Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas
Abstract
:1. Introduction
2. Botanical Characterisation
3. Antioxidant Properties of Mangifera indica L.
4. Anti-Inflammatory Effects of Mango
5. Anticancer Effects of Mango
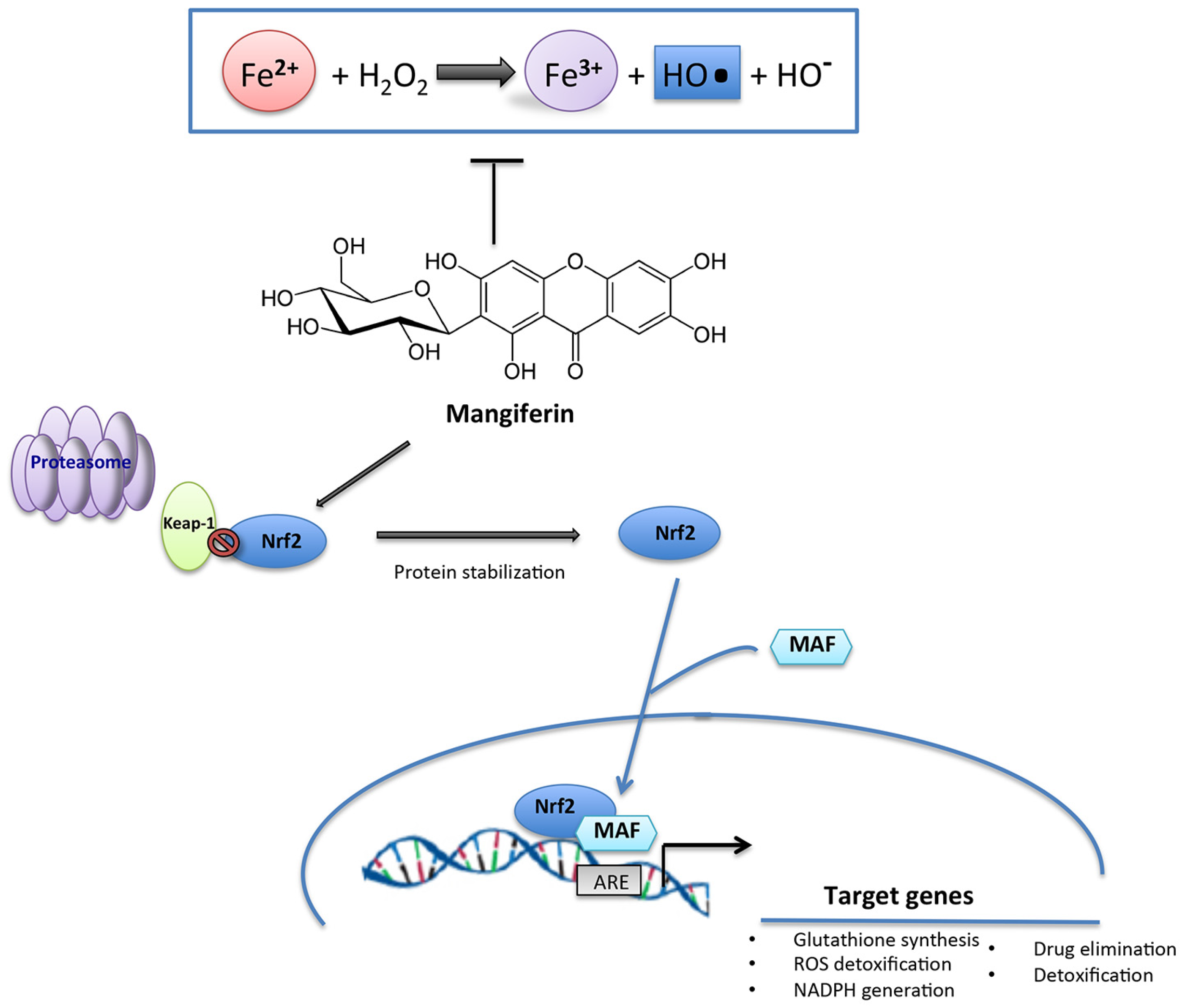
6. Mangiferin: An Unusual Natural Plant Polyphenol by Pleotropic Nutraceutical Features
7. Vimang: The Cuban Mango Plant Extract with Antioxidant Potential and Beneficial Effects for Human Health
8. Diffusion of Mangifera indica Cultivations in the Mediterranean Sicilian Area and its Impact on Sicilian Life
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Diet, nutrition, and avoidable cancer. Environ. Health Perspect. 1995, 103, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. Antioxidants and disease: more questions than answers. Nutr. Res. 2000, 20, 449–459. [Google Scholar] [CrossRef]
- Usman, M.; Fatima, B.; Muhammad, M.J. Breeding in Mango. Int. J. Agric. Biol. 2001, 3, 522–526. [Google Scholar]
- Kostermans, A.J.G.H.; Bompard, J.M. The Mangoes, Their Botany, Nomenclature, Horticulture and Utilization; Academic Press: London, UK, 1993; Available online: https://books.google.it/books/about/The_Mangoes.html?id=UpstquPSMYoC&redir_esc=y (accessed on 23 March 2017).
- Mukherjee, S.K. Origin, distribution and phylogenetic affinities of the species of Mangifera indica L. J. Linn. Soc. Bot. 1953, 55, 65–83. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexican ‘Ataulfo’ mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Mongi, R.J.; Sørensen, K.M.; Ndabikunze, B.K.; Chove, B.E.; Engelsen, S.B. A comprehensive and comparative GC-MS metabolomics study of non-volatiles in Tanzanian grown mango, pineapple, jackfruit, baobab and tamarind fruits. Food Chem. 2016, 213, 691–699. [Google Scholar] [CrossRef] [PubMed]
