Calcium Supplement Derived from Gallus gallus domesticus Promotes BMP-2/RUNX2/SMAD5 and Suppresses TRAP/RANK Expression through MAPK Signaling Activation
Abstract
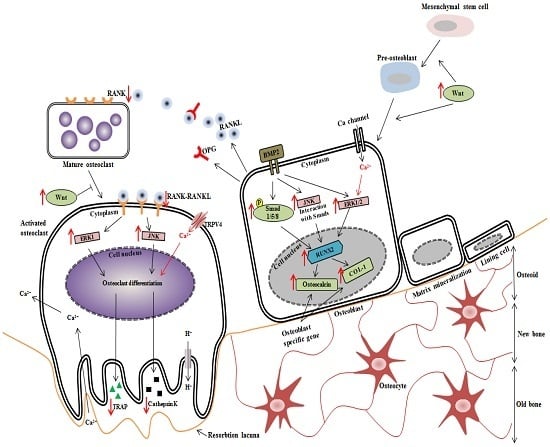
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sample
2.2. Animals and Diet
2.3. Biochemical Analyses
2.4. Biomechanical Testing of Tibia
2.5. Ca Content of Tibia
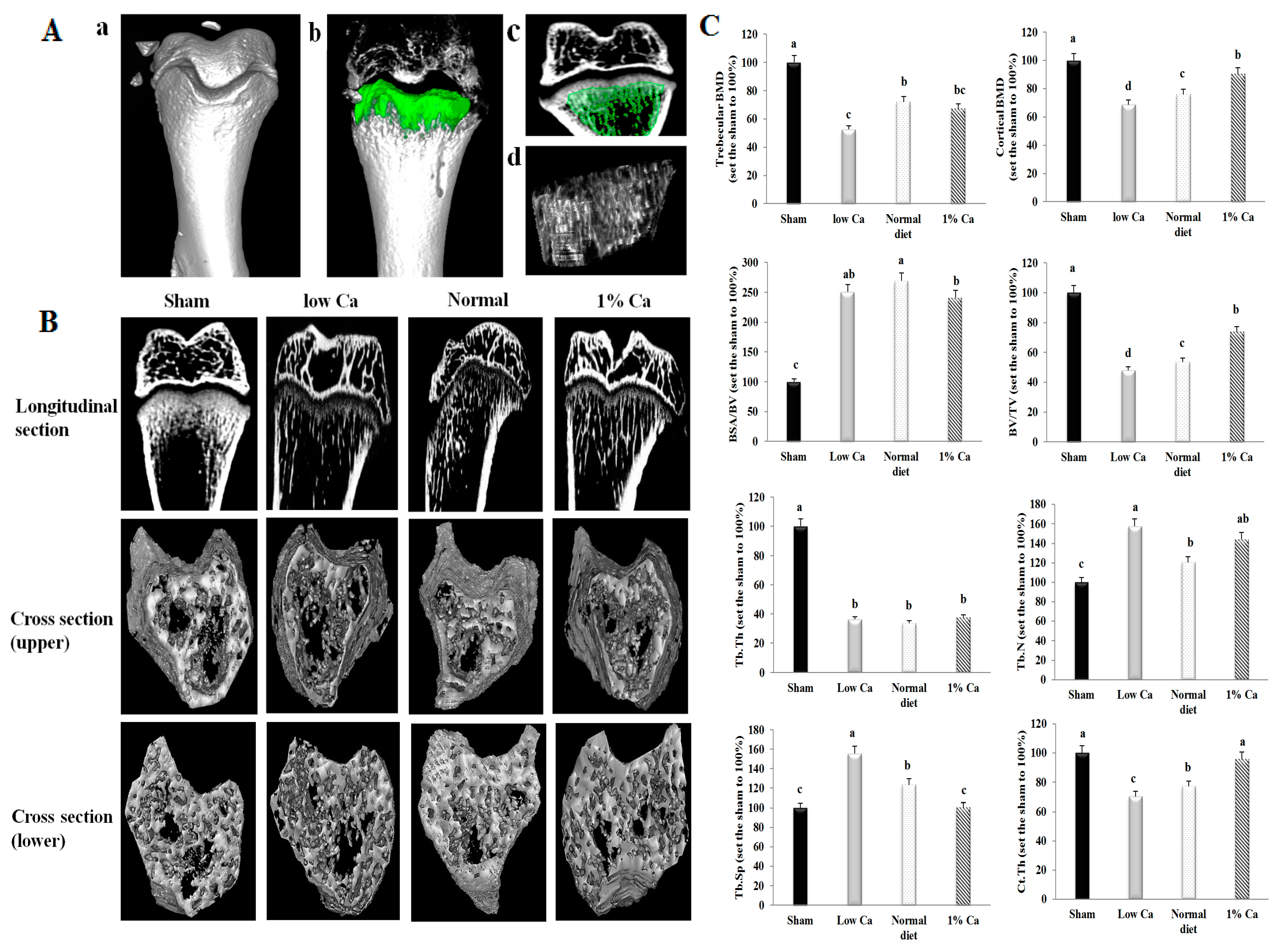
2.6. Micro-Computed Tomography
2.7. Histology and Tartrate-Resistant Acid Phosphatase Staining
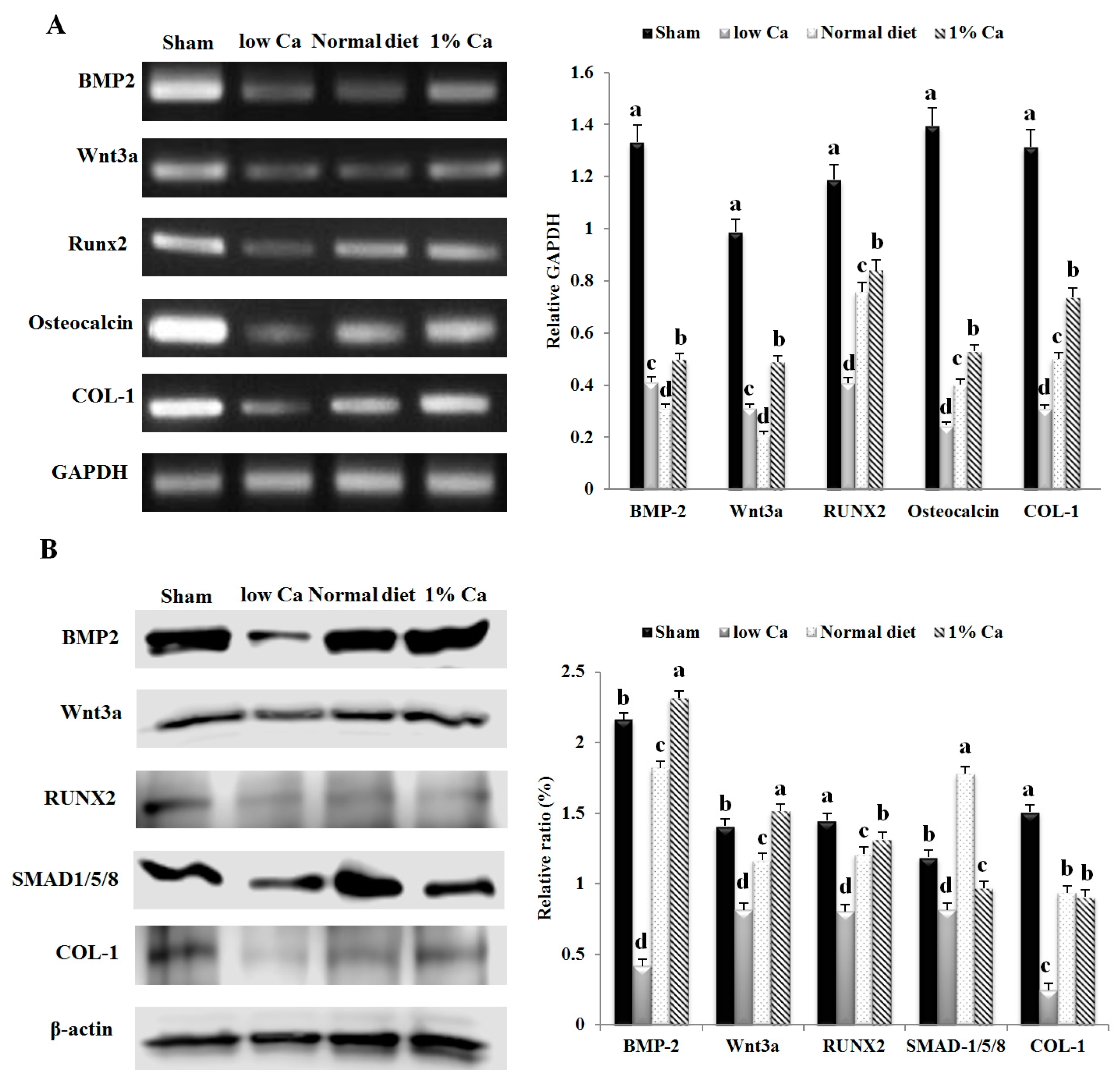
2.8. Reverse Transcription-Polymerase Chain Reaction Assay
2.9. Western Blot Analysis
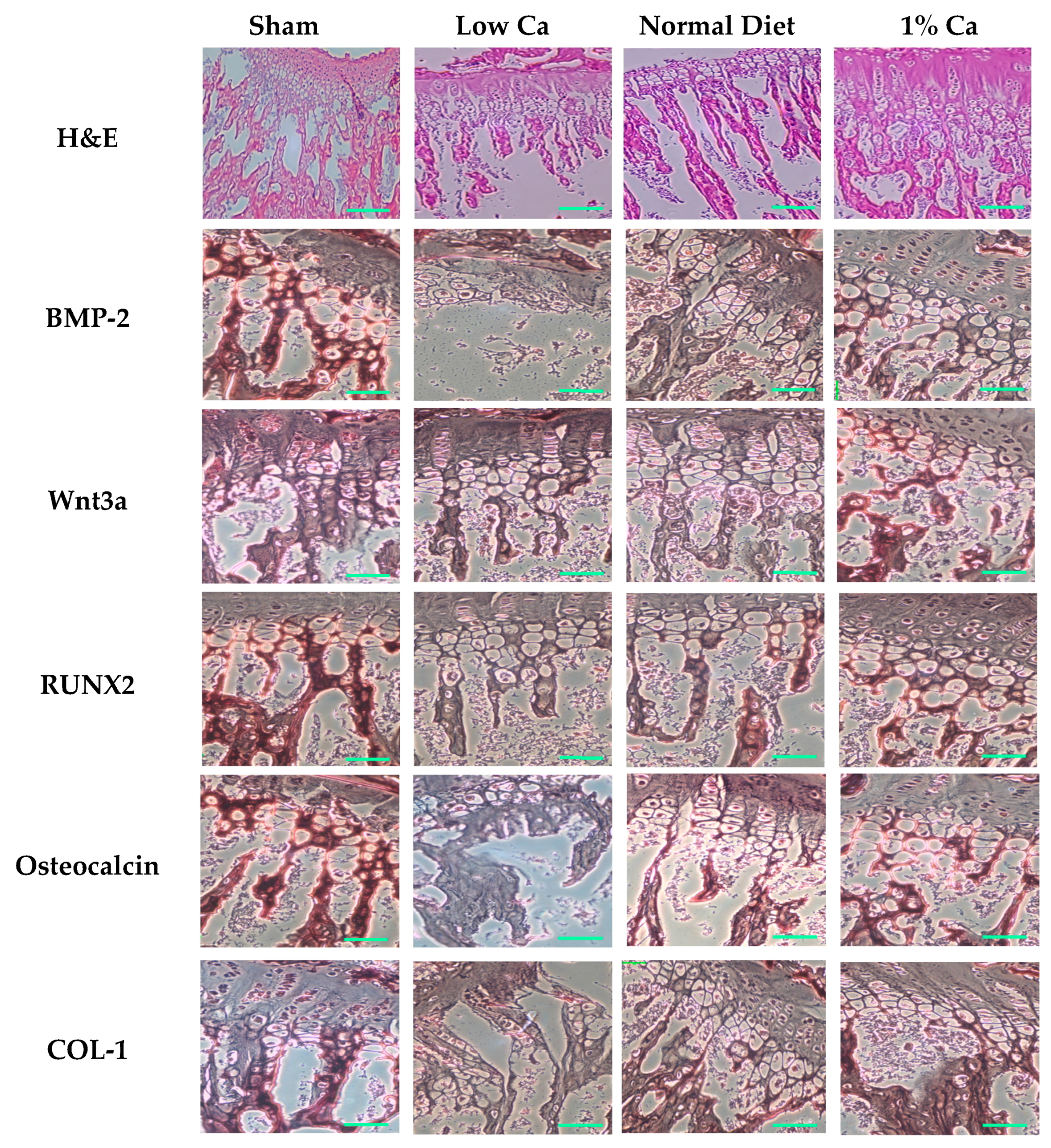
2.10. Immunohistochemical Stain
2.11. Statistical Analysis
3. Results
3.1. Serum Estrogen Concentrations of Tibia
3.2. Feed Efficiency Ratio
3.3. Tibia Bone Strength and Ca Content of Tibia
3.4. Effects on Bone Morphometric Parameters
3.5. Expression of Osteoblastogenesis Marker Genes
3.6. Effects of Ca on the Expression of Osteoclast Specific Gene Markers
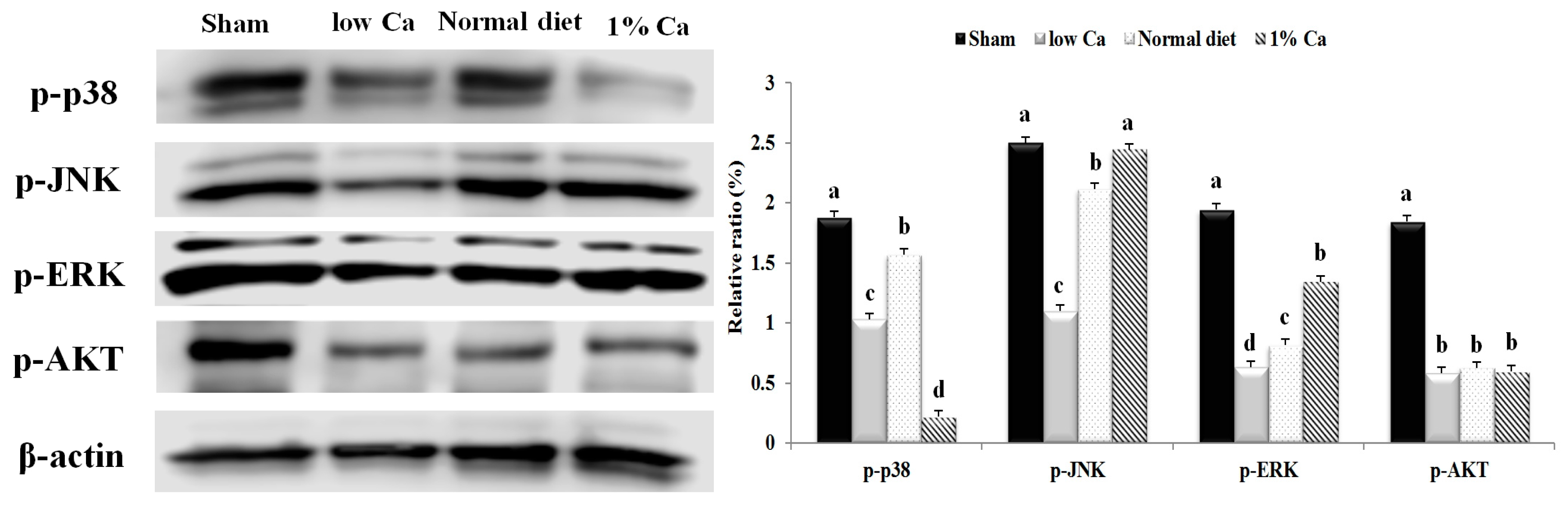
3.7. Effects of 1% Ca Supplement on Phosphorylation of AKT, ERK and p38 MAPKs
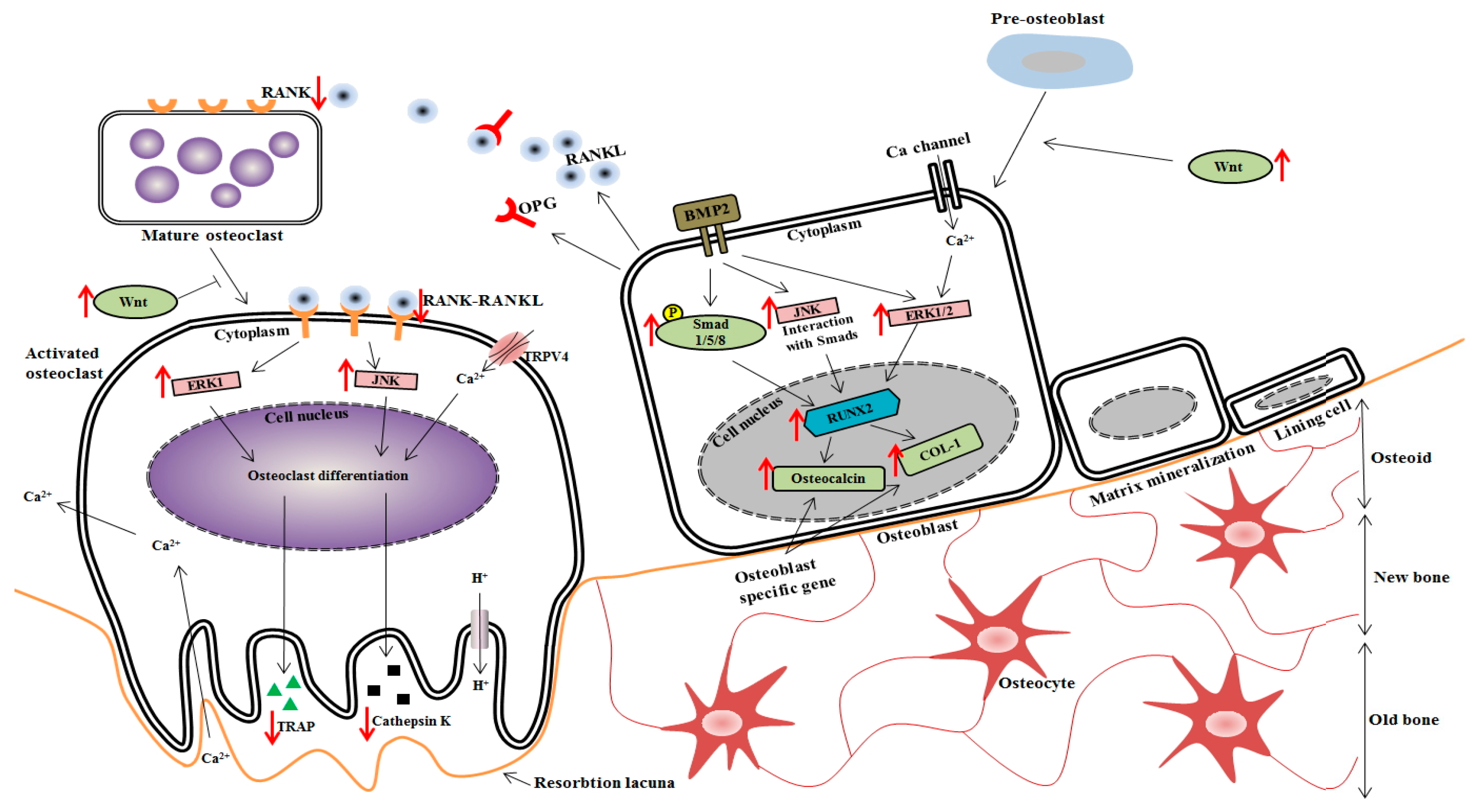
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, C.H.; Song, G.S.; Kim, Y.S. Effects of cheonggukjang and doenjang on bone loss in ovariectomized rats. Food Sci. Biotechnol. 2008, 17, 553–557. [Google Scholar]
- Bart, L.; Clarke, M.D.; Khosla, S. Physiology of bone loss. Radiol. Clin. N. Am. 2010, 48, 483–495. [Google Scholar] [CrossRef]
- Goodman, M.T.; Wilkens, L.R.; Hankin, J.H.; Lyu, L.C.; Wu, A.H.; Kolonel, L.N. Association of soy and fiber consumption with the risk of endometrial cancer. Am. J. Epidemiol. 1997, 146, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.; Davis, S.R. Hormone replacement theraphy: Current controversies. Clin. Endocrinol. 2003, 58, 249–261. [Google Scholar] [CrossRef]
