Desalted Duck Egg White Peptides Promote Calcium Uptake and Modulate Bone Formation in the Retinoic Acid-Induced Bone Loss Rat and Caco-2 Cell Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Desalted Duck Egg White Peptides (DPs)
2.2. Animals and Experimental Design
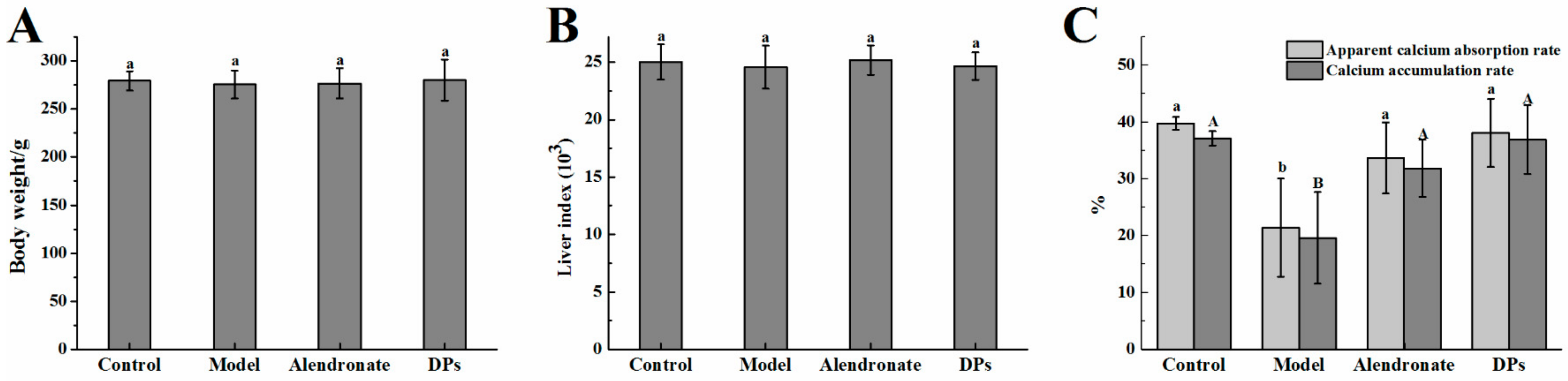
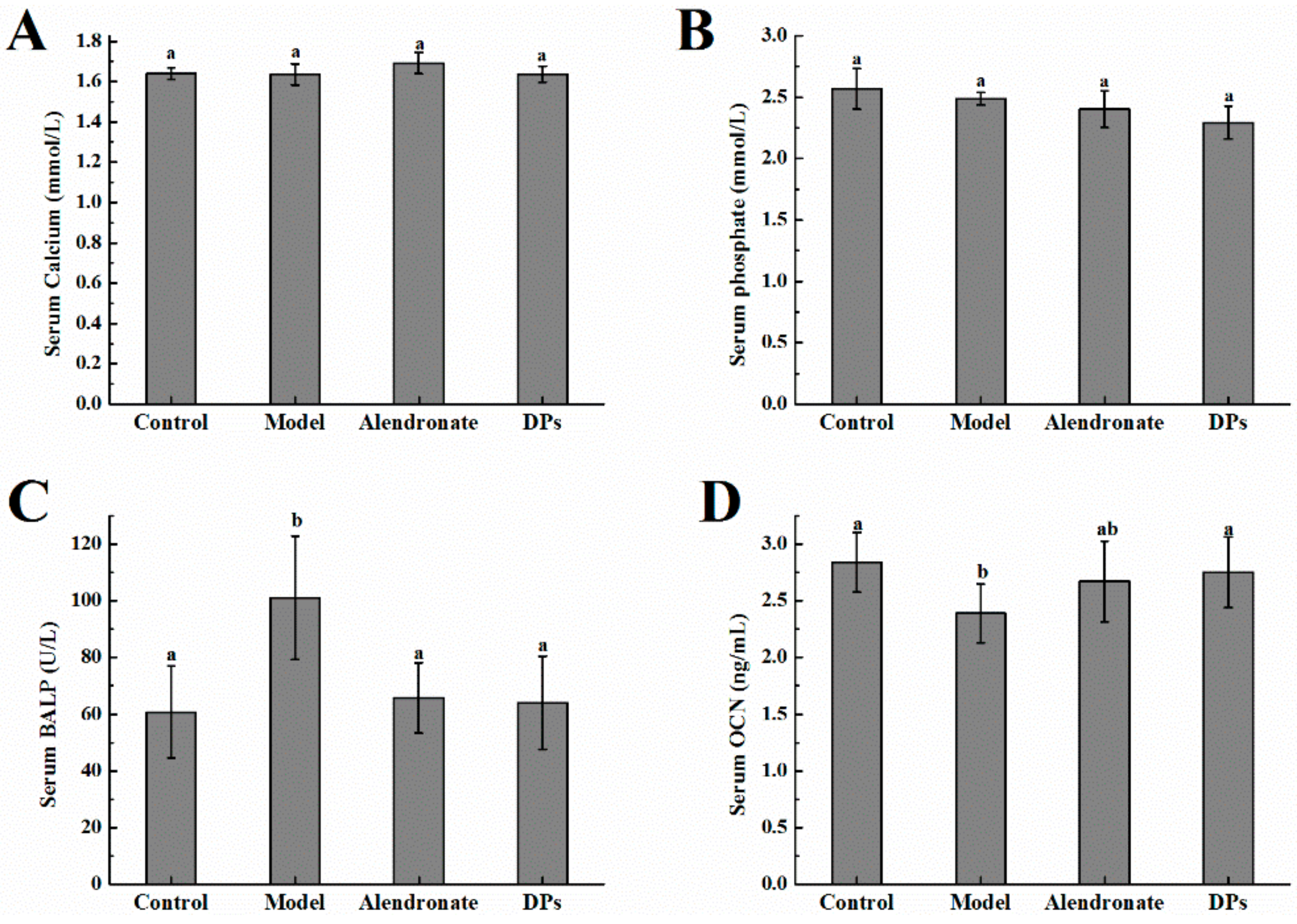
2.3. Serum Parameters
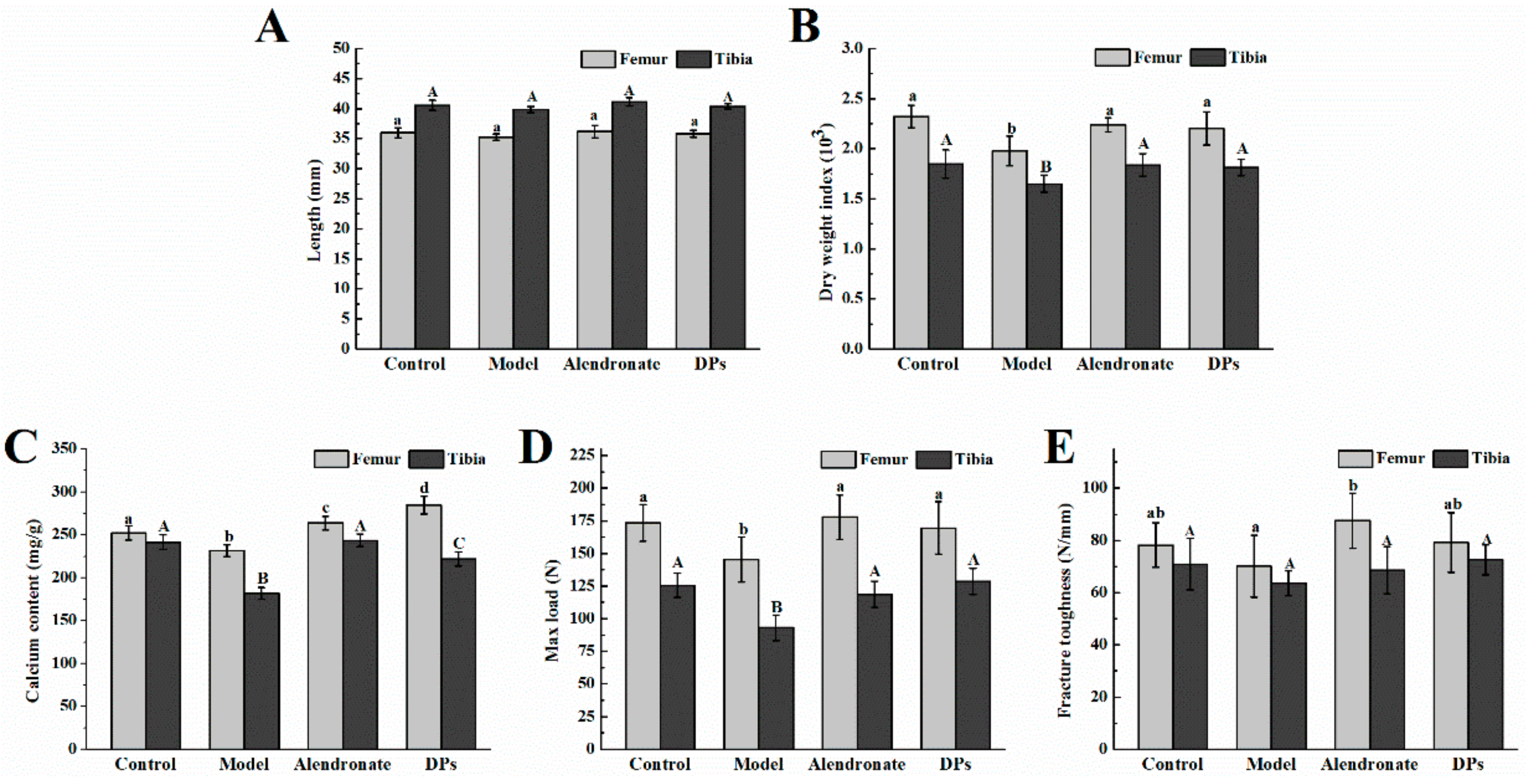
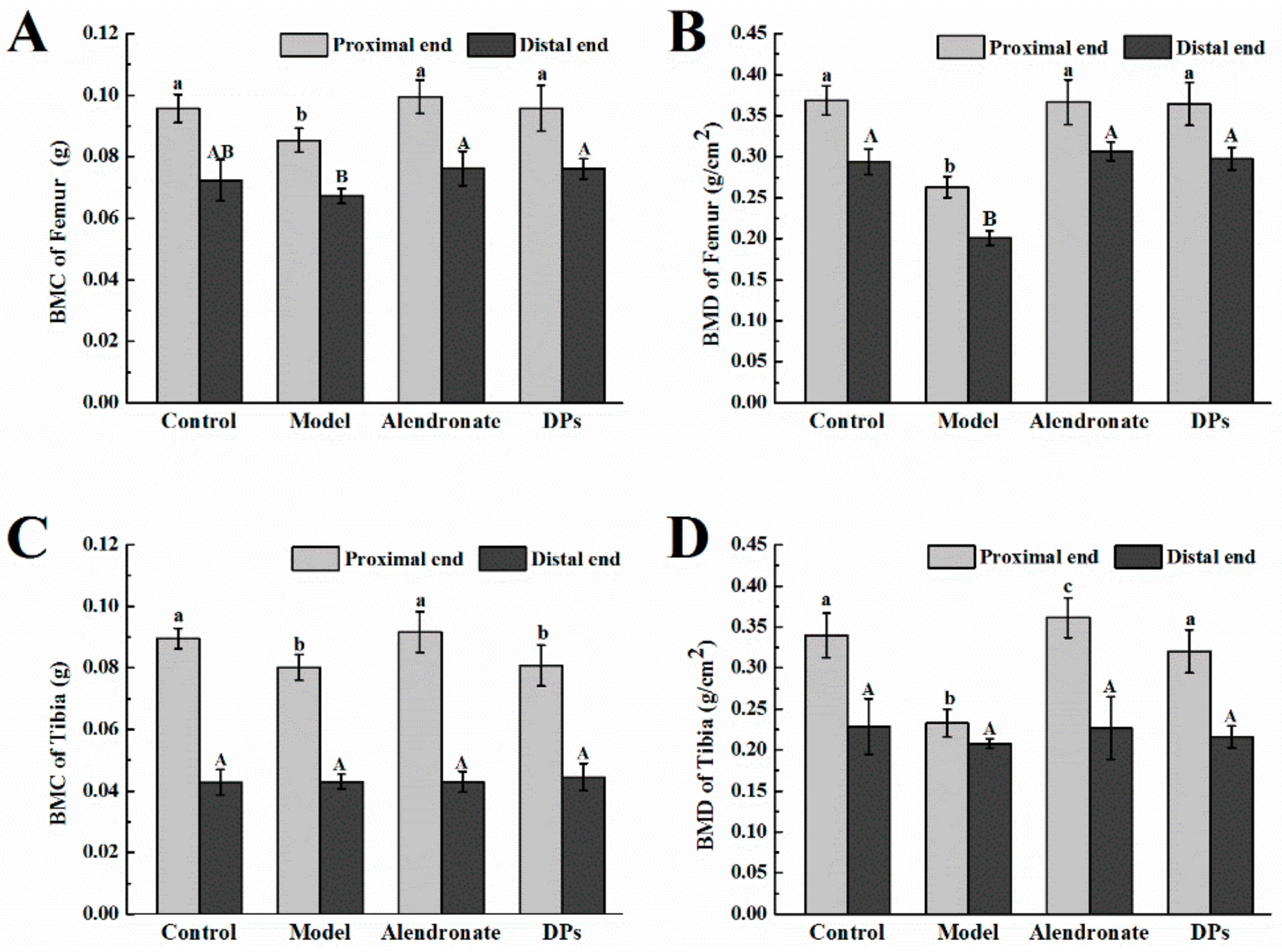
2.4. Bone Parameters
2.5. Establishment of Caco-2 Cell Monolayers
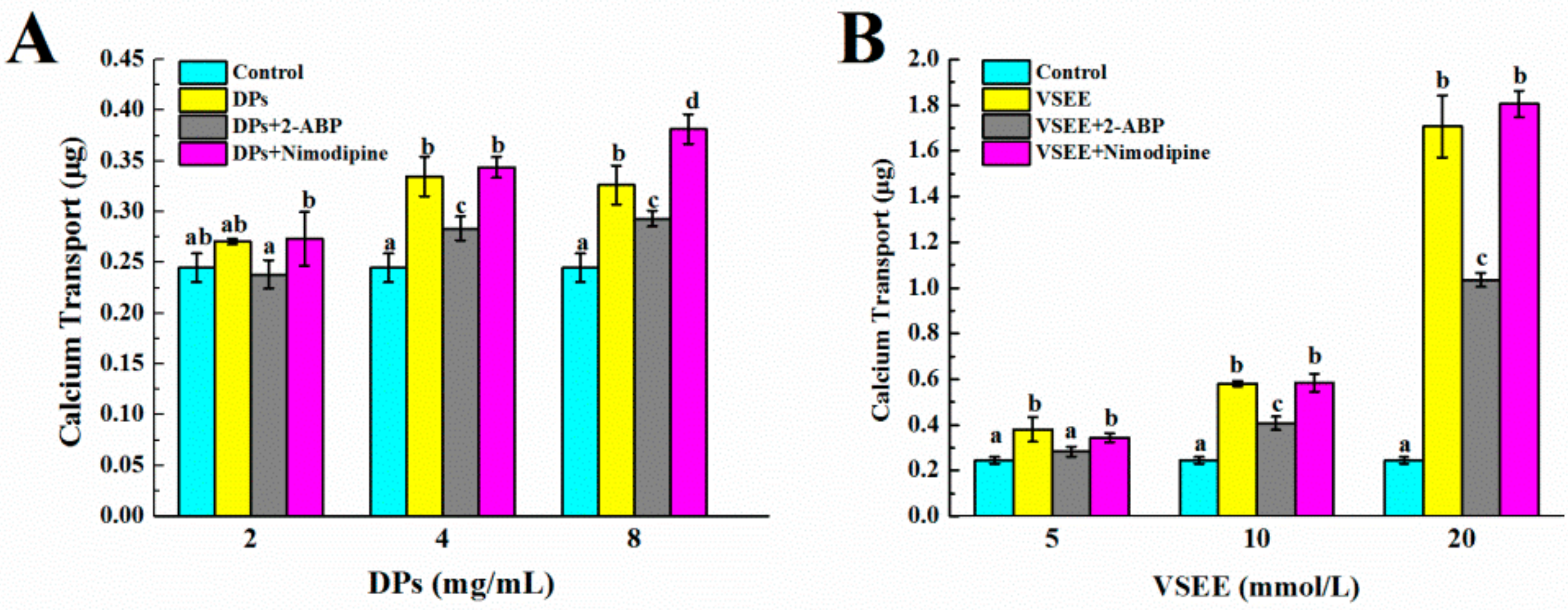
2.6. Calcium Transport Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physiological Variables and Apparent Absorption Rate
3.2. The Values of Calcium, Phosphate, BALP, and OCN in Serum
3.3. Bone Test Results
3.3.1. Bone Calcium Content, Bone Length, Biomechanical Parameters, BMC, and BMD
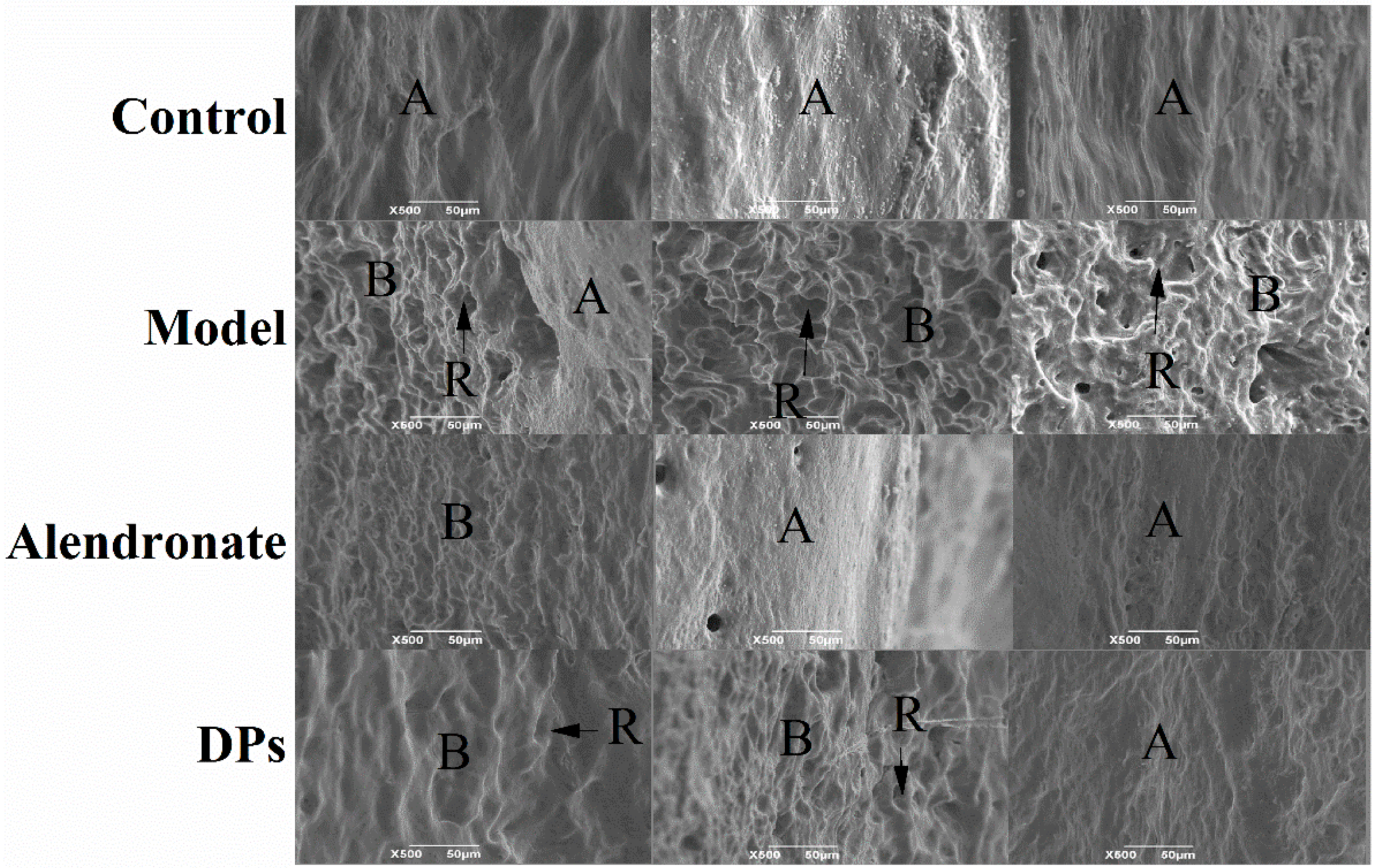
3.3.2. The SEM of Bones
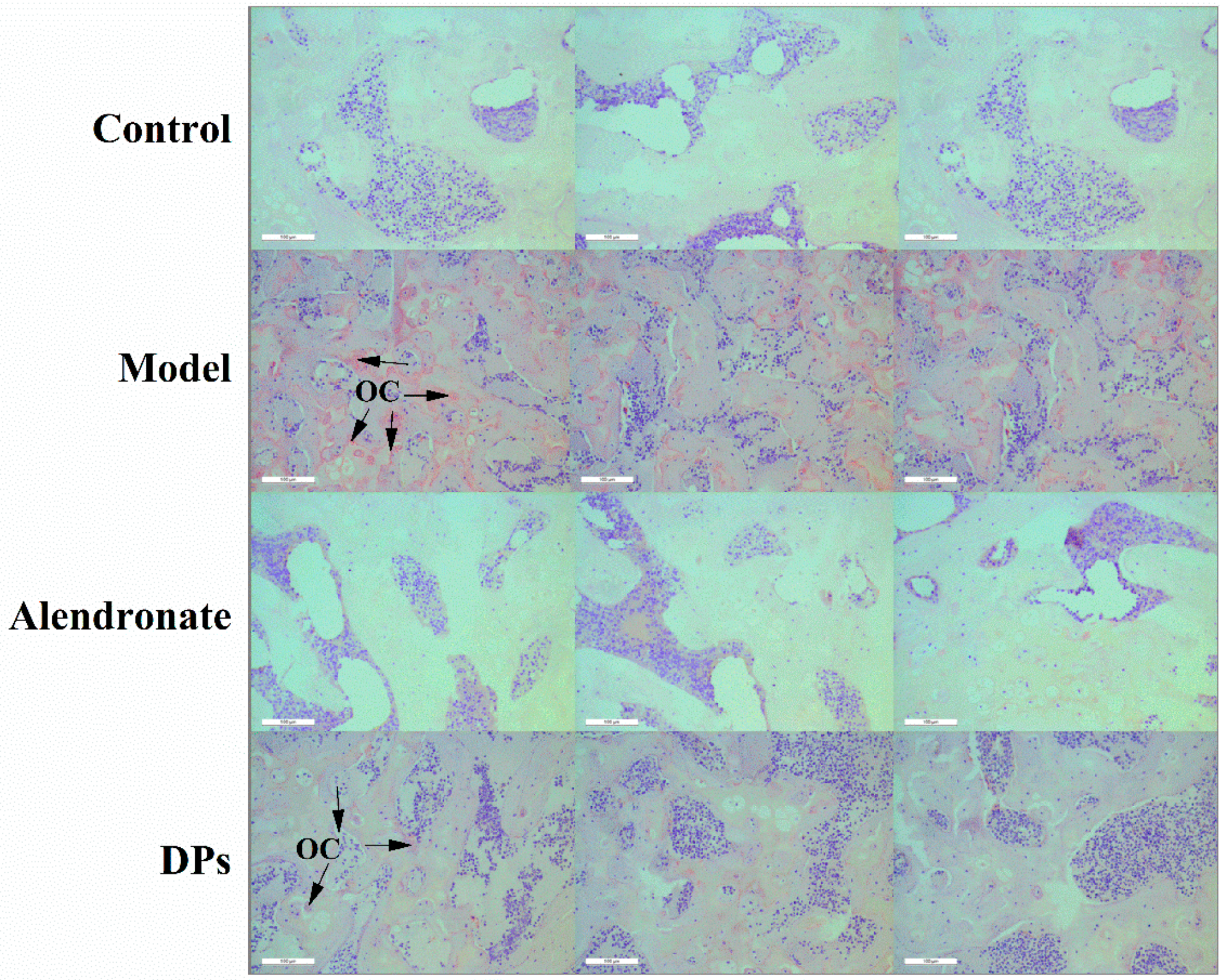
3.3.3. The Tartrate-Resistant Acid Phosphate (TRAP) Staining of Bones
3.4. The Pathway of Calcium Absorption by DPs
