Potential Impact of Nutrition on Immune System Recovery from Heavy Exertion: A Metabolomics Perspective
Abstract
:1. Background
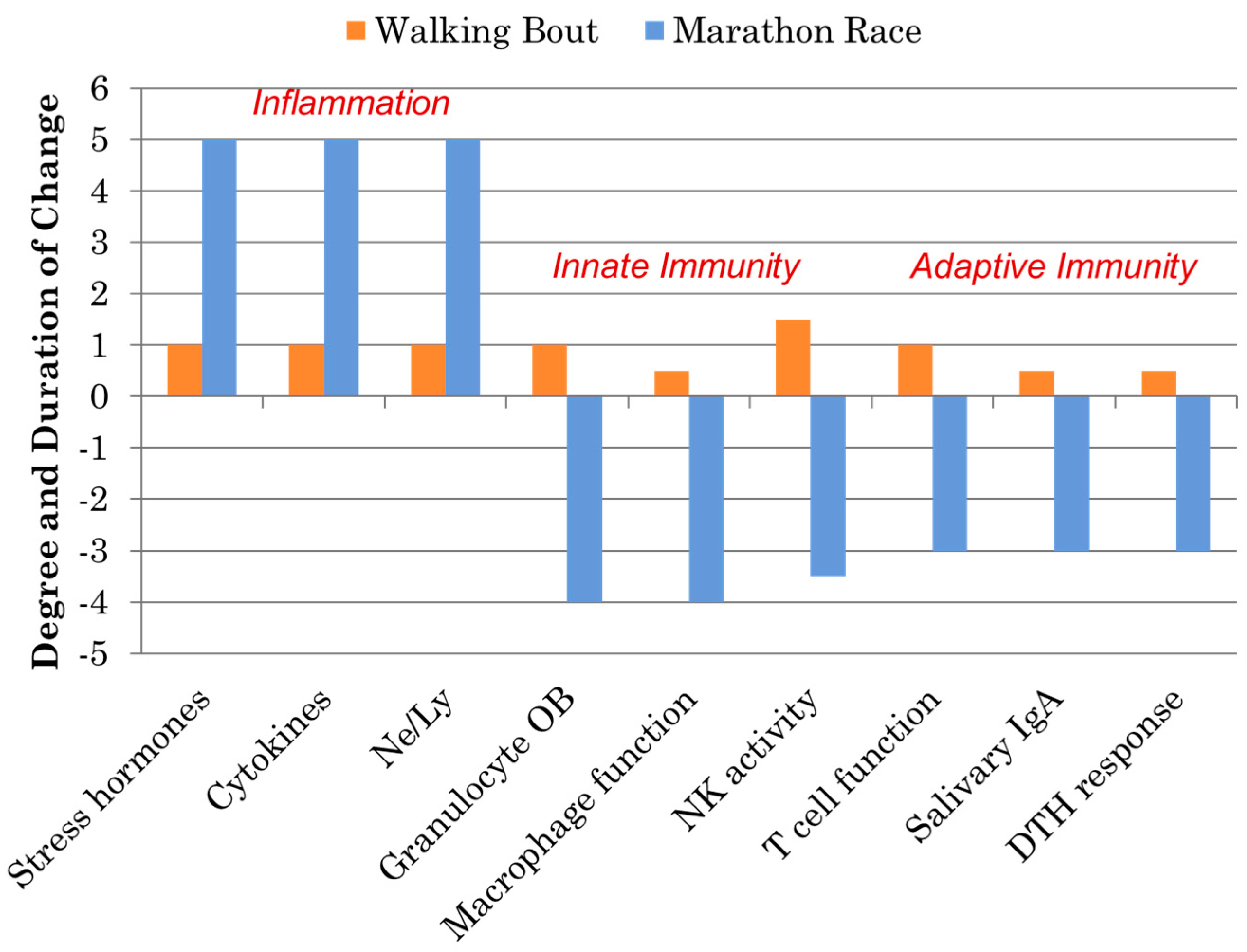
Immune Response to Intensive Exercise, Overreaching, and Overtraining
2. Immunonutrition Strategies
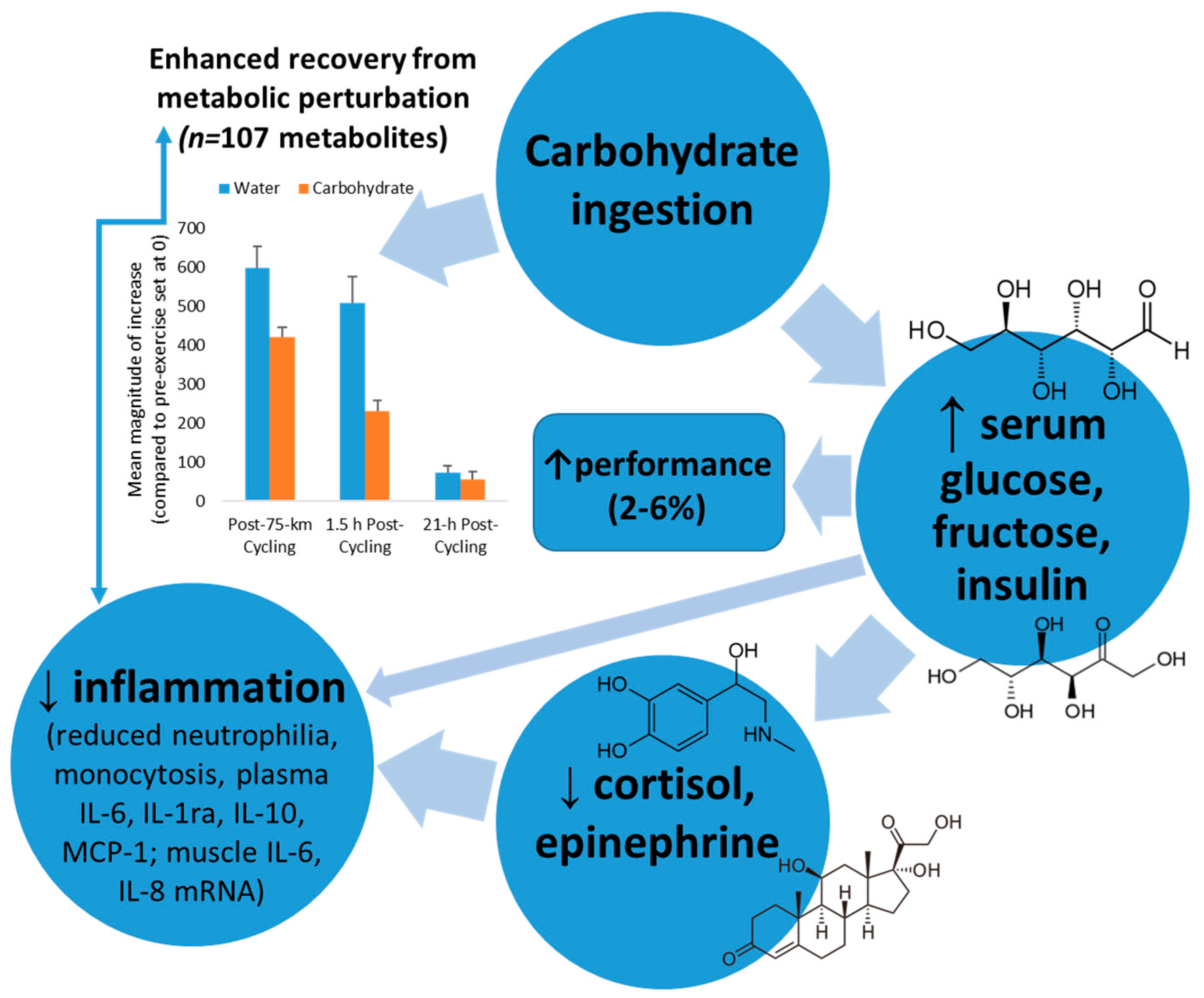
Carbohydrates
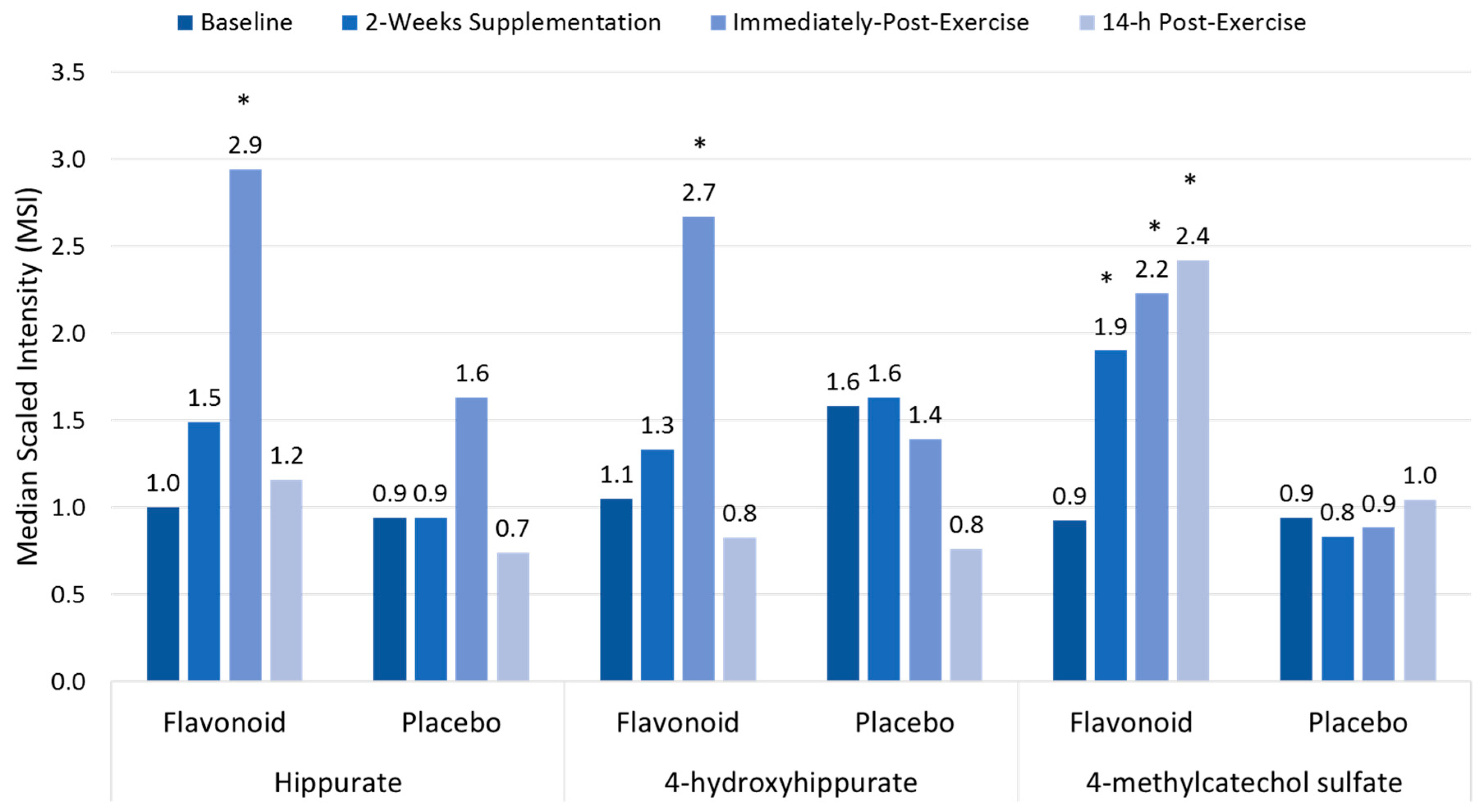
Polyphenols
3. Metabolomics and Immunometabolism Relationships
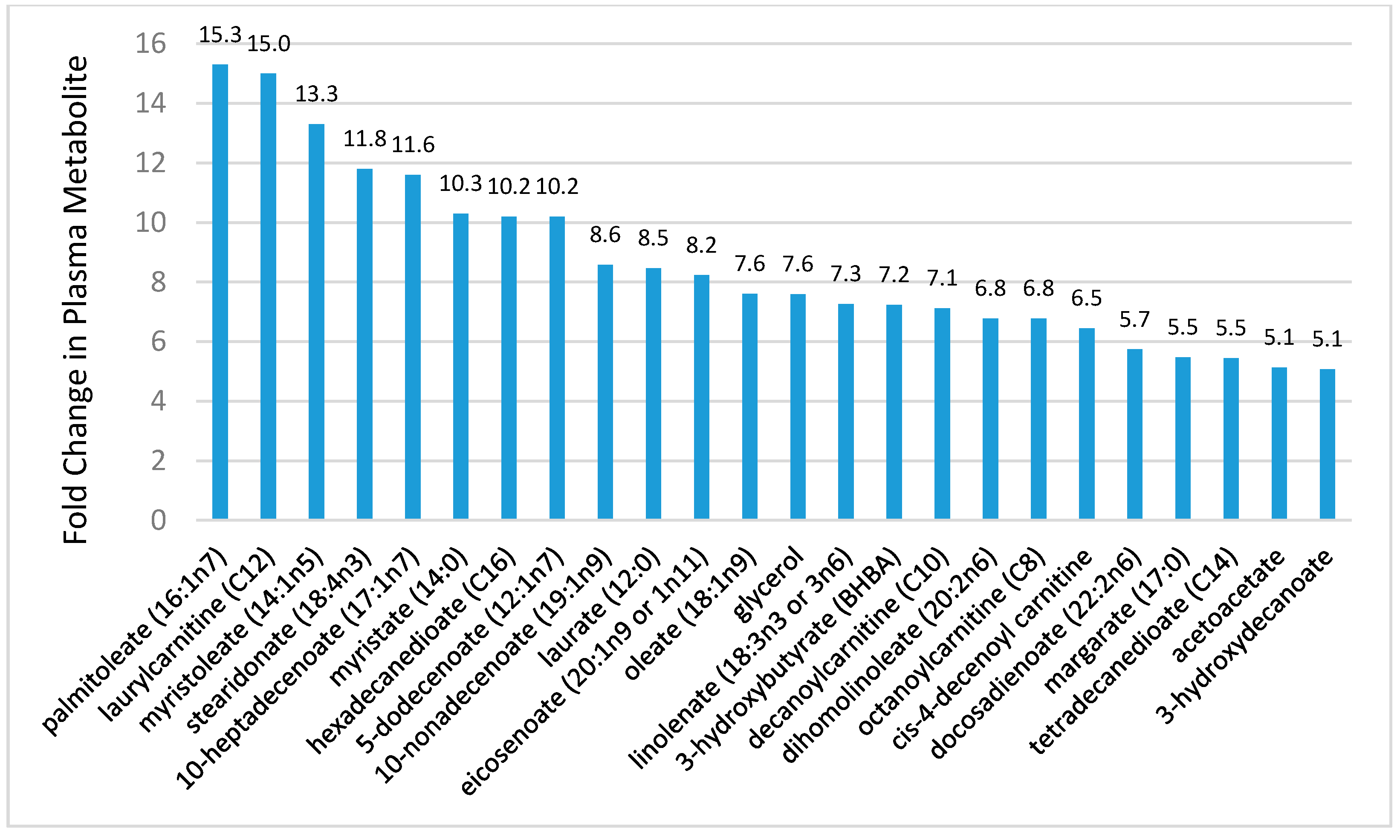
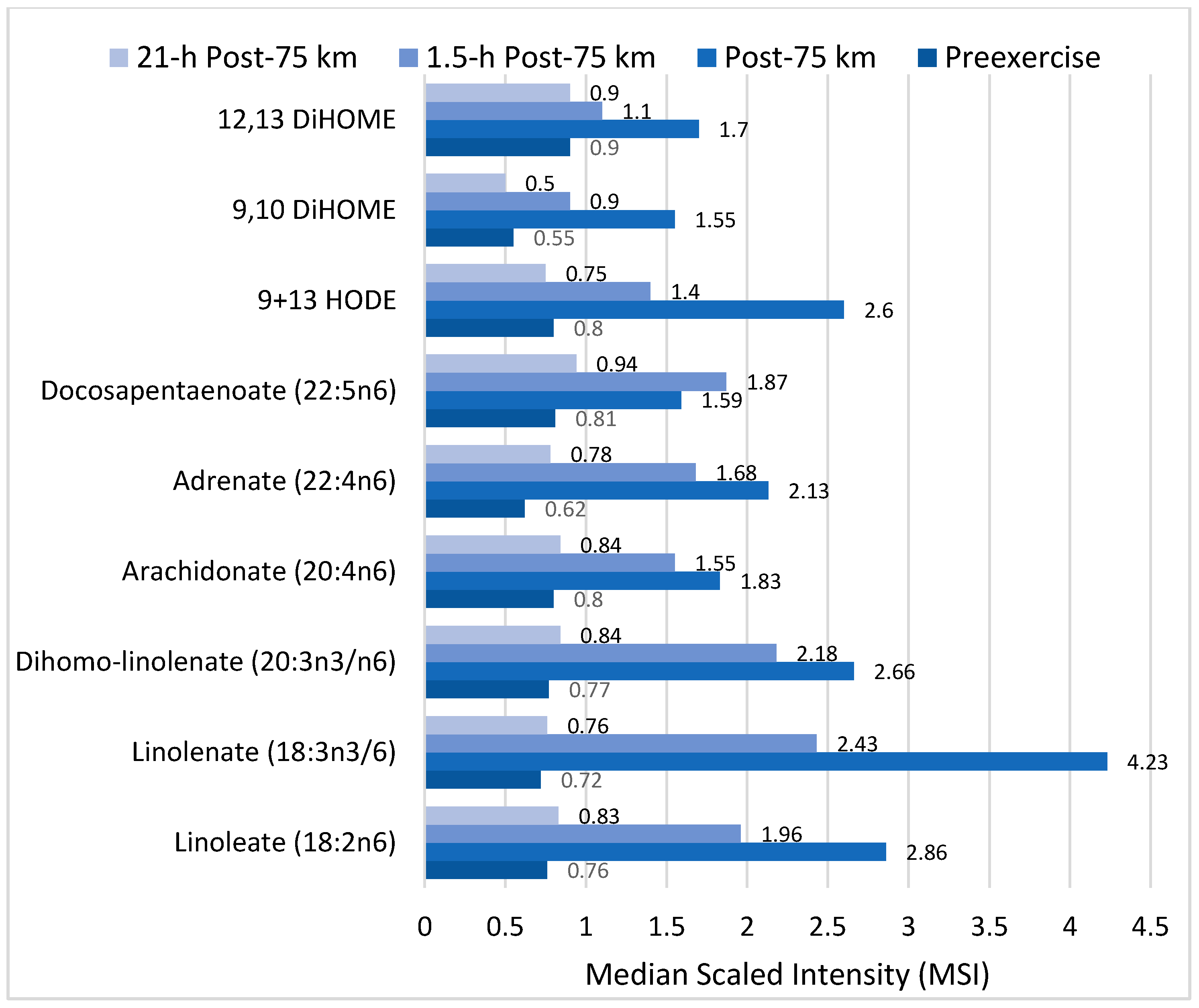
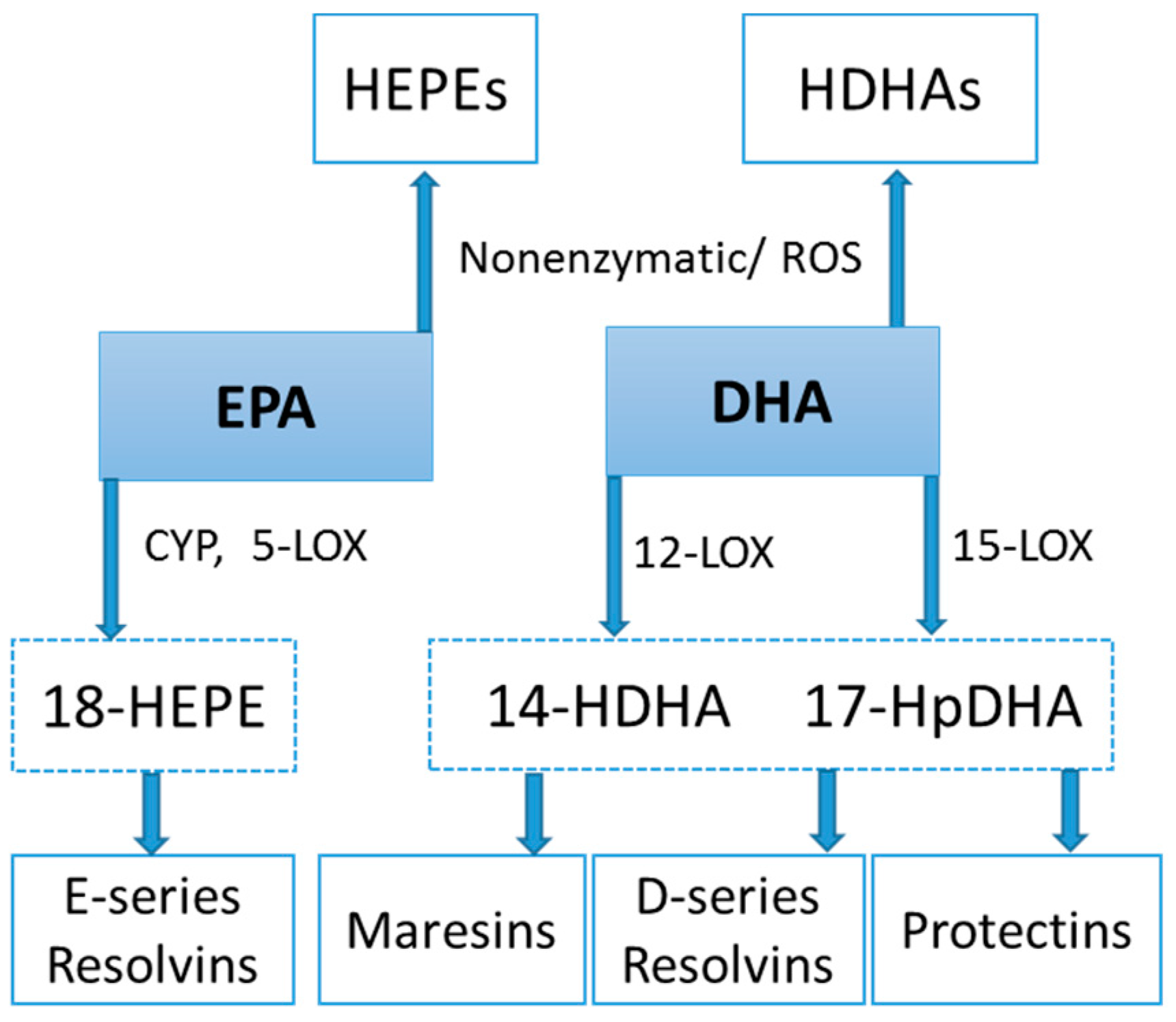
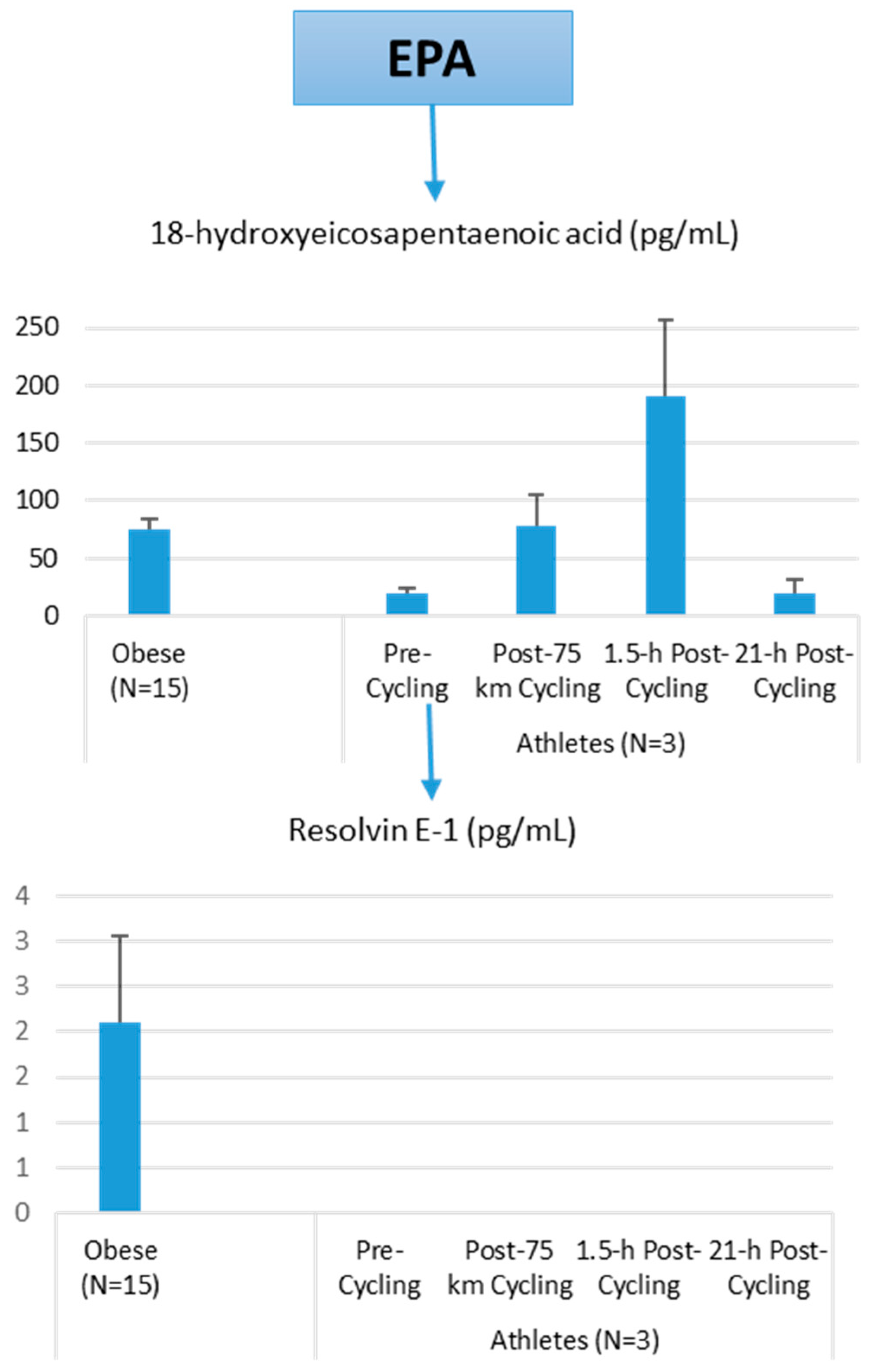
Lipid Mediators
Lipid Mediators, Exercise, Nutrition, and Obesity
4. Conclusions
Conflicts of Interest
References
- Calder, P.C. Feeding the immune system. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Immunonutrition support for athletes. Nutr. Rev. 2008, 66, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2030; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016.
- Srinivasan, B.; Lee, S.; Erickson, D.; Mehta, S. Precision nutrition—Review of methods for point-of-care assessment of nutritional status. Curr. Opin. Biotechnol. 2016, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.; Barbul, A. Immunonutrition: Role in wound healing and tissue regeneration. Adv. Wound Care 2014, 3, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Montemuiño, S.; Luna, J.; de Torres, M.V.; Amaya, E. The role of immunonutritional support in cancer treatment: Current evidence. Clin. Nutr. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Trivax, B.; Tandon, P.; Alkam, B.; Hanouneh, I.; Steiger, E. Should perioperative immunonutrition for elective surgery be the current standard of care? Gastroenterol. Rep. 2016, 4, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cialdella-Kam, L.; Nieman, D.C.; Knab, A.M.; Shanely, R.A.; Meaney, M.P.; Jin, F.; Sha, W.; Ghosh, S. A mixed flavonoid-fish oil supplement induces immune-enhancing and anti-inflammatory transcriptomic changes in adult obese and overweight women-a randomized controlled trial. Nutrients 2016, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Berk, L.S.; Simpson-Westerberg, M.; Arabatzis, K.; Youngberg, S.; Tan, S.A.; Lee, J.W.; Eby, W.C. Effects of long-endurance running on immune system parameters and lymphocyte function in experienced marathoners. Int. J. Sports Med. 1989, 10, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Luo, B.; Dréau, D.; Henson, D.A.; Shanely, R.A.; Dew, D.; Meaney, M.P. Immune and inflammation responses to a 3-day period of intensified running versus cycling. Brain Behav. Immun. 2014, 39, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.; Soligard, T.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S.; Castell, L.M.; Calder, P.C.; Bishop, N.C.; Blomstrand, E.; Mooren, F.C.; Krüger, K.; Kavazis, A.N.; Quindry, J.C.; Senchina, D.S.; et al. Consensus statement: Immunutrition and exercise. Exerc. Immunol. Rev. 2017, 23, 8–50. [Google Scholar] [PubMed]
