Calculation of Haem Iron Intake and Its Role in the Development of Iron Deficiency in Young Women from the Australian Longitudinal Study on Women’s Health
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.1.1. Dietary Questionnaire for Epidemiological Studies
2.1.2. Exposure Variable: Calculating Haem Iron
2.1.3. Outcome Variable: Self-Reported Diagnosed Iron Deficiency
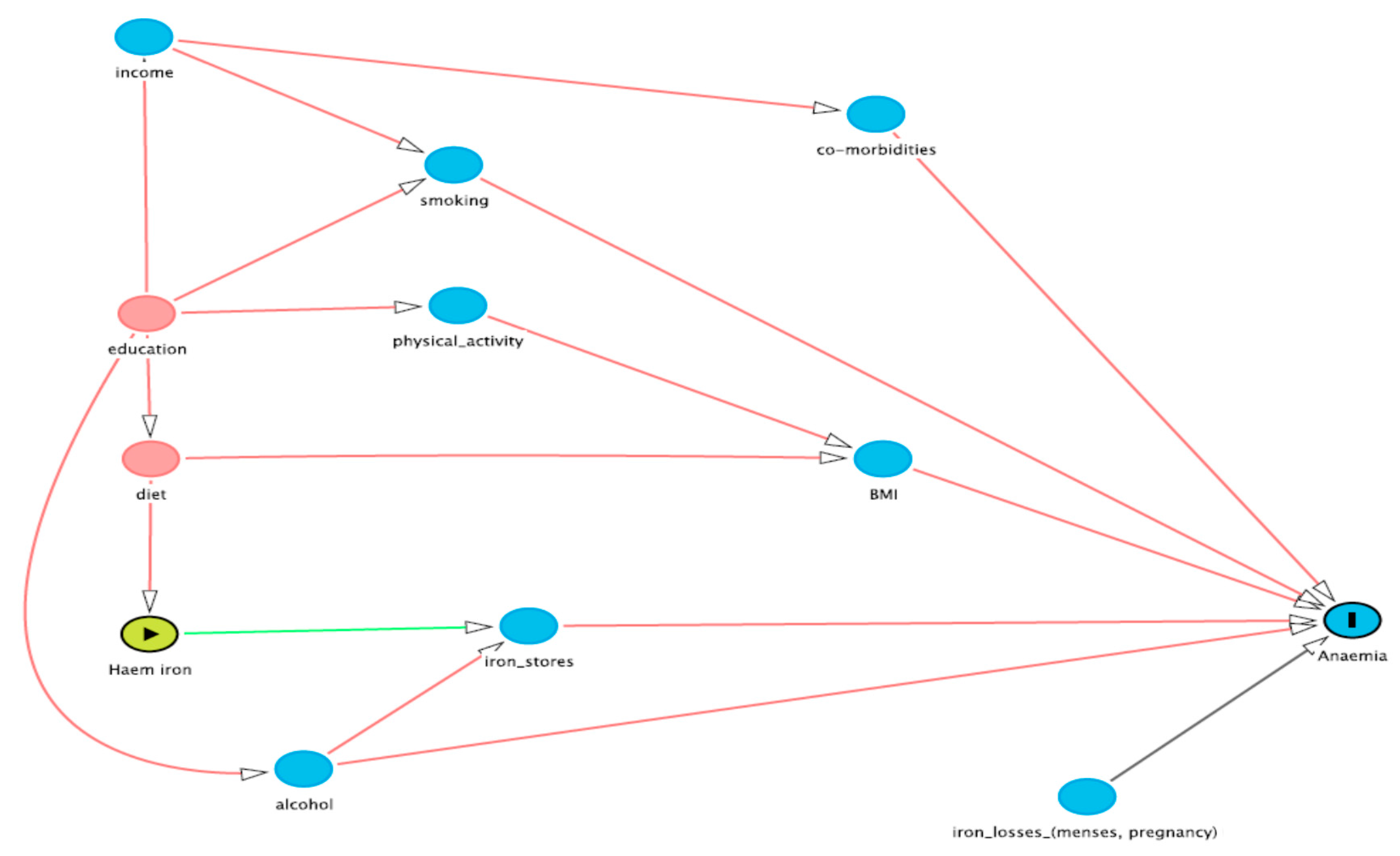
2.1.4. Covariate Selection
2.2. Statistical Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coad, J.; Conlon, C. Iron deficiency in women: assessment, causes and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Coyne, T.; Dobson, A.; McClintock, C. Iron status among Australian adults: Findings of a population based study in Queensland, Australia. Asia Pac. J. Clin. Nutr. 2008, 17, 40–47. [Google Scholar] [PubMed]
- Geissler, C.; Singh, M. Iron, meat and health. Nutrients 2011, 3, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L. Iron requirements and bioavailability of dietary iron. Exp. Suppl. 1983, 44, 223–244. [Google Scholar]
- Hallberg, L.; Rossander-Hulthen, L.; Brune, M.; Gleerup, A. Inhibition of haem-iron absorption in man by calcium. Brit. J. Nutr. 1993, 69, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Bjorn-Rasmussen, E.; Howard, L.; Rossander, L. Dietary haem iron absorption: A discussion of possible mechanisms for the absorption-promoting effect of meat and for the regulation of iron absorption. Scand. J. Gastroenterol. 1979, 14, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91 (Suppl. S5), 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.E.; Mahoney, A.W. Contributions of haem and nonhaem iron to human nutrition. Crit. Rev. Food Sci. Nutr. 1992, 31, 333–367. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R. High-, but not low-bioavailability diets enable substantial control of women’s iron absorption in relation to body iron stores, with minimal adaptation within several weeks. Am. J. Clin. Nutr. 2003, 78, 1168–1177. [Google Scholar] [PubMed]
- Alexander, D.; Ball, M.J.; Mann, J. Nutrient intake and haematological status of vegetarians and age-sex matched omnivores. Eur. J. Clin. Nutr. 1994, 48, 538–546. [Google Scholar] [PubMed]