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a who report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Orsolic, N.; Goluza, E.; Dikic, D.; Lisicic, D.; Sasilo, K.; Rodak, E.; Jelec, Z.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Chavassieux, P.; Santora, A.; Yates, J.; Meunier, P. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 2000, 27, 687–694. [Google Scholar] [CrossRef]
- Liao, E.Y.; Luo, X.H.; Wang, W.B.; Wu, X.P.; Zhou, H.D.; Dai, R.C.; Liao, H.J.; Yang, C. Effects of different nylestriol/levonorgestrel dosages on bone metabolism in female sprague–dawley rats with retinoic acid-induced osteoporosis. Endocr. Res. 2003, 29, 23–42. [Google Scholar] [CrossRef] [PubMed]
- García Vieyra, M.I.; Del Real, A.; López, M.G. Agave fructans: Their effect on mineral absorption and bone mineral content. J. Med. Food 2014, 17, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, M.D.; Espinosa Martos, I.; Préstamo, G.; Rupérez, P. Soybean whey enhance mineral balance and caecal fermentation in rats. Eur. J. Nutr. 2010, 49, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; McCabe, G.P.; McCabe, L.D.; Peacock, M.; Weaver, C.M. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: A randomised controlled trial using dual stable isotopic tracers. Brit. J. Nutr. 2014, 112, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, K.; Jamaluddin, R.; Karimi, G.; Erfani, R. Effect of probiotics supplementation on bone mineral content and bone mass density. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Zhao, L.N.; Cai, X.; Wang, S.Y.; Huang, Y.F.; Hong, J.; Rao, P.F. Purification and characterisation of a glutamic acid-containing peptide with calcium-binding capacity from whey protein hydrolysate. J. Dairy Res. 2015, 82, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Harnedy, P.A.; O’Keeffe, M.B.; Zhang, L.; Li, B.; Hou, H.; FitzGerald, R.J. Fractionation and identification of alaska pollock skin collagen-derived mineral chelating peptides. Food Chem. 2015, 173, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ren, L.; Jiang, J. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur. Food Res. Technol. 2011, 232, 281–287. [Google Scholar] [CrossRef]
- Jeon, S.J.; Lee, J.H.; Song, K.B. Isolation of a calcium-binding peptide from chlorella protein hydrolysates. J. Food Sci. Nutr. 2010, 15, 282–286. [Google Scholar] [CrossRef]
- Choi, D.W.; Lee, J.H.; Chun, H.H.; Bin Song, K. Isolation of a calcium-binding peptide from bovine serum protein hydrolysates. Food Sci. Biotechnol. 2012, 21, 1663–1667. [Google Scholar] [CrossRef]