- Tolstikov, V. Metabolomics: Bridging the gap between pharmaceutical development and population health. Metabolites 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Zhao, X.; Weigert, C.; Simon, P.; Fehrenbach, E.; Fritsche, J.; Machann, J.; Schick, F.; Wang, J.; Hoene, M.; et al. Medium-chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS ONE 2010, 5, e11519. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Meaney, M.P.; John, C.; Pappan, K.L.; Kinchen, J.M. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J. Proteome Res. 2015, 14, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
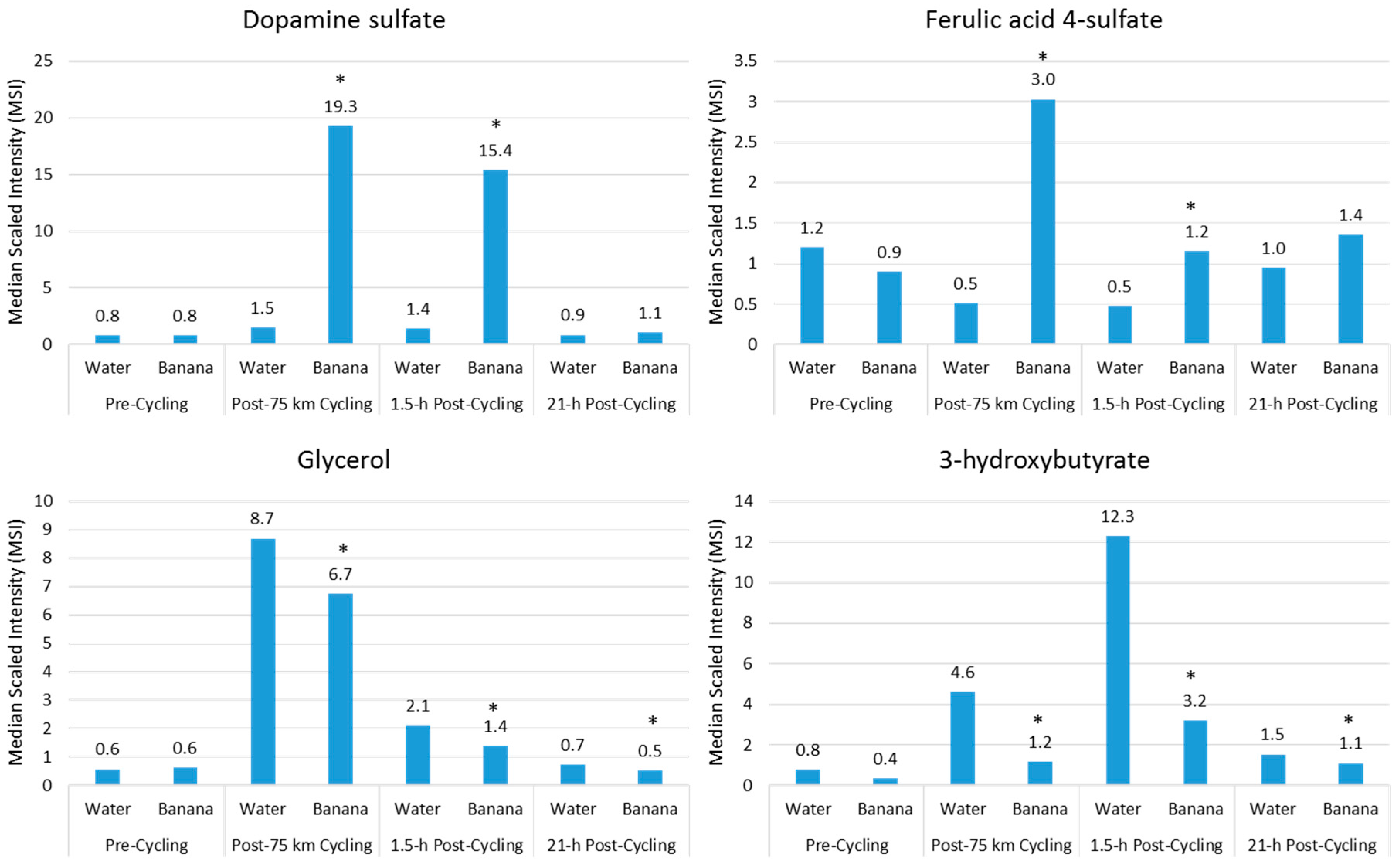
- Nieman, D.C.; Gillitt, N.D.; Henson, D.A.; Sha, W.; Shanely, R.A.; Knab, A.M.; Cialdella-Kam, L.; Jin, F. Bananas as an energy source during exercise: A metabolomics approach. PLoS ONE 2012, 7, e37479. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Scherr, J.; Luo, B.; Meaney, M.P.; Dréau, D.; Sha, W.; Dew, D.A.; Henson, D.A.; Pappan, K.L. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: A randomized, crossover trial. PLoS ONE 2014, 9, e113725. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Sha, W.; Pappan, K.L. IL-6 linkage to exercise-induced shifts in lipid-related metabolites: A metabolomics-based analysis. J. Proteome Res. 2017, 16, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Gillitt, N.D.; Pappan, K.L.; Lila, M.A. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J. Proteome Res. 2013, 12, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Meaney, M.P.; Dew, D.A.; Pappan, K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R68–R74. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Nieman, D.C.; Gillitt, N.D.; Shanely, R.A.; Cialdella-Kam, L.; Henson, D.; Sha, W.; Meaney, M.P. Effects of a freeze-dried juice blend powder on exercise-induced inflammation, oxidative stress, and immune function in cyclists. Appl. Physiol. Nutr. Metab. 2014, 39, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Maddipati, K.R.; Cameron-Smith, D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. 2016, 22, 110–134. [Google Scholar] [PubMed]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Sha, W. Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sports Med. 2011, 45, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Brown, V.A. Immune response to a 30-min walk. Med. Sci. Sports Exerc. 2005, 37, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Nieman, D.C.; Henson, D.A.; Butterworth, D.E.; Schmitt, R.L.; Bailey, E.M.; Warren, B.J.; Utter, A.; Davis, J.M. Carbohydrate and the cytokine response to 2.5 h of running. J. Appl. Physiol. 1997, 82, 1662–1667. [Google Scholar] [PubMed]