- Pynaert, I.; Delanghe, J.; Temmerman, M.; De Henauw, S. Iron intake in relation to diet and iron status of young adult women. Ann. Nutr. Metab. 2007, 51, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Hogdahl, A.; Nilsson, L.; Rybo, G. Menstrual blood loss and iron deficiency. Acta Med. Scand. 1996, 180, 639–650. [Google Scholar] [CrossRef]
- Snyder, A.C.; Dvorak, L.L.; Roepke, J.B. Influence of dietary iron source on measures of iron status among female runners. Med. Sci. Sports Exerc. 1989, 21, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.L.M.; Skeaff, C.M.; O’Brien, S.M.; Williams, S.M.; Gibson, R.S. Can dietary treatment of non-anemic iron deficiency improve iron status? J. Am. Coll. Nutr. 2001, 20, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Brown, W.J.; Roberts, D.C.K. Dietary and lifestyle factors influencing iron stores in Australian women: An examination of the role of bio-available dietary iron. Aust. J. Nutr. Diet 2001, 58, 107–113. [Google Scholar]
- Monsen, E.; Hallberg, L.; Layrisse, M.; Hegsted, D.M.; Cook, J.D.; Mertz, W.; Finch, C.A. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978, 31, 134–141. [Google Scholar] [PubMed]
- Rangan, A.M.; Ho, R.W.L.; Blight, G.D.; Binns, C.W. Haem iron content of Australian meats and fish. Food Aust. 1997, 49, 508–511. [Google Scholar]
- Women’s Health Australia. Australian Longitudinal Study on Women’s Health. Available online: http://www.alswh.org.au/ (accessed on 15 October 2013).
- Lee, C.; Dobson, A.J.; Brown, W.J.; Bryson, L.; Byles, J.; Warner-Smith, P.; Young, A.F. Cohort profile: The Australian Longitudinal Study on Women’s Health. Int. J. Epidemiol. 2005, 34, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.; Loxton, D. The impact of attrition in an 11-year prospective longitudinal study of younger women. Ann. Epidemiol. 2010, 20, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Cancer Council Victoria. Dietary Questionnaires. Available online: http://www.cancervic.org.au/about-our/research/epidemiology/nutritional_assessment_services (accessed on 3 May 2013).
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti Cancer council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public. Health. 2000, 24, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Women’s Health Australia Surveys: 1973–78 Cohort Survey 3 (25–30 Years). 2003. Available online: http://www.alswh.org.au/images/content/pdf/Surveys_and_Databooks/Surveys/2003Yng3Survey.pdf (accessed on 15 October 2013).
