q. Identification of lung-specific genes by meta-analysis of multiple tissue RNA-seq data. FEBS Open Bio 2016, 6, 774–781. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Dellon, E.S. Eosinophilic esophagitis: Diagnostic testsandcriteria. Curr. Opin. Gastroenterol. 2012, 28, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.A.; David, L.; Correa, P.; Carneiro, F.; de Bolós, C.; Garcia, E.; Mandel, U.; Clausen, H.; Sobrinho-Simões, M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999, 59, 1003–1007. [Google Scholar] [PubMed]
- Corfield, A.P.; Carroll, D.; Myerscough, N.; Probert, C.S. Mucins in the gastrointestinal tract in health and disease. Front. Biosci. 2001, 6, D1321–D1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lupski, J.R. Non-coding genetic variants in human disease. Hum. Mol. Genet. 2015, 24, R102–R110. [Google Scholar] [CrossRef] [PubMed]
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Age (years) | 12 | 2.5 | 4 |
| Sex | male | male | male |
| Age at stricture diagnosis | 8 years | 1 month | 2 years |
| Cause | Caustic agent | Post-surgery (esophageal atresia) | Post-surgery (Hiatal hernia) |
| Initial stenosis diameter (mm) | 1 | 5 | <1 |
| EBD (times) | 18 | 7 | 6 |
| Esophageal mucosal eosinophil/hpf | 9 | 35 | 12 |
| sIgE | |||
| Milk | + | + | + |
| Egg | + | + | + |
| Wheat | + | - | + |
| Peanut | + | + | + |
| Soy | - | - | + |
| Fish | - | - | + |
| Others | rice | crab | rice |
| Treatment | Dietary allergen exclusion | Elemental formula | Dietary allergen exclusion |
| Follow-up duration (months) | 14 | 12 | 26 |
| Resolution of esophageal stricture | Yes | Yes | Yes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Xiong, M.; Chen, H.; Geng, L.; Chen, P.; Xie, J.; Ye, S.Q.; Li, D.-Y.; Gong, S. Novel Genetic Variants Associated with Child Refractory Esophageal Stricture with Food Allergy by Exome Sequencing. Nutrients 2017, 9, 390. https://doi.org/10.3390/nu9040390
Yang M, Xiong M, Chen H, Geng L, Chen P, Xie J, Ye SQ, Li D-Y, Gong S. Novel Genetic Variants Associated with Child Refractory Esophageal Stricture with Food Allergy by Exome Sequencing. Nutrients. 2017; 9(4):390. https://doi.org/10.3390/nu9040390
Chicago/Turabian StyleYang, Min, Min Xiong, Huan Chen, Lanlan Geng, Peiyu Chen, Jing Xie, Shui Qing Ye, Ding-You Li, and Sitang Gong. 2017. "Novel Genetic Variants Associated with Child Refractory Esophageal Stricture with Food Allergy by Exome Sequencing" Nutrients 9, no. 4: 390. https://doi.org/10.3390/nu9040390