- San, A.T.; Joyce, D.C.; Hofman, P.J.; Macnish, A.J.; Webb, R.I.; Matovic, N.J.; Williams, C.M.; De Voss, J.J.; Wong, S.H.; Smyth, H.E. Stable isotope dilution assay (SIDA) and HS-SPME-GCMS quantification of key aroma volatiles for fruit and sap of Australian mango cultivars. Food Chem. 2017, 221, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Pleguezuelo, C.R.; Durán Zuazo, V.H.; Muriel Fernández, J.L.; Franco Tarifa, D. Physico-chemical Quality Parameters of Mango (Mangifera indica L.) Fruits Grown in a Mediterranean Subtropical Climate (SE Spain). J. Agric. Sci. Technol. 2012, 14, 365–374. [Google Scholar]
- Ismail, A.M.; Cirvilleri, G.; Lombard, L.; Crous, P.W.; Groenewald, J.Z.; Polizzi, G. Characterisation of neofusicoccum species causing mango dieback in Italy. J. Plant Pathol. 2013, 95, 549–557. [Google Scholar] [CrossRef]
- Aiello, D.; Ferrante, P.; Vitale, A.; Polizzi, G.; Scortichini, M.; Cirvilleri, G. Characterization of Pseudomonas syringae pv. syringae isolated from mango in Sicily and occurrence of copper-resistant strains. J. Plant Pathol. 2015, 97, 273–282. [Google Scholar] [CrossRef]
- Farina, V.; Corona, O.; Mineo, V.; D’Asaro, A.; Barone, F. Qualitative characteristics of Mango fruits (Mangifera indica L.), which have undergone preservation (Italian). Acta Italus Hortus 2013, 12, 70–73. [Google Scholar]
- Masud Parvez, G.M. Pharmacological Activities of Mango (Mangifera indica): A Review. J. Pharmacogn. Phytochem. 2016, 5, 1–7. [Google Scholar]
- United States Department of Agriculture (USDA). National Nutrient Database for Standard Reference, SR-28, Full Report (All Nutrients): 09176, Mangos, Raw National Agricultural Library. USDA. Available online: https://ndb.nal.usda.gov/ndb/foods/show/2271. (accessed on 25 January 2016).