- Nieman, D.C. Influence of carbohydrate on the immune response to intensive, prolonged exercise. Exerc. Immunol. Rev. 1998, 4, 64–76. [Google Scholar] [PubMed]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, K. H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Med. 2014, 44 (Suppl. 1), S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Castell, L. Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med. 2003, 33, 323–345. [Google Scholar] [CrossRef] [PubMed]
- He, C.S.; Handzlik, M.; Fraser, W.D.; Muhamad, A.; Preston, H.; Richardson, A.; Gleeson, M. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 2013, 19, 86–101. [Google Scholar] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gojanovich, G.; Davis, J.M.; Murphy, E.A.; Mayer, E.P.; Pearce, S.; Dumke, C.L.; Utter, A.C.; McAnulty, S.R.; et al. Influence of carbohydrate on immune function following 2 h cycling. Res. Sports Med. 2006, 14, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; McAnulty, S.R.; McAnulty, L.S.; Morrow, J.D.; Ahmed, A.; Heward, C.B. Vitamin E and immunity after the Kona Triathlon World Championship. Med. Sci. Sports Exerc. 2004, 36, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Stear, S.J.; Castell, L.M.; Burke, L.M. A-Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance: Part 15, flavonoids. Br. J. Sports Med. 2010, 44, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar] [PubMed]
- McFarlin, B.K.; Carpenter, K.C.; Davidson, T.; McFarlin, M.A. Baker’s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J. Diet. Suppl. 2013, 10, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus): An effective nutritional supplement against upper respiratory tract infections? Med. Sport Sci. 2012, 59, 57–61. [Google Scholar] [PubMed]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine colostrum modulates cytokine production in human peripheral blood mononuclear cells stimulated with lipopolysaccharide and phytohemagglutinin. J. Interferon Cytokine Res. 2009, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Thatcher, R.; March, D.S.; Davison, G. Influence of 4 weeks of bovine colostrum supplementation on neutrophil and mucosal immune responses to prolonged cycling. Scand. J. Med. Sci. Sports 2015, 25, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The effect of krill oil supplementation on exercise performance and markers of immune function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.; Gabriel, B.; Thies, F.; Gray, S.R. Fish oil supplementation augments post-exercise immune function in young males. Brain Behav. Immun. 