- Women’s Health Australia Surveys: 1973–78 Cohort Survey 4 (28–33 Years). 2006. Available online: http://www.alswh.org.au/images/content/pdf/Surveys_and_Databooks/Surveys/2006Yng4Survey.pdf (accessed on 15 October 2013).
- Women’s Health Australia Surveys: 1973–78 Cohort Survey 5 (31–36 Years). 2009. Available online: http://www.alswh.org.au/images/content/pdf/Surveys_and_Databooks/Surveys/2009Yng5Survey.pdf (accessed on 15 October 2013).
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiological research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Howards, P.P.; Schisterman, E.F.; Poole, C.; Kaufman, J.S.; Weinberg, C.R. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am. J. Epidemiol. 2012, 176, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Willet, W.; Stampfer, M.J. Total energy intake: Implications for epidemiological analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.; Curtin, J.; Zimmerman, T.; McNutt, S.; Risch, A.; Holden, J. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol. Nutr. Food Res. 2005, 49, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Harnly, J.M.; Ferrucci, L.M.; Risch, A.; Mayne, S.T.; Sinha, R. Developing a heme iron database for meats according to meat type, cooking method and doneness level. Nutr. Sci. 2012, 3, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; McMillin, K.W.; Godber, J.S.; Bidner, T.D.; Younathan, M.T.; Marshall, D.L.; Hart, L.T. Iron distribution in heated beef and chicken muscles. J. Food Sci. 1993, 58, 697–700. [Google Scholar] [CrossRef]
- Jackson, J.; Williams, R.; McEvoy, M.; MacDonald-Wicks, L.; Patterson, A. Is Higher Consumption of Animal Flesh Foods Associated with Better Iron Status among Adults in Developed Countries? A Systematic Review. Nutrients 2016, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Eat for Health: The Guidelines. Available online: https://www.eatforhealth.gov.au/sites/default/files/files/the_guidelines/n55_australian_dietary_guidelines.pdf (accessed on 1 October 2013).
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.M.; Pierre, F.H.F.; Corpet, D.E. Haem iron from meat and risk of colorectal cancer: A meta-analysis and a review of the mechanisms involved. Cancer Prev. Res. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Cross, A.J.; Park, Y.; Schatzkin, A.; Hollenbeck, A.R.; Rohan, T.E.; Sinha, R. Intakes of dietary iron and haem-iron and risk of postmenopausal breast cancer in the National Institutes of Health-AARP Diet and Health Study. Am. J. Clin. Nutr. 2010, 92, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A prospective study of intakes of zinc and haem iron and colorectal cancer risk in men and women. Cancer Causes Control 2011, 22, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Eat for Health: Australian Dietary Guidelines. Available online: http://www.eatforhealth.gov.au/ (accessed on 1 October 2013).
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.K.; Patterson, A.J.; Smith, R.; Collins, C.E. Disparities exist between National food group recommendations and the dietary intakes of women. BMC Women’s Health 2011, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Conlon, C.A.; Kruger, R.; Coad, J. Dietary Determinants of and Possible Solutions to Iron Deficiency for Young Women Living in Industrialized Countries: A Review. Nutrients 2014, 6, 3747–3776. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Bryant, C.E.; Rooney, K.B.; Steinbeck, K.S.; Griffin, H.J.; Petocz, P.; O’Connor, H.T. Iron, Hepcidin and Inflammatory Status of Young Healthy Overweight and Obese Women in Australia. PLoS ONE 2013, 8, e68675. [Google Scholar] [CrossRef] [PubMed]