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Berardini, N.; Carle, R.; Schieber, A. Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. ‘Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O- and xanthone C-glycosides, anthocyanins, and pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Masibo, M.; He, Q. Major Mango Polyphenols and Their Potential Significance to Human Health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Jahurul, M.H.; Zaidul, I.S.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Guevara Garcia, M.; Gonzalez Laime, S.; Alvarez Leon, A.; Riano Montalvo, A.; Garrido, G.G.; Nunez Selles, A.J. Ethnomedical uses of Mangifera indica L. stem bark extract in Cuba (spanish). Rev. Cuba Plant Med. 2004, 9, 27–32. [Google Scholar]
- Sairam, K.; Hemalatha, S.; Kumar, A.; Srinivasan, T.; Ganesh, J.; Sarkar, M.; Venkataraman, S. Evaluation of anti-diarrhoeal activity in seed extracts of Mangifera indica. J. Ethnopharmacol. 2003, 84, 11–15. [Google Scholar] [CrossRef]
- Thambi, P.A.; John, S.; Lydia, E.; Iyer, P.; Sarah Jane Monica, S.J. Antimicrobial efficacy of mango peel powder and formulation of recipes using mango peel powder (Mangifera indica L.). Int. J. Home Sci. 2016, 2, 155–161. [Google Scholar]
- Bbosa, G.S.; Kyegombe, D.B.; Ogwal-Okeng, J.; Bukenya-Ziraba, R.; Odyek, O.; Waako, P. Antibacterial activity of Mangifera indica (L.). Afric. J. Ecol. 2007, 45, 13–16. [Google Scholar] [CrossRef]
- Masibo, M.; He, Q. Mango Bioactive Compounds and Related Nutraceutical Properties—A Review. Food Rev. Int. 2009, 25, 346–370. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Done, A.J.; Traustadóttir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Afifa, K.; Kamruzzaman, M.; Mahfuza, I.; Afzal, H.; Arzina, H.; Roksana, H. A comparison with antioxidant and functional properties among five mango (Mangifera indica L.) varieties in Bangladesh. Int. Food Res. J. 2014, 21, 1501–1506. [Google Scholar]
- Carlisi, D.; D’Anneo, A.; Martinez, R.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. The oxygen radicals involved in the toxicity induced by parthenolide in MDA-MB-231 cells. Oncol. Rep. 2014, 32, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Carlisi, D.; Giuliano, M.; Calvaruso, G.; Cernigliaro, C.; Vento, R.; D’Anneo, A. The analysis of estrogen receptor-α positive breast cancer stem-like cells unveils a high expression of the serpin proteinase inhibitor PI-9: Possible regulatory mechanisms. Int. J. Oncol. 2016, 49, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.; Sabella, S.; Pellerito, O.; Vento, R.; Calvaruso, G.; Giuliano, M. The secreted protein acidic and rich in cysteine is a critical mediator of cell death program induced by WIN/TRAIL combined treatment in osteosarcoma cells. Int. J. Oncol. 2016, 48, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; D’Anneo, A.; Marino Gammazza, A.; Caruso Bavisotto, C.; Barone, R.; Emanuele, S.; Lo Cascio, F.; Mocciaro, E.; Fais, S.; Conway De Macario, E.; et al. The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells. Oncotarget 2016, 7, 28849–28867. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A.; et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, O.; Notaro, A.; Sabella, S.