- Liu, F.R.; Wang, L.; Wang, R.; Chen, Z.X. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide–calcium complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef] [PubMed]
- Erba, D.; Ciappellano, S.; Testolin, G. Effect of caseinphosphopeptides on inhibition of calcium intestinal absorption due to phosphate. Nutr. Res. 2001, 21, 649–656. [Google Scholar] [CrossRef]
- Erba, D.; Ciappellano, S.; Testolin, G. Effect of the ratio of casein phosphopeptides to calcium (w/w) on passive calcium transport in the distal small intestine of rats. Nutrition 2002, 18, 743–746. [Google Scholar] [CrossRef]
- Parkinson, G.; Cransberg, P. Effect of casein phosphopeptide and 25-hydroxycholecalciferol on tibial dyschondroplasia in growing broiler chickens. Brit. Poultry Sci. 2004, 45, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Tsuchita, H.; Goto, T.; Shimizu, T.; Yonehara, Y.; Kuwata, T. Dietary casein phosphopeptides prevent bone loss in aged ovariectomized rats. J. Nutr. 1996, 126, 86. [Google Scholar] [PubMed]
- Cross, K.; Huq, N.; Reynolds, E. Casein phosphopeptides in oral health-chemistry and clinical applications. Curr. Pharm. Des. 2007, 13, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Hu, J.; Hou, T.; Ma, Z.; Wang, C.; He, H. Effects of desalted duck egg white peptides and their products on calcium absorption in rats. J. Funct. Foods 2014, 8, 234–242. [Google Scholar] [CrossRef]
- Hou, T.; Liu, W.; Shi, W.; Ma, Z.; He, H. Desalted duck egg white peptides promote calcium uptake by counteracting the adverse effects of phytic acid. Food Chem. 2017, 219, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, C.; Ma, Z.; Shi, W.; Lui, W.; He, H. Desalted duck egg white peptides: Promotion of calcium uptake and structure characterization. J. Agric. Food Chem. 2015, 63, 8170–8176. [Google Scholar] [CrossRef] [PubMed]
- Pitts, C.J.D.; Kearns, A.E. Update on medications with adverse skeletal effects. Mayo Clin. Proc. 2011, 86, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yang, Z.; Li, P.; Zhang, Y.; Sse, W.C. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 2007, 35, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Hosking, D.; Devogelaer, J.P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Guo, X.; Zhang, Y.G.; Li, Y.F.; Cao, J.L.; Xiong, Y.M. The effect of retinoic acid on induction of osteoporotic model rats and the possible mechanism. J. Sichuan Univ. 2005, 36, 229–232. [Google Scholar]
