1. Introduction
The branched-chain amino acids (BCAA) leucine (Leu), isoleucine (Ile) and valine (Val) are an important class of essential amino acids. These amino acids play an important role in the human body for protein synthesis, production of energy and synthesis of many neurotransmitters [
1,
2]. Moreover, the BCAA contribute to dietary protein intake [
3], with animal protein being the main source
Several studies have shown that elevated levels of BCAA in blood, including Leu, Ile and Val, may be related to the risk of developing type 2 diabetes, insulin resistance and cardiovascular diseases [
4,
5,
6,
7]. These noncommunicable diseases (NCD) are the major causes of death worldwide. The World Health Organization has projected an increase in mortality from NCD from 38 million in 2012 to 52 million in 2030 [
8].
Studies evaluating the dietary intake of BCAA are scarce. Moreover, despite the literature demonstrating the importance of identifying the relationship between dietary intake and socioeconomic status for advances of public health [
9], there is a gap of knowledge regarding the association between macronutrient and socioeconomic status [
10].
To our knowledge, there have been no population-based studies that examined the association of dietary BCAA intake with demographic, socioeconomic and lifestyle factors in Brazil. Therefore, the aim of this study was to investigate BCAA consumption and their association with demographic, socioeconomic and lifestyle factors, and to identify the main foods contributing to BCAA intake.
2. Materials and Methods
This study was approved by the Ethics Committee of the Public Health School of the University of São Paulo (Certificate of Presentation for Ethical Appreciation—CAAE # 26800414.1.0000.5421). All participants provided written informed consent before all procedures.
2.1. Study Design
Data were drawn from the 2008 Health Survey of São Paulo (Inquérito de Saúde de São Paulo, ISA-Capital 2008). This is a cross-sectional population-based survey that used a complex, stratified, multistage probability sample of individuals living in permanent households from urban areas of Sao Paulo, in southeastern Brazil. In this survey, health, nutritional information and lifestyle conditions were collected.
The sample was obtained in two stages. As the primary sampling units, 70 census tracts were randomly selected from all urban census tracts in the city of São Paulo. In the second stage, 16,607 households were randomly selected within these census tracts and stratified. Eight domains were fixed according to age and sex: infants (<one year; both genders); children (1–11 years; both genders); male adolescents (12–19 years); female adolescents (12–19 years); male adults (20–59 years); female adults (20–59 years); male older adults (60 years or more) and female older adults (60 years or more). To preserve the representativeness of each domain and to correct differences in the relative participation of the age groups in the population of the city of Sao Paulo, different sampling fractions were applied. The sample size was calculated to estimate proportions of 0.5 with a sample error of 0.07 at a 5% significance level and design effect of 1.5.
This ISA-Capital study included 2691 individuals aged 12 years and older for both sexes (605 adolescents, 1162 adults and 924 older adults). In the present study, we included only data obtained from adolescents (n = 560), adults (n = 585) and older adults (n = 517) of both sexes, who answered a structured questionnaire and at least one 24-h dietary recall (24HR). The survey response rate was 77%. The findings presented here are based on the complete case analysis.
2.2. Data Collecting and Processing
Characteristics such as weight, height, physical activity level, sex, age, the household head’s level of education, household per capita income, self-reported race, alcohol consumption and smoking status were collected by trained interviewers using a structured questionnaire formulated for this study.
Age was calculated by the difference between the interview date and the date of birth. Self-reported race was classified as
white and
non-white (black, brown, yellow and indigenous). The household head’s level of education was measured in years of study. Household per capita income was calculated by adding the monetary income reported by all family members and dividing by the number of family members (1 minimum wage = US
$260.00; R
$415.00). The smoking status was obtained from questions about current or former smoking habits and the number of cigarettes smoked per day were classified as
never,
current and
former smoker status. The intake of alcoholic beverages was obtained by asking questions about the amount, frequency and preference of the consumption of alcohol, and the individuals were classified as
consumer or
non consumer of alcoholic beverages [
11].
Level of physical activity was collected by the long version of the International Physical Activity Questionnaire [
12]. Leisure time physical activity was classified as
insufficiently active or
sufficiently active (
sufficiently active was defined as physical activity practiced at least 30 min daily, five days per week, at a moderate intensity or at least 20 min daily, three days per week, at a vigorous intensity).