2012, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; McAnulty, S.R.; Jin, F.; Maxwell, K.R. n-3 polyunsaturated fatty acids do not alter immune and inflammation measures in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Shi, J.J.; Li, Y.M.; Zhang, X.Y.; Chen, Y.; Calder, P.C.; Tang, L.J. What is the impact of n-3 PUFAs on inflammation markers in Type 2 diabetic mellitus populations? A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.; Gleeson, M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Zwetslootm K, A.; Meaney, M.P.; Lomiwes, D.D.; Hurst, S.M.; Hurst, R.D. Post-exercise skeletal muscle glycogen related to plasma cytokines and muscle IL-6 protein content, but not muscle cytokine mRNA expression. Front. Nutr. 2015, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Akerstrom, T.C.; Fischer, C.P.; Plomgaard, P.; Thomsen, C.; van Hall, G.; Pedersen, B.K. Glucose ingestion during endurance training does not alter adaptation. J. Appl. Physiol. 2009, 106, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Morton, J.P. Ramping up the signal: Promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin. Exp. Pharmacol. Physiol. 2014, 41, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014, 44 (Suppl. 1), S87–S96. [Google Scholar] [CrossRef] [PubMed]
- Vandenbogaerde, T.J.; Hopkins, W.G. Effects of acute carbohydrate supplementation on endurance performance: A meta-analysis. Sports Med. 2011, 41, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Balentine, D.A.; Dwyer, J.T.; Erdman, J.W., Jr.; Ferruzzi, M.G.; Gaine, P.C.; Harnly, J.M.; Kwik-Uribe, C.L. Recommendations on reporting requirements for flavonoids in research. Am. J. Clin. Nutr. 2015, 101, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T.; Lin, H.; Jacques, P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015, 102, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Croft, K.D.; Lewis, J.R.; Prince, R.L. Flavonoid intake and all-cause mortality. Am. J. Clin. Nutr. 2015, 101, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.S.; Braakhuis, A.J.; Hopkins, W.G. Effect of flavonoids on upper respiratory tract infections and immune function: A systematic review and meta-analysis. Adv. Nutr. 2016, 7, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Henson, D.A.; Sanderson, M.C.; Nieman, D.C.; Gillitt, N.D.; Lila, M.A. The protective effects of a polyphenol-enriched protein powder on exercise-induced susceptibility to virus infection. Phytother. Res. 2014, 28, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sayers, T.J.; Colburn, N.H.; Milner, J.A.; Young, H.A. Impact of dietary components on NK and Treg cell function for cancer prevention. Mol. Carcinog. 