- Ranhotra, H.S. The interplay between retinoic acid receptor-related orphan receptors and human diseases. J. Recept. Sig. Transd. 2012, 32, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nieves, J. Skeletal effects of nutrients and nutraceuticals, beyond calcium and vitamin D. Osteoporos. Int. 2013, 24, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Yang, L.; Meng, G.L.; Yuan, Z.; Fan, J.; Li, D.; Chen, J.Z.; Shi, T.Y.; Hu, H.M.; Wei, B.-Y. Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS ONE 2013, 8, e57251. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R.; Soliman, A.M. Oxidative stress as a risk factor of osteoporotic model induced by vitamin A in rats. Aust. J. Basic. Appl. Sci. 2009, 3, 1559–1568. [Google Scholar]
- Marks, S.C.; Cielinski, M.J.; Sundquist, K.T. Bone surface morphology reflects local skeletal metabolism. Microsc. Res. Tech. 1996, 33, 121–127. [Google Scholar] [CrossRef]
- Boyde, A.; Hobdell, M. Scanning electron microscopy of primary membrane bone. Z. Zellforsch. Mikrosk. Anat. 1969, 99, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Cerri, P.S.; Boabaid, F.; Katchburian, E. Combined tunel and trap methods suggest that apoptotic bone cells are inside vacuoles of alveolar bone osteoclasts in young rats. J. Periodontal Res. 2003, 38, 223–226. [Google Scholar] [CrossRef] [PubMed]
- de Barboza, G.D.; Guizzardi, S.; de Talamoni, N.T. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142. [Google Scholar] [CrossRef]
- Kovacs, G.; Montalbetti, N.; Simonin, A.; Danko, T.; Balazs, B.; Zsembery, A.; Hediger, M.A. Inhibition of the human epithelial calcium channel TRPV6 by 2-aminoethoxydiphenyl borate (2-APB). Cell Calcium 2012, 52, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Perego, S.; Cosentino, S.; Fiorilli, A.; Tettamanti, G.; Ferraretto, A. Casein phosphopeptides modulate proliferation and apoptosis in HT-29 cell line through their interaction with voltage-operated l-type calcium channels. J. Nutr. Biochem. 2012, 23, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Wikman Larhed, A.; Artursson, P. Co-cultures of human intestinal goblet (HT29-h) and absorptive (Caco-2) cells for studies of drug and peptide absorption. Eur. J. Pharm. Sci. 1995, 3, 171–183. [Google Scholar] [CrossRef]
