Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Chemicals
2.3. Metabolic Characterization
2.4. Blood and Tissues Collection
2.5. Liver Pathology and Triglyceride Content
2.6. Cell Size and Weight, Glucose Uptake, and Lipolysis in Isolated Epididymal Adipocytes
2.7. Liver, Skeletal Muscle, and Adipose Tissue Gene and Protein Expression
2.8. Statistical Analysis
3. Results
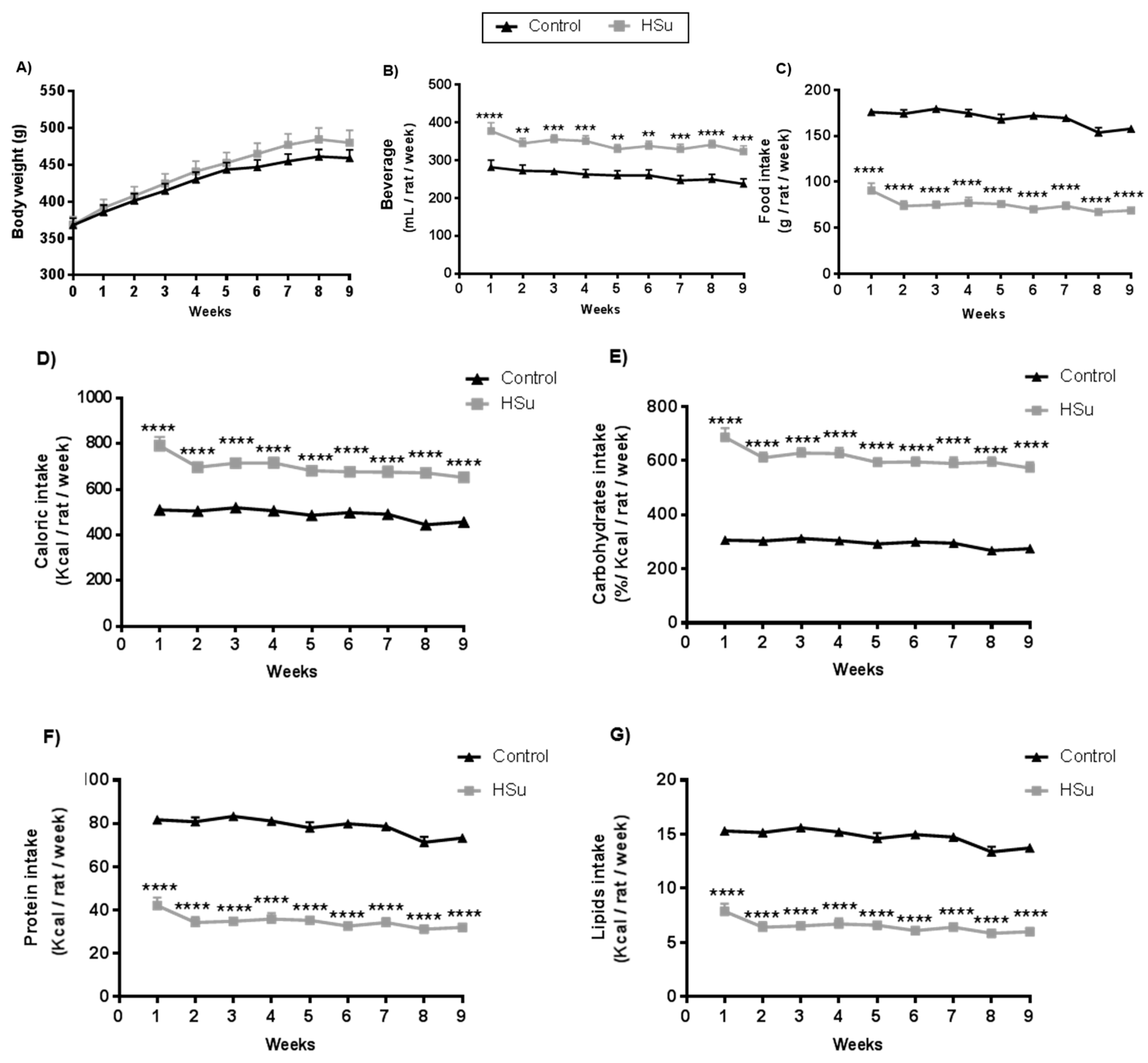
3.1. HSu Diet Increases Beverage Consumption and Caloric Intake, Maintaining Body Weight
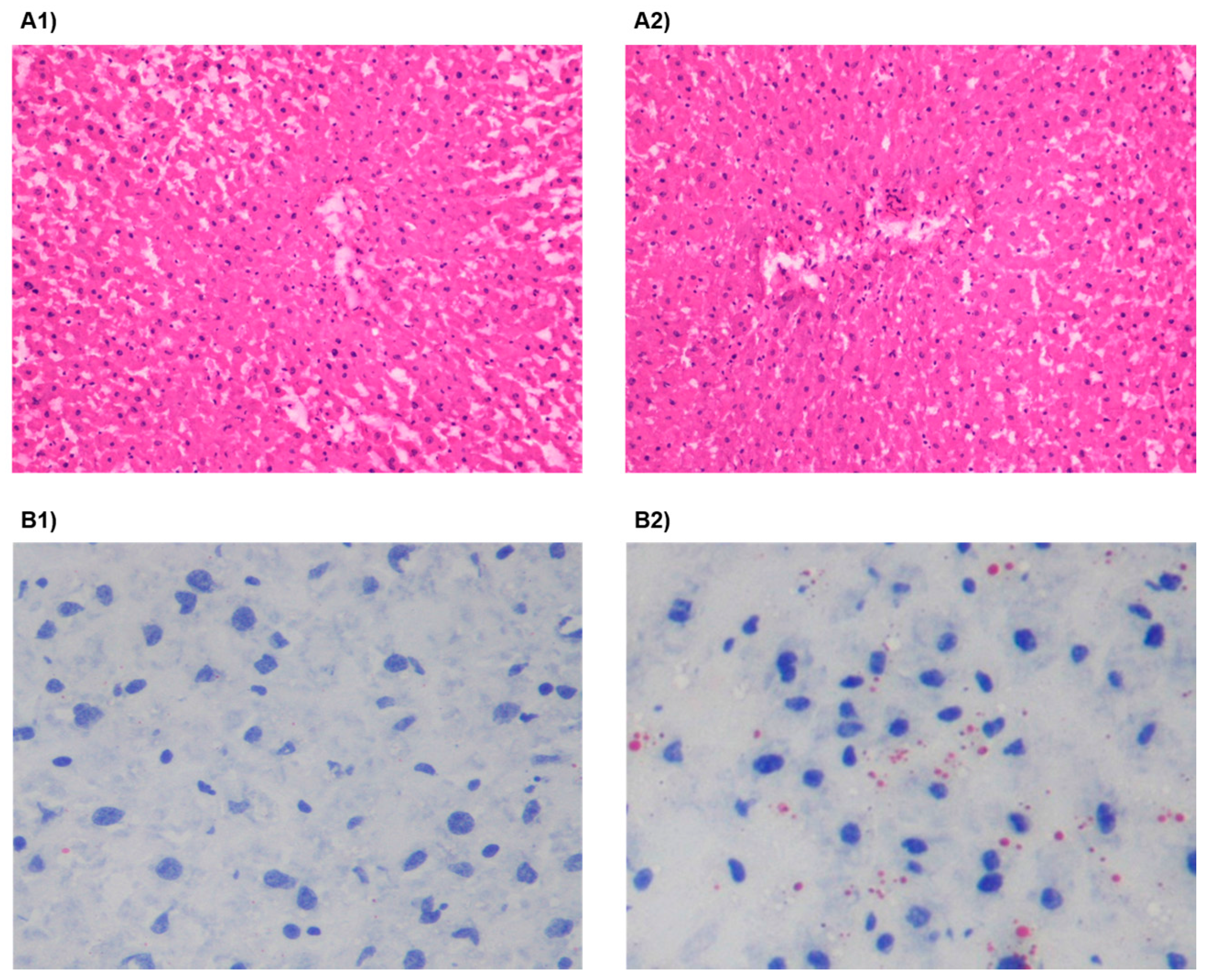
3.2. HSu Diet Increases Serum Triglyceride Levels and Impairs Liver Function