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. WIN induces apoptotic cell death in human colon cancer cells through a block of autophagic flux dependent on PPARγ down-regulation. Apoptosis 2014, 19, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Marino Gammazza, A.; Campanella, C.; Barone, R.; Caruso Bavisotto, C.; Gorska, M.; Wozniak, M.; Carini, F.; Cappello, F.; D’Anneo, A.; Lauricella, M.; et al. Doxorubicin anti-tumor mechanisms include Hsp60 post-translational modifications leading to the Hsp60/p53 complex dissociation and instauration of replicative senescence. Cancer Lett. 2017, 385, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, B.L.; Shivalingaiah, S. The anti-inflammatory activity of standard aqueous stem bark extract of Mangifera indica L. as evident in inhibition of Group IA sPLA2. An. Acad. Bras. Cienc. 2016, 88, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Talero, E.; Siracusa, R.; Alcaide, A.; Cordaro, M.; Maria Zubelia, J.; Bruschetta, G.; Crupi, R.; Esposito, E.; Cuzzocrea, S.; et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br. J. Nutr. 2015, 114, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Duricova, D. What Can We Learn from Epidemiological Studies in Inflammatory Bowel Disease? Dig. Dis. 2017, 35, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inlammatory bowel disease. Ann. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Andus, T. Cytokines in inlammatory bowel disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Andresen, L.; Jørgensen, V.; Perner, A.; Hansen, A.; Eugen-Olsen, J.; Rask-Madsen, J. Activation of nuclear factor kB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005, 54, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Márquez, L.; Pérez-Nievas, B.G.; Gárate, I.; García-Bueno, B.; Madrigal, J.L.; Menchén, L.; Garrido, G.; Leza, J.C. Anti-inflammatory effects of Mangifera indica L. extract in a model of colitis. World J. Gastroenterol. 2010, 16, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Blanco-Molina, M.; Sancho, R.; Macho, A.; Delgado, R.; Muñoz, E. An aqueous stem bark extract of Mangifera indica (Vimang) inhibits T cell proliferation and TNF-induced activation of nuclear transcription factor NF-kappaB. Phytother. Res. 2005, 19, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016, 60, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2017, 56, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Severi, J.A.; Lima, Z.P.; Kushima, H.; Brito, A.R.; dos Santos, L.C.; Vilegas, W.; Hiruma Lima, C.A. Polyphenols with antiulcerogenic action from aqueous decoction of mango leaves (Mangifera indica L.). Molecules 2009, 14, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud-Awny, M.; Attia, A.S.; Abd-Ellah, M.F.; El-Abhar, H.S. Mangiferin Mitigates Gastric Ulcer in Ischemia/Reperfused Rats: Involvement of PPAR-γ, NF-κB and Nrf2/HO-1 Signaling Pathways. PLoS ONE 2015, 10, e0132497. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Dwivedi, S.K.; Swarup, D. Hypoglycemic potential of Mangifera indica leaves in rats. Int. J. Pharmacol. 1997, 35, 130–133. [Google Scholar] [CrossRef]
- Aderibigbe, A.O.; Emudianughe, T.S.; Lawal, B.A. Antihyperglycaemic effect of Mangifera indica in rat. Phytother. Res. 1999, 13, 504–507. [Google Scholar] [CrossRef]
- Perpetuo, G.F.; Salgado, J.M. Effect of mango (Mangifera indica L.) ingestion on blood glucose levels of normal and diabetic rats. J. Plant Foods Hum. Nutr. 2003, 58, 1–12. [Google Scholar] [CrossRef]
- Gondi, M.; Prasada Rao, U.J.S. Ethanol extract of mango (Mangifera indica L.) peel inhibits α-amylase and α-glucosidase activities, and ameliorates diabetes related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. J. Food Sci. Technol. 2015, 52, 7883–7889. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Do, T.N.; Le, T.H.; Nguyen, M.T.; Nguyen, N.T.; Esumi, H.; Awale, S. Chemical Constituents of Mangifera indica and Their Antiausterity Activity against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2016, 79, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Mosaddik, A.; Gyawali, R.; Ahn, K.S.; Cho, S.K. Induction of apoptosis by ethanolic extract of mango peel and comparative analysis of the chemical constitutes of mango peel and flesh. Food Chem. 2012, 133, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Bernal, A.; Amparo Urango, L.; Rojano, B.; Maldonado, M.E. In vitro and in vivo effects of mango pulp (Mangifera indica cv. Azucar) in colon carcinogenesis. Arch. Latinoam. Nutr. 2014, 64, 16–23. [Google Scholar] [PubMed]