2015, 54, 669–678. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhangm, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyaniding-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.F.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J. Nutr. 2016, 146, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D. Rethinking paradigms for studying mechanisms of action of plant bioactives. Nutr. Bull. 2015, 40, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, E.; Blackburn, G.; Kalna, G.; Zhang, T.; Anthony, N.; Watson, D.G. A study of the effects of exercise on the urinary metabolome using normalization to individual metabolic output. Metabolites 2015, 5, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Wientzek, A.; Bachlechner, U.; Jacobs, S.; Drogan, D.; Prehn, C.; Adamski, J.; Krumsiek, J.; Schulze, M.B.; Pischon, T.; et al. Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: Findings from a population-based study. Int. J. Obes. 2014, 38, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Koves, T.R.; Hubal, M.J.; Abouassi, H.; Beri, N.; Bateman, L.A.; Stevens, R.D.; Ilkayeva, O.R.; Hoffman, E.P.; Muoio, D.M.; et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 2014, 57, 2282–2295. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Meaney, M.P.; John, C.S.; Knagge, K.J.; Chen, H. 9- and 13-Hydroxy-octadecadienoic acids (9+13 HODE) are inversely related to granulocyte colony stimulating factor and IL-6 in runners after 2h running. Brain Behav. Immun. 2016, 56, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.W.; Carey, A.L.; Sacchetti, M.; Steinberg, G.R.; Macaulay, S.L.; Febbraio, M.A.; Pedersen, B.K. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am. J. Physiol. Endocrinol. Metab. 2004, 288, E155–E162. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Ostermann, A.I.; Rund, K.; Thoms, S.; Blume, C.; Stahl, F.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and oxylipins in obese subjects. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Spindler, S.A.; Clark, K.S.; Callewaert, D.M.; Reddy, R.G. Significance and immunoassay of 9- and 13-hydroxyoctadecadienoic acids. Biochem. Biophys. Res. Commun. 1996, 218, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G. Linoleic acid peroxidation—The dominant lipid peroxidation process in low density lipoprotein—And its relationship to chronic diseases. Chem. Phys. Lipids 1998, 95, 105–162. [Google Scholar] [CrossRef]