- Barnes, S. Phyto-oestrogens and osteoporosis; what is a safe dose? Brit. J. Nutr. 2003, 89, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Orawan, W.; Kanchanit, B.; Peraphan, P.; Vichai, R.; Prachya, K. Sesamin stimulates osteoblast differentiation through p38 and ERK1/2 MAPK signaling pathways. BMC Complem. Altern. Med. 2012, 12, 71. [Google Scholar] [CrossRef]
- Kang, S.C.; Kim, H.J.; Kim, M.H. Effects of astragalus membranaceus with supplemental calcium on bone mineral density and bone metabolism in calcium-deficient ovariectomized rats. Biol. Trace Elem. Res. 2013, 151, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rasch, L.A.; Schueren, M.A.E.; Tuyl, L.H.D.; Bultink, I.E.M.; Vries, J.H.M.; Lems, W.F. Content validity of a short calcium intake list to estimate daily dietary calcium intake of patients with osteoporosis. Calcif. Tissue Int. 2017, 100, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Adluri, R.S.; Zhan, L.; Bagchi, M.; Maulik, N.; Maulik, G. Comparative effects of a novel plant-based calcium supplement with two common calcium salts on proliferation and mineralization in human osteoblast cells. Mol. Cell. Biochem. 2010, 740, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.S.; Lee, H.S.; Kim, K.Y.; Kim, M.J.; Kang, P.D. Effects of silkworm pupae on bone mineral density in ovariectomized rat model of osteoporesis. Int. J. Indust. Entomol. 2012, 24, 63–68. [Google Scholar] [CrossRef]
- Petenuci, M.E.; Stevanato, F.B.; Visentainer, J.E.; Matsushita, M.; Gracia, E.E.; de Souza, N.E.; Visentainer, J.V. Fatty acid concentration, proximate composition, and mineral composition in fishbone flour of Nile Tilapia. Arch. Latinoam. Nutr. 2008, 58, 87–90. [Google Scholar] [PubMed]