- Abdullah, A.S.; Mohammed, A.S.; Rasedee, A.; Mirghani, M.E.; Al-Qubaisi, M.S. Induction of apoptosis and oxidative stress in estrogen receptor-negative breast cancer, MDA-MB231 cells, by ethanolic mango seed extract. BMC Complement. Altern. Med. 2015, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Kim, H.; Krenek, K.; Talcott, S.T.; Mertens-Talcott, S.U. Mango polyphenolics suppressed tumor growth in breast cancer xenografts in mice: role of the PI3K/AKT pathway and associated microRNAs. Nutr. Res. 2015, 35, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Saito, F.; Yasuhara, T.; Sugimoto, A. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermat. 2004, 51, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Kuś, P.; Góralska, E.; Woźniak, D. Mangiferin—A bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013, 13, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+—Citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.C.; Trevisan, M.T.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, A.K.; Tan, Z.W.; Shimada, R.; Shaw, P.N.; Flanagan, B.M. Between fruit variability of the bioactive compounds, β-carotene and mangiferin, in mango (Mangifera indica). Nutr. Diet. 2013, 70, 158–163. [Google Scholar] [CrossRef]
- Lei, J.; Zhou, C.; Hu, H.; Hu, L.; Zhao, M.; Yang, Y.; Chuai, Y.; Ni, J.; Cai, J. Mangiferin aglycone attenuates radiation-induced damage on human intestinal epithelial cells. J. Cell. Biochem. 2012, 113, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Kawpoomhae, K.; Sukma, M.; Ngawhirunpat, T.; Opanasopit, P.; Sripattanaporn, A. Antioxidant and neuroprotective effects of standardized extracts of Mangifera indica leaf. Thai J. Pharm. Sci. 2010, 34, 32–43. [Google Scholar]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and Cancer: Mechanisms of Action. Nutrients 2016, 28, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-P.; Zhao, J.; Li, S.-S.; Yang, L.-J.; Zeng, L.-L.; Chen, Y.; Fang, J. Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro. Acta Pharmacol. Sin. 2014, 35, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, B.; Li, S.; Zeng, L.; Chen, Y.; Fang, J. Mangiferin increases Nrf2 protein stability by inhibiting its ubiquitination and degradation in human HL60 myeloid leukemia cells. Int. J. Mol. Med. 2014, 33, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.K.; Kaur, R.; Lohan, S.; Singh, K.K.; Singh, B. Mangiferin: a promising anticancer bioactive. Pharm. Pat. Anal. 2016, 5, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Núñez Selles, A.J.; Daglia, M.; Rastrelli, L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors 2016, 42, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Telang, M.; Dhulap, S.; Mandhare, A.; Hirwani, R. Therapeutic and cosmetic applications of mangiferin: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, C.M.; Gaspar, L.R. Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J. Photochem. Photobiol. B 2015, 151, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; El-Maraghy, N.; Reda, E.; Baraka, W. Modulation of Diabetes and Dyslipidemia in Diabetic Insulin-Resistant Rats by Mangiferin: Role of Adiponectin and TNF-α. Ann. Braz. Acad. Sci. 2014, 86, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zheng, D.; Fung, G.; Deng, H.; Chen, L.; Liang, J.; Jiang, Y.; Hu, Y. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can. J. Physiol. Pharmacol. 2016, 94, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Zhu, X.; Zhang, L.; Lu, Q.; Wang, J.Y.; Zhang, F.; Guo, H.; Yin, J.L.; Yin, X.X. Up-regulation of glyoxalase 1 by mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2013, 721, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Rodeiro, I.; Hernández, I.; García, G.; Pérez, G.; Merino, N.; Núñez-Sellés, A.; Delgado, R. In vivo acute toxicological studies of an antioxidant extract from Mangifera indica L. (Vimang). Drug Chem. Toxicol. 2009, 32, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Andreu, G.L.; Dorta, D.J.; Delgado, R.; Cavalheiro, R.A.; Santos, A.C.; Vercesi, A.E.; Curti, C. Vimang (Mangifera indica L. extract) induces permeability transition in isolated mitochondria, closely reproducing the effect of mangiferin, Vimang’s main component. Chem. Biol. Interact. 2006, 159, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Andreu, G.L.; Paim, B.A.; Castilho, R.F.; Velho, J.A.; Delgado, R.; Vercesi, A.E.; Oliveira, H.C. Mangifera indica L. extract (Vimang) and its main polyphenol mangiferin prevent mitochondrial oxidative stress in atherosclerosis-prone hypercholesterolemic mouse. Pharmacol. Res. 2008, 57, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Delgado, R.; Pérez, G.; Garrido, G.; Núñez Sellés, A.J.; León, O.S. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother. Res. 2000, 4, 424–427. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W.; Ingkaninan, K.; Wittaya-Areekul, S. Mangifera indica fruit extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in animal model of mild cognitive impairment. Oxid. Med. Cell. Longev. 2014, 2014, 132097–132104. [Google Scholar] [CrossRef] [PubMed]
- Farina, V.; Mazzaglia, A.; Padoan, D.; Barone, F. Chemical-physical and sensory characterization of mango fruits (Mangifera indica L.) cultivated in Sicily (Italian). Acta Italus Hortus 2013, 12, 113. [Google Scholar]
- Mazzaglia, A.; Piva, G.; D’Asaro, A.; Farina, V. Sensory profile of mango fruits (Mangifera indica L.) Cultivated in Sicily (Italian). Acta Italus Hortus 2013, 12, 113–115. [Google Scholar]



| Mangifera indica L. Nutrition value per 100 g | |
|---|---|
| Energy | 60 Kcal |
| Fruit composition | Quantity |
| Carbohydrates | 14.98 g |
| Protein | 0.82 g |
| Fat | 0.38 g |
| Fiber | 1.6 g |
| Vitamins | |
| Vitamin C | 36.4 mg |
| Vitamin E | 1.12 mg |
| Vitamin A | 1082 IU |
| Niacin (vit B3) | 669 µg |
| Pantothenic acid (vit B5) | 160 µg |
| Pyridoxine (vit B6) | 119 µg |
| Riboflavin (vit B2) | 38 µg |
| Thiamin (vit B1) | 28 µg |
| Folates | 43 µg |
| Vitamin K | 4.2 µg |
| Minerals | |
| Potassium | 168 mg |
| Phosphorus | 14 mg |
| Calcium | 11 mg |
| Magnesium | 10 mg |
| Sodium | 1 mg |
| Copper | 110 µg |
| Iron | 160 µg |
| Manganese | 27 µg |
| Zinc | 90 µg |
| Carotenoids | |
| β−Carotene | 445 µg |
| α−Carotene | 17 µg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. https://doi.org/10.3390/nu9050525
Lauricella M, Emanuele S, Calvaruso G, Giuliano M, D’Anneo A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients. 2017; 9(5):525. https://doi.org/10.3390/nu9050525
Chicago/Turabian StyleLauricella, Marianna, Sonia Emanuele, Giuseppe Calvaruso, Michela Giuliano, and Antonella D’Anneo. 2017. "Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas" Nutrients 9, no. 5: 525. https://doi.org/10.3390/nu9050525