- Thompson, D.A.; Hammock, B.D. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J. Biosci. 2007, 32, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Gouveia-Figueira, S.; Späth, J.; Zivkovic, A.M.; Nording, M.L. Profiling the oxylipin and endocannabinoid metabolome by UPLC-ESI-MS/MS in human plasma to monitor postprandial inflammation. PLoS ONE 2015, 10, e0132042. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Miao, H.; Cheng, X.L.; Wei, F. Lipidomics: Novel insight into the biochemical mechanism of lipid metabolism and dysregulation-associated disease. Chem. Biol. Interact. 2015, 240, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Barquissau, V.; Ghandour, R.A.; Ailhaud, G.; Klingenspor, M.; Langin, D.; Amri, E.Z.; Pisani, D.F. Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, K.; Suga, T.; Mano, N. Bioanalytical insights into mediator lipidomics. J. Pharm. Biomed. Anal. 2015, 113, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ali, A.; Khan, S.A.; Zahran, S.A.; Damanhouri, G.; Azhar, E.; Qadri, I. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediat. Inflamm. 2014, 2014, 502749. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 2014, 307, C39–C54. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Le Faouder, P.; Baillif, V.; Spreadbury, I.; Motta, J.P.; Rousset, P.; Chêne, G.; Guigné, C.; Tercé, F.; Vanner, S.; Vergnolle, N.; et al. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 932, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Nieman, D.C.; Henson, D.A.; Jin, F.; Knab, A.M.; Sha, W. Inflammation and oxidative stress are lower in physically fit and active adults. Scand. J. Med. Sci. Sports 2013, 23, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lieb, D.C.; Brotman, J.J.; Hatcher, M.A.; Aye, M.S.; Cole, B.K.; Haynes, B.A.; Wohlgemuth, S.D.; Fontana, M.A.; Beydoun, H.; Nadler, J.L.; et al. Adipose tissue 12/15 lipoxygenase pathway in human obesity and diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E1713–E1720. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Kaur, G.; Miller, E.G.; Larsen, A.E.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J. 2016, 30, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Nording, M.L.; Yang, J.; Georgi, K.; Hegedus Karbowski, C.; German, J.B.; Weiss, R.H.; Hogg, R.J.; Trygg, J.; Hammock, B.D.; Zivkovic, A.M. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PLoS ONE 2013, 24, e76575. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Gammelmark, A.; Madsen, T.; Obel, T.; Aardestrup, I.; Schmidt, E.B. The effect of low-dose marine n-3 fatty acids on the biosynthesis of pro-inflammatory 5-lipoxygenase pathway metabolites in overweight subjects: A randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 43–48. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Brennan, L. The role of metabolomics in determination of new dietary biomarkers. Proc. Nutr. Soc. 2017, in press. [Google Scholar]