- Larsen, T.; Thilsted, S.H.; Kongsbak, K.; Hansen, M. Whole small fish as a rich calcium source. Brit. J. Nutr. 2000, 83, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jeon, B.J.; Ahn, J.; Kwak, H.S. Effects of nanocalcium supplemented milk on bone calcium metabolism in ovariectomized rats. Asian Aust. J. Anim. Sci. 2007, 20, 1266–1271. [Google Scholar] [CrossRef]
- Fujita, T.; Fukase, M.; Miyamoto, H.; Matsumoto, T.; Ohue, T. Increase of bone mineral density by calcium supplement with oyster shell electrolysate. Bone Miner. 1990, 11, 85–91. [Google Scholar] [CrossRef]
- Jung, W.K.; Kim, W.K. Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius. belengerii) frame. Eur. Food Res. Technol. 2007, 224, 763–767. [Google Scholar] [CrossRef]
- Lee, J.G.; Lim, Y.S.; Joo, D.S.; Jeong, I.H. Effects of diet with sea tangle (Kjellemaniella. crassifolia) on calcium absorption, serum composition and feces in rats. Korean J. Fish Aquat. Sci. 2002, 35, 601–607. [Google Scholar] [CrossRef]
- Cho, C.M.; Park, C.K.; Lee, M.Y.; Lew, I.D. Physicochemical characteristics of silky fowl (gallusdomesticus var. silkies). Korean J. Food Sci. Anim. Res. 2006, 26, 306–314. [Google Scholar]
- Chae, H.S.; An, J.N.; Yoo, Y.M.; Park, Y.B.; Cho, S.H.; Kim, J.H. Quality stability of high pressure boiled extract of ogol chicken during storage periods. Korean J. Poult. Sci. 2002, 29, 279–286. [Google Scholar]
- Liu, W.; Gu, R.; Lin, F.; Lu, J.; Yi, W.; Ma, Y.; Dong, Z.; Cai, M. Isolation and identification of antioxidative peptides from pilot-scale black-bone silky fowl (Gallus gallus domesticus Brisson) muscle oligopeptides. J. Sci. Food Agric. 2013, 93, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Chung, K.H.; Lee, K.J.; Kim, D.H.; An, J.H. Effect of gallus gallus var. domesticus (Yeonsan ogolgye) extracts on osteoblast differentiation and osteoclast formation. J. Microbiol. Biotechnol. 