| Flesh Food or Mixed Food (FFQ) | Total Iron (mg/100 g) | Haem Iron (mg/100 g) | Median Food Item Intake at Survey Three (g/Day) | Median Haem Iron Intake at Survey Three (mg/Day) |
|---|---|---|---|---|
| Beef | 2.8 | 1.7 | 28 | 0.48 |
| Lamb | 2.4 | 1.4 | 9 | 0.13 |
| Veal | 2.1 | 1.3 | 0 | 0 |
| Pork | 1.4 | 0.9 | 2 | 0.02 |
| Ham | 1.4 | 0.9 | 3 | 0.03 |
| Sausages/Frankfurters | 2.5 | 0.9 | 3 | 0.03 |
| Bacon | 1.1 | 0.7 | 3 | 0.02 |
| Salami | 2.0 | 0.7 | 1 | 0.01 |
| Chicken | 1.2 | 0.7 | 26 | 0.18 |
| Fish; fried | 0.7 | 0.4 | 3 | 0.01 |
| Fish; steamed, grilled, or baked | 0.4 | 0.3 | 9 | 0.03 |
| Fish; canned | 1.4 | 0.3 | 5 | 0.02 |
| Hamburger * | 2.5 | 0.9 | 4 | 0.04 |
| Pizza † | 1.3 | 0.3 | 16 | 0.05 |
| Pies ‡ | 1.2 | 0.2 | 12 | 0.02 |
| Characteristic | No Iron Deficiency (n = 4599) | Diagnosis of Iron Deficiency (n = 766) | p-Value |
|---|---|---|---|
| Age in years | 27.6 (1.45) | 27.6 (1.45) | 0.271 |
| Mean (SD) | |||
| Individual Income (%) | |||
| <$37,000 annually | 56.2 | 59.1 | |
| >$37,000 annually | 43.8 | 40.9 | 0.144 |
| Highest education level (%) | 33.6 | 29.9 | |
| Year 10 or equivalent, trade/apprenticeship or diploma | 17.5 | 17.8 | |
| Year 12 or equivalent Bachelors or higher degree | 49.0 | 52.3 | 0.124 |
| Body Mass Index (kg/m2) | 24.6 (5.29) | 24.6 (5.58) | 0.787 |
| Mean (SD) | |||
| Haem iron intake (mg/day) | 1.38 (1.21) | 1.26 (1.15) | 0.0001 |
| Median (IQR) | |||
| Heavy menstruation (%) | |||
| Sometimes | 65.5 | 63.5 | |
| Often | 34.5 | 36.5 | 0.637 |
| Alcohol (drinks/week) | 0 (7) | 0 (6) | 0.0257 |
| Median (IQR) * | |||
| Smoking (cigarettes/week) | 0 (0) | 0 (0) | 0.4754 |
| Median (IQR) * | |||
| Type 2 diabetes (%) | 0.330 | 0.400 | 0.774 |
| Asthma (%) | 9.68 | 12.3 | 0.0260 |
| Hypertension (%) | 1.92 | 2.25 | 0.548 |
| Depression (%) | 11.2 | 12.5 | 0.319 |
| Predictor | Unadjusted Odds Ratio | Unadjusted 95% CI (p-Value) | Adjusted Odds Ratio * | Adjusted 95% CI (p-Value) * | Energy-Adjusted Odds Ratio † | Energy-Adjusted 95% CI (p-Value) † |
|---|---|---|---|---|---|---|
| Survey 4 | ||||||
| Haem Iron Intake (mg/day) | 0.96 | 0.90, 1.02 (0.172) | 0.91 | 0.84, 0.99 (0.020) | 0.90 | 0.82, 1.00 (0.044) |
| Survey 5 | ||||||
| Haem Iron Intake (mg/day) | 0.95 | 0.89, 1.01 (0.102) | 0.89 | 0.82, 0.97 (0.007) | 0.87 | 0.78, 0.96 (0.007) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeves, A.J.; McEvoy, M.A.; MacDonald-Wicks, L.K.; Barker, D.; Attia, J.; Hodge, A.M.; Patterson, A.J. Calculation of Haem Iron Intake and Its Role in the Development of Iron Deficiency in Young Women from the Australian Longitudinal Study on Women’s Health. Nutrients 2017, 9, 515. https://doi.org/10.3390/nu9050515
Reeves AJ, McEvoy MA, MacDonald-Wicks LK, Barker D, Attia J, Hodge AM, Patterson AJ. Calculation of Haem Iron Intake and Its Role in the Development of Iron Deficiency in Young Women from the Australian Longitudinal Study on Women’s Health. Nutrients. 2017; 9(5):515. https://doi.org/10.3390/nu9050515
Chicago/Turabian StyleReeves, Angela J., Mark A. McEvoy, Lesley K. MacDonald-Wicks, Daniel Barker, John Attia, Allison M. Hodge, and Amanda J. Patterson. 2017. "Calculation of Haem Iron Intake and Its Role in the Development of Iron Deficiency in Young Women from the Australian Longitudinal Study on Women’s Health" Nutrients 9, no. 5: 515. https://doi.org/10.3390/nu9050515