| Immunonutrition Supplement | Underlying Rationale | Recommendation Based on Current Evidence |
|---|---|---|
| Carbohydrate | Maintains blood glucose during exercise, lowers release of stress hormones; partially counters post-exercise inflammation and related immune changes. | Recommended: 30–70 g per hour of heavy exertion depending on exercise intensity and duration. |
| High fruit and vegetable intake, with extracts rich in polyphenols | Augment oxidative capacity, anti-viral defenses; gut-derived phenolics aid inherent defenses against long-term inflammation and oxidative stress, improve vascular health, and decrease risk of chronic disease. | Recommended, but the focus should be on long term benefits; extracts reserved for periods of heavy training and competition. |
| Bovine colostrums | Mix of immune, growth, and hormonal factors in fluid produced by the mammary glands for 24–72 h following calving improves immune function and lower illness risk. | Results are mixed; more data from well-designed studies needed. |
| Probiotics, prebiotics | Non-pathogenic bacteria in probiotics colonize the gut, improve intestinal microbial flora, and thereby enhance gut and systemic immune function, with a reduction in infection rates; prebiotics provide non-digestible food ingredients that promote the growth of beneficial microorganisms. | Results are mixed; more data from well-designed studies needed. |
| β-glucan | Receptors on intestinal wall immune cells interact with β-glucan improving innate immunity. | Results are mixed; more data needed comparing different sources. |
| n-3-PUFAs (fish oil) rich in EPA 20:5n3 and DHA 22:6n3 | Exert anti-inflammatory and immune-regulatory effects post-exercise; incorporated into cell membranes, partly replacing arachidonic acid and decreasing omega-6-derived mediators. | Results are mixed; data needed with longer duration and improved selection of outcome biomarkers. |
| Vitamin D | Plays a key role in both innate and acquired immunity through gene expression modulation; athletes may have low vitamin D levels, especially during the winter months. | Results are mixed; data needed on what actually constitutes a deficiency and benefits for correcting low levels. |
| Glutamine | Important immune cell substrate that may be lowered with prolonged exercise. | Results are mixed; more data from well-designed studies needed. |