Body mass index (BMI) was calculated using the Quetelet equation (BMI = weight (kg)/height (m
2)), from self-reported weight and height and classified according to the cut-off for adolescents (underweight, BMI < 3rd percentile; healthy weight, BMI > 3rd percentile and <85th percentile; overweight, BMI > 85th percentile and <97th percentile; obese, BMI > 97th percentile) [
13], adults (BMI < 18.5 kg/m
2; healthy weight, BMI 18.5–24.9 kg/m
2; overweight, BMI 25.0–29.9 kg/m
2; obese, BMI ≥ 30 kg/m
2) [
14] and older adults (underweight, BMI < 23 kg/m
2; healthy weight, BMI 23.0–27.9 kg/m
2; overweight, BMI 28–29.9 kg/m
2; obese, BMI ≥ 30 kg/m
2) [
15]. This information was validated by Carvalho et al. [
16] in a previous study with the same population, that found high sensitivity (>91%) and specificity (>83%) in all ages and sex groups.
2.3. Assessment of Dietary Intake
Dietary intake was assessed based on two 24HRs. Both were collected by trained interviewers on non-consecutive days, during all seasons of the year and days of the week. The first 24HR was collected in the household and the second was collected by telephone. These 24HRs were collected using, respectively, the Multiple-Pass Method and the Automated Multiple Pass Method, which have been described in detail by Guenther et al. and Blanton et al. [
17,
18]. The Nutrition Data System for Research (NDSR) software, 2014 version for the analyses of dietary intake data was used. This software was created at the University of Minnesota by the Nutrition Coordinating Center (Minneapolis, MN, USA) [
19]. The NDSR software uses the American food composition database developed by the United States Department of Agriculture (USDA) [
20]. Brazilian food compositions were compared with the USDA table and, if necessary, the BCAA value in food was corrected [
21].
The prevalence of inadequate dietary intake of BCAA was determined using the estimated average requirement (EAR) cut-off point approach, a simplification of the probability method, which estimates the proportion of individuals with usual intakes below the EAR (median requirement).
Food contributors to BCAA intake were investigated using the method of considering the sampling design, proposed by Block et al. [
22]. This method estimates the corresponding percentage of foods or food groups consumed by the population from the total intake of the nutrient assessed. A total of 45 groups were arranged by similarity in nutritional composition of BCAA.
The misreporting percentage was estimated using the equation: Energy intake—EER (estimated energy requirements)/EER∙100 [
23]. The equations used to calculate EER on an individual basis were from Institute of Medicine of the National Academies [
24].
2.4. Statistical Analyses
The characteristics of subjects were presented as medians and interquartile range (IQR) for continuous variables, and percentages for categorical variables. The total BCAA was defined as the sum of the energy-adjusted dietary Leu, Ile and Val. The usual BCAA intake was estimated using the Multiple Source Method (MSM) [
25]. This web-based tool provides usual food intake distributions by linking the probability and the amount of consumption with the integration of covariates into the model [
26]. Multiple linear regression models were used to evaluate the associations between the total and each individual’s BCAA intake with demographic, socioeconomic and lifestyle variables in all age groups (adolescents, adults and older adults). For these regression models, the total and each BCAA were adjusted for total energy intake using the nutrient residual model [
27]. Furthermore, all models were adjusted for misreporting and the models were accepted after residual analysis.
All analyses considered the complexity of the sample design and were performed with STATA version 13.0 (StataCorp LLC., College Station, TX, USA), considering p-values < 0.05 as statistically significant.
3. Results
The sample (n = 1662) was predominantly composed of the female sex (56.8%), the adults age group (35.2%), never smoked status (69.5%), self-reported white skin colour (58.0%) and non-consumer of alcohol beverages (58.3%). The median of the household head’s education was eight years (IQR 4–11) and household per capita income was US$275.0 (IQR 164.0–476.0). Excess body weight was observed in 35.7% of the individuals and 84.7% had insufficient leisure time physical activity.