- Suzuki, Y.; Landowski, C.P.; Hediger, M.A. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 2008, 70, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L. Alternative perspective on intestinal calcium absorption: Proposed complementary actions of Cav1. 3 and TRPV6. Nutr. Rev. 2011, 69, 347–370. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, T.; Liu, Y.; Kolba, N.; Guo, D.; He, H. Desalted Duck Egg White Peptides Promote Calcium Uptake and Modulate Bone Formation in the Retinoic Acid-Induced Bone Loss Rat and Caco-2 Cell Model. Nutrients 2017, 9, 490. https://doi.org/10.3390/nu9050490
Hou T, Liu Y, Kolba N, Guo D, He H. Desalted Duck Egg White Peptides Promote Calcium Uptake and Modulate Bone Formation in the Retinoic Acid-Induced Bone Loss Rat and Caco-2 Cell Model. Nutrients. 2017; 9(5):490. https://doi.org/10.3390/nu9050490
Chicago/Turabian StyleHou, Tao, Yanshuang Liu, Nikolai Kolba, Danjun Guo, and Hui He. 2017. "Desalted Duck Egg White Peptides Promote Calcium Uptake and Modulate Bone Formation in the Retinoic Acid-Induced Bone Loss Rat and Caco-2 Cell Model" Nutrients 9, no. 5: 490. https://doi.org/10.3390/nu9050490