| Vitamin E | Quenches exercise-induced reactive oxygen species (ROS) and augments immunity. | Not recommended; may be pro-oxidative and pro-inflammatory at high doses. |
| Vitamin C | Quenches ROS and augments immunity. | Not recommended; not consistently different from placebo. |
| Multiple vitamins and minerals (zinc, magnesium, iron, selenium, manganese) | Work together to quench ROS and reduce inflammation; co-factors for immune responses. | Not recommended; not different from placebo; balanced diet typically sufficient, but may be beneficial if the diet is insufficient. Concerns over blocking ROS signaling for training adaptations. |
| Amino acids (especially leucine, isoleucine, valine) | Metabolism provides nitrogen for glutamine synthesis. | Not recommended; lack of quality data from controlled studies to recommend amino acid supplementation with exercise. |
| Herbal supplements (e.g., ginseng, Echinacea) | Contain bioactive molecules that augment immunity and counter infection. | Not recommended; humans studies do not show consistent support within an athletic context. |
| Flavonoids | Sample Polyphenols | Food Sources |
|---|---|---|
| Simple Flavonoids | ||
| Flavan-3-ols | (+)-catechins, (−)-epicatechin, (−)-epigallocatechin-3-gallate | Green tea, chocolate, tree fruits, grapes, red wine |
| Flavanones | Hesperetin, Naringenin, Eriodictyol | Citrus fruits and juices |
| Flavones | Luteolin, Apigenin | Parsley, celery seed, oregano |
| Isoflavones | Daidzein, Genistein, Glycitein | Soybeans, soy-based foods, legumes |
| Flavonols | Quercetin, Kaempferol, Myricetin, Isorhamnetin | Onions, apples, tea, berries |
| Anthocyanidins | Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin | Most berries, stone fruits |
| Complex Flavonoids | ||
| Condensed Tannins (Proanthocyanidins) | Procyanidins, Prodelphinidins, Propelargonidin | Chocolate, stone fruit (apples, pears), grapes, strawberries, cranberries, nut skins, cinnamon, beer, wine, barley, legumes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieman, D.C.; Mitmesser, S.H. Potential Impact of Nutrition on Immune System Recovery from Heavy Exertion: A Metabolomics Perspective. Nutrients 2017, 9, 513. https://doi.org/10.3390/nu9050513
Nieman DC, Mitmesser SH. Potential Impact of Nutrition on Immune System Recovery from Heavy Exertion: A Metabolomics Perspective. Nutrients. 2017; 9(5):513. https://doi.org/10.3390/nu9050513
Chicago/Turabian StyleNieman, David C., and Susan Hazels Mitmesser. 2017. "Potential Impact of Nutrition on Immune System Recovery from Heavy Exertion: A Metabolomics Perspective" Nutrients 9, no. 5: 513. https://doi.org/10.3390/nu9050513