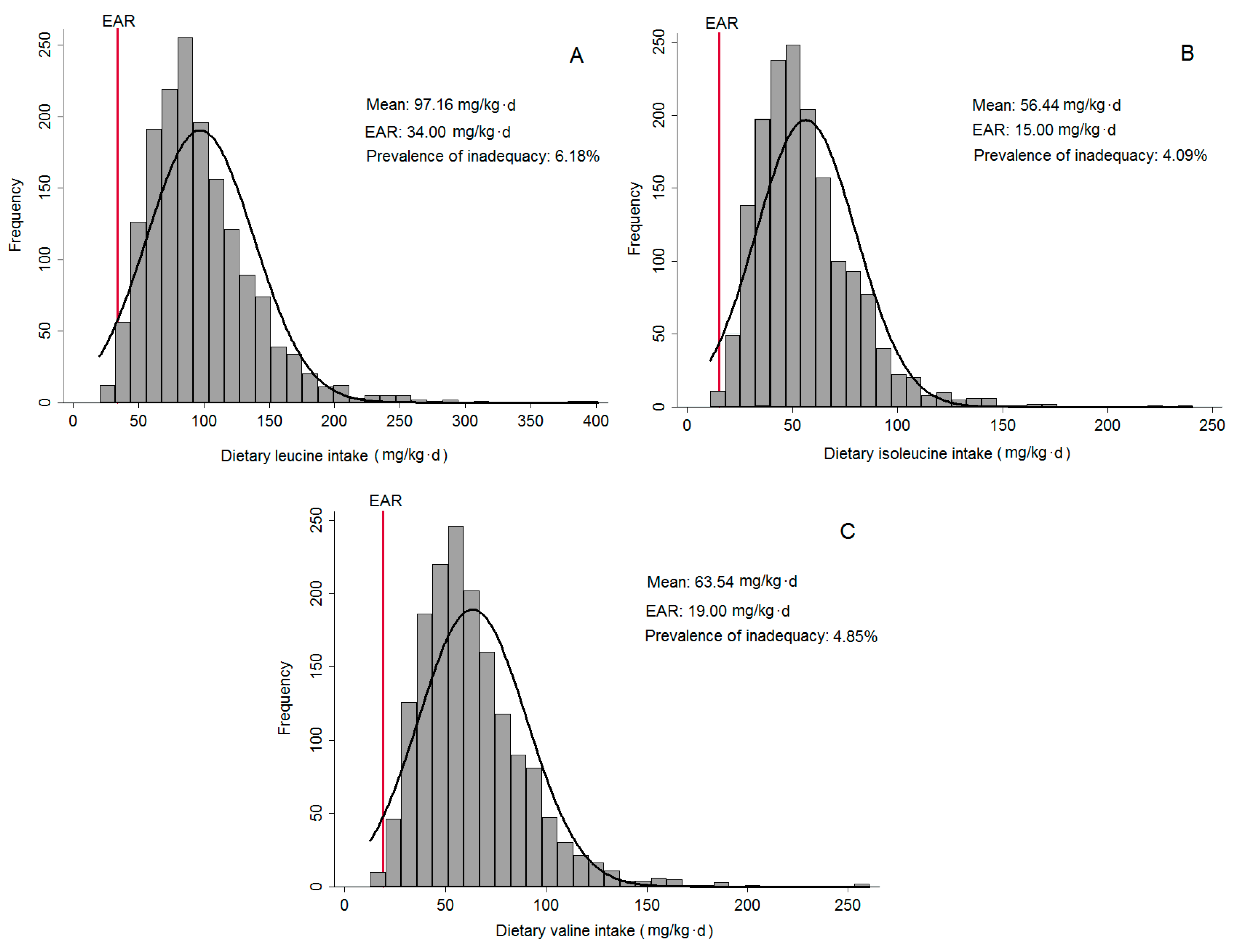
The mean of total BCAA intake was 217.14 mg/kg·day, with 97.16 mg/kg·day from Leu; 56.44 mg/kg·day from Ile; and 63.54 mg/kg·day from Val. In
Figure 1, it is possible to observe that deficiency of BCAA is low in the residents of São Paulo. Regarding age groups, the mean of total protein was 85.64 g/day for adolescents, 80.01 g/day for adults and 69.81 g/day for older adults. Considering the total BCAA by groups, the means for adolescents, adults and older adults were, respectively, 268.04 mg/kg·day, 203.13 mg/kg·day and 178.37 mg/kg·day.
The association between individuals’ BCAA (Leu, Ile and Val) and the total BCAA with socioeconomic, demographic and lifestyle conditions are shown in
Table 1,
Table 2 and
Table 3. After further adjustment for potential confounders, in adolescents, Leu, Ile, Val and total BCAA intakes were negatively associated with the female sex, non-white race and were positively associated with household per capita income. In adults, Leu, Ile, Val, and total BCAA intakes were negatively associated with the female sex and former smoker status and were positively associated with household per capita income. No associations were observed for older adults.
The main food contributors to Leu, Ile, Val, and total BCAA intakes were unprocessed red meat, unprocessed poultry, bread and toast, and beans and rice (
Table 4,
Table 5 and
Table 6).