2015, 43, 322–329. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.K.; Saleh, H.A. Olive oil effectively mitigates ovariectomy-induced osteoporosis in rats. BMC Complem. Altern. Med. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell. Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Osyczka, A.M.; Leboy, P.S. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology 2005, 146, 3428–3437. [Google Scholar] [CrossRef] [PubMed]
- Bobick, B.E.; Kulyk, W.M. Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Res. C Embryo Today 2008, 84, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Baslé, M.F.; Legrand, E.; Audran, M. Trabecular bone microarchitecture: A review. Morphologie 2008, 92, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Valenti, M.T.; Bertoldo, F.; Zanatta, M.; Zenari, S.; Realdi, G.; Lo Cascio, V.; Giannini, S. Bone microarchitecture evaluated by histomorphometry. Micron 2005, 36, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Liu, Y.S.; Zhou, Y.S. Bone micro-architectural analysis of mandible and tibia in ovariectomised rats: A quantitative structural comparison between undecalcified histological sections and micro-CT. Bone Jt. Res. 2016, 5, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Sawyer, R.K.; Moore, A.J.; Morris, H.A.; O’Loughlin, P.D.; Anderson, P.H. Adequate dietary vitamin D and calcium are both required to reduce bone turnover and increased bone mineral volume. J. Steroid Biochem. Mol. Biol. 2014, 144, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Sakurai, T.; Kashima, I. Effects of a supplement containing vitamin K2 (MK7) and calcium phosphate (salmon bone powder) on the femur trabecular structure in ovariectomized mice: A study using three-dimensional imaging analysis. Oral Radiol. 2004, 20, 37–43. [Google Scholar] [CrossRef]