4. Discussion
In this study, we observed consistent associations of BCAA consumption, including Leu, Ile and Val, with socioeconomic, demographic and lifestyle factors in adolescents and adults. Compared to dietary reference intake (DRI), residents of São Paulo appear to have a low prevalence of inadequacy. It is important to emphasize that some authors have raised questions about the DRI for BCAA intake and suggested higher values of requirement. Riazi et al. [
28], for example, indicated a total BCAA requirement for adults of 144 mg/kg·day, a value more than twice as high as the estimation by the DRI Committee (68 mg/kg·day). A group from the University of Toronto had suggested values 48% higher than the DRI value for young children [
29]. Some factors could be responsible for the distinct amino acids requirement, such as energy level, protein (or nitrogen) level, and fibre type [
30]. These facts impair the ability to determine precise requirements for amino acids in humans and animal models [
31,
32].
In adults, protein requirement estimations has been described in the literature and depend on one of two main approaches, namely, the factorial method and nitrogen balance. The BCAA-requirement estimation methods have limitations and are inconclusive. The DRI community uses the average of the values found by the methods [
24]. Besides this, considering all values of BCAA intake observed here, it is important to investigate the tolerable upper intake level of these amino acids for each age, mainly in older adults, once BCAA consumption can be linked to NCD.
Regardless of age, women had a lower association with the consumption of these amino acids when compared with men. Micha et al. [
33] observed a similar result when quantifying global intake of key foods related to NCD in adults from 187 countries and supposed that women consumed less unprocessed red meat (−4.2 g/day) in the Tropical Latin America region—unprocessed red meat is one of the main sources of BCAA. The lower protein consumption of women could be explained by the lower amount of food consumed in this sex group [
34], probably because females generally need a lower energy intake than males, due to smaller average body weight and lower resting metabolic rate [
35]. Despite these differences between the sexes, EAR does not consider different values for both sexes, which highlights this difference.
Former smoker status was associated with higher intake of individual and total BCAA in adults. Smoking cessation appears to be associated with being overweight and having a higher intake of energy (calories), cholesterol, saturated fatty acids, and alcohol [
36]. In addition, being overweight or obese may contribute to a higher consumption of animal protein; western dietary habits; lower consumption of fruits, vegetables, whole grains; and a higher consumption of sweets [
37,
38].
The results obtained in the present study pointed to the association between self-reported race, household per capita income, and higher intake of all these amino acids. This association corroborates with the results of Carvalho et al. [
39], which showed that Brazilians ingest more red meat for all age groups and these results are associated with higher household per capita income. The high consumption of red meat has been described in the literature. Souza et al. [
40] observed during the National Dietary Survey, which used the database of the National Family Budgets Survey, that the percentage of red meat consumption varies from 43% to 50%. It is important to emphasize that meat, rice, beans, coffee and bread constitute the dietary pattern of the Brazilian population.
Our findings demonstrate that unprocessed red meat is the main source of dietary Leu, Ile, Val and total BCAA intake for all age groups, constituting 22% of food intake. Regarding the BCAA intake, our results were similar to the US adult population, in that the major food contributors were red meat (~37%), milk (~12%) and fish (~8%) [
6]. However, these results are different from those observed in Japan, whose lowest food contributor with total BCAA intake in the adult population was red meat with approximately 14.9% of consumption in men and 13.7% in women [
5].
In older adults, no significant results were found. This age group generally consumes less protein and some of the major dangers of this is the loss of muscle mass, strength and sarcopenia, which is a function that progressively occurs with aging. Several studies have identified animal protein, which contains essential amino acids, as a key nutrient for muscle health in older adults. These populations are less responsive to the anabolic stimulus of low doses of amino acid intake compared to younger individuals [
41]. Therefore, the requirement for older adults needs to be higher for better anabolic responsiveness and the consumption of high-quality proteins must be proposed.
In a recent population-based cohort study, Zheng et al. [
6] showed that high circulating levels of BCAA can be a biomarker of type 2 diabetes and a risk marker of other NCDs. In this context, over nutrition affects the rising of BCAA circulating in the plasma, leading to increase flux of these amino acids through their catabolic pathways [
6]. In humans, these amino acids play regulatory roles in insulin and glucose metabolism. Thus, diet is an important source of these amino acids, and meat is an important food item for human nutrition; however, excessive meat intake, especially red and processed red meat, has been linked to the risk of cancer and to potentially carcinogenic substances [
42].
Our study has limitations. First, this is a cross-sectional study, consequently, it is impossible to determine causality between the factors evaluated and BCAA intake. In addition, the dietary assessment used was the 24HR, which is a method that has a source of errors such as recollection of memory, omissions and possible errors in portion size estimation. However, we sought to minimize the influence of the potentially confounding variables. We measured the database from two 24HRs, through the Multiple Pass Method and finally, we used the MSM to estimate usual intake.