- Farlay, D.; Boivin, G.; Panczer, G.; Lalande, A.; Meunier, P.J. Long-term strontium ranelate administration in monkeys preserves characteristics of bone mineral crystals and degree of mineralization of bone. J. Bone Miner. Res. 2005, 20, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporosis Int. 2003, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, H.J.; Feng, J.; Shi, Z.L.; Wu, L.D. Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J. Int. Med. Res. 2009, 37, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, L.; Wu, J.; Zhang, J.; Su, J. Linarin promotes osteogenic differentiation by activating the BMP-2/RUNX2 pathway via protein kinase a signaling. Int. J. Mol. Med. 2016, 37, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, J.; Ghayor, C.; Guicheux, J.; Caverzasio, J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J. Biol. Chem. 2004, 279, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, Z.; Ye, Z. Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells. Biol. Pharm. Bull. 2008, 31, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, W.; Lin, Z.; Zhou, P.; Zhang, X.; Zhang, W.; Chen, Q.; Kou, D.; Ying, X.; Shen, Y.; et al. Effects of extracellular calcium on viability and osteogenic differentiation of bone marrow stromal cells in vitro. Hum. Cell. 2013, 26, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Welldon, K.J.; Findlay, D.M.; Evdokiou, A.; Ormsby, R.T.; Atkins, G.J. Calcium induces pro-anabolic effects on human primary osteoblasts associated with acquisition of mature osteocyte markers. Mol. Cell. Endocrinol. 2013, 376, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Maehira, F.; Miyagi, I.; Eguchi, Y. Effects of calcium sources and soluble silicate on bone metabolism and the related gene expression in mice. Nutrition 2009, 25, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, W.W.; Chiu, P.K.; Lam, R.W.; Xu, B.; Cheung, K.M.; Leong, J.C.; Luk, K.D. Strontium-calcium coadministration stimulates bone matrix osteogenic factor expression and new bone formation in a large animal model. J. Orthop. Res. 2009, 27, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, M.; Li, L.; Choi, H.; Gerwick, W.H.; Soh, Y. Bromo-honaucin A inhibits osteoclastogenic differentiation in RAW 264.7 cells via Akt and ERK signaling pathways. Eur. J. Pharmacol. 2015, 769, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.E.; Dai, S.; Alhawagri, M.A.; Darwech, I.; Abu-Amer, Y. IKKβ activation is sufficient for RANK-independent osteoclast differentiation and osteolysis. J. Bone Miner. Res. 2010, 25, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.M.; Kim, Y.H.; Kim, S.N.; Kim, G.S.; Baek, J.H. High extracellular Ca2+ alone stimulates osteoclast formation but inhibits in the presence of other osteoclastogenic factors. Exp. Mol. Med. 2003, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Liou, H.M.; Lin, C.J.; Kuo, K.L.; Hung, Y.S.; Weng, R.C.; Hsu, F.Y. MG63 osteoblast-like cells exhibit different behavior when grown on electrospun collagen matrix versus electrospun gelatin matrix. PLoS ONE 2012, 7, e31200. [Google Scholar] [CrossRef] [PubMed]
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. Collagen I induces the expression of alkaline phosphatase and osteopontin via independent activations of FAK and ERK signalling pathways. Arch. Oral Biol. 2014, 59, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Kim, M.K.; Bae, Y.S.; Jo, I.; Lee, S.J.; Chung, C.P.; Park, Y.J.; Min, D.S. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell. Signal 2008, 20, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Salasznyk, R.M.; Klees, R.F.; Hughlock, M.K.; Plopper, G.E. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell. Commun. Adhes. 2004, 11, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Chuderland, D.; Seger, R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun. Integr. Biol. 2008, 1, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Tsai, Y.H.; Liu, Y.; Lin, S.Y.; Liang, Y.C. Calcium-containing crystals enhance receptor activator of nuclear factor κB ligand/macrophage colony stimulating factor-mediated osteoclastogenesis via extracellular-signal-regulated kinase and p38 pathways. Rheumatology 2015, 54, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yin, Z.; Mao, L.; Wang, X.; Liu, J.; Jiang, X.; Zhang, Z.; Lin, K.; Chang, J.; Fang, B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci. Rep. 2016, 6, 22005. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Guo, F.; Zhou, X.; Gong, L.; Zhang, Y.; Zhai, W.; Chen, L.; Cen, L.; Yin, S.; Chang, J.; et al. The stimulation of osteogenic differentiation of human adipose-derived stem cells by ionic products from akermanite dissolution via activation of the ERK pathway. Biomaterials 2011, 32, 7023–7033. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Rotwein, P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J. Cell. Sci. 2009, 122, 716–726. [Google Scholar] [CrossRef] [PubMed]



| Composition | Sham | Low Ca | Normal Diet | 1% Ca |
|---|---|---|---|---|
| Casein (g/kg) | 200.0 | 200.0 | 200.0 | 200.0 |
| L-Cystine (g/kg) | 3.0 | 3.0 | 3.0 | 3.0 |
| Sucrose (g/kg) | 334.288 | 334.288 | 334.288 | 334.288 |
| Corn Starch (g/kg) | 313.0 | 320.0 | 313.0 | 320.0 |
| Soybean Oil (g/kg) | 60.0 | 60.0 | 60.0 | 60.0 |
| Cellulose (g/kg) | 40.0 | 40.0 | 40.0 | 40.0 |
| Mineral Mix (g/kg) a | 13.37 | 13.37 | 13.37 | 13.37 |
| Potassium Phosphate, monobasic (g/kg) | 11.43 | 11.43 | 11.43 | 11.43 |
| Vitamin Mix (g/kg) b | 10.0 | 10.0 | 10.0 | 10.0 |
| Calcium (%) | 0.6 | 0.01 | 0.6 | 1.0 |
| P (%) | 0.4 | 0.4 | 0.4 | 0.4 |
| Sham | Low Ca | Normal Diet | 1% Ca | |
|---|---|---|---|---|
| Feed intake (g/day) | 33.60 ± 4.19 a | 34.80 ± 3.84 a | 33.20 ± 3.64 a | 33.30 ± 4.74 a |
| Body weight (g) | ||||
| Initial | 117.33 ± 8.4 a | 118.75 ± 7.68 a | 118.50 ± 7.0 a | 113.66 ± 5.8 a |
| Final | 339.75 ± 16.34 a | 324.25 ± 30.89 a | 338.25 ± 32.34 a | 320.33 ± 50.58 a |
| Body weight gain (g/week) | 32.90 ± 2.24 a | 30.81 ± 3.21 a | 33.14 ± 3.97 a | 32.59 ± 6.54 a |
| FER * | 0.14 ± 0.02 a | 0.13 ± 0.01 a | 0.14 ± 0.02 a | 0.13 ± 0.03 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.S.; Kim, G.-J.; Song, D.H.; Chung, K.-H.; Lee, K.-J.; Kim, D.-H.; An, J.H. Calcium Supplement Derived from Gallus gallus domesticus Promotes BMP-2/RUNX2/SMAD5 and Suppresses TRAP/RANK Expression through MAPK Signaling Activation. Nutrients 2017, 9, 504. https://doi.org/10.3390/nu9050504
Yoo HS, Kim G-J, Song DH, Chung K-H, Lee K-J, Kim D-H, An JH. Calcium Supplement Derived from Gallus gallus domesticus Promotes BMP-2/RUNX2/SMAD5 and Suppresses TRAP/RANK Expression through MAPK Signaling Activation. Nutrients. 2017; 9(5):504. https://doi.org/10.3390/nu9050504
Chicago/Turabian StyleYoo, Han Seok, Gyung-Ji Kim, Da Hye Song, Kang-Hyun Chung, Kwon-Jai Lee, Dong-Hee Kim, and Jeung Hee An. 2017. "Calcium Supplement Derived from Gallus gallus domesticus Promotes BMP-2/RUNX2/SMAD5 and Suppresses TRAP/RANK Expression through MAPK Signaling Activation" Nutrients 9, no. 5: 504. https://doi.org/10.3390/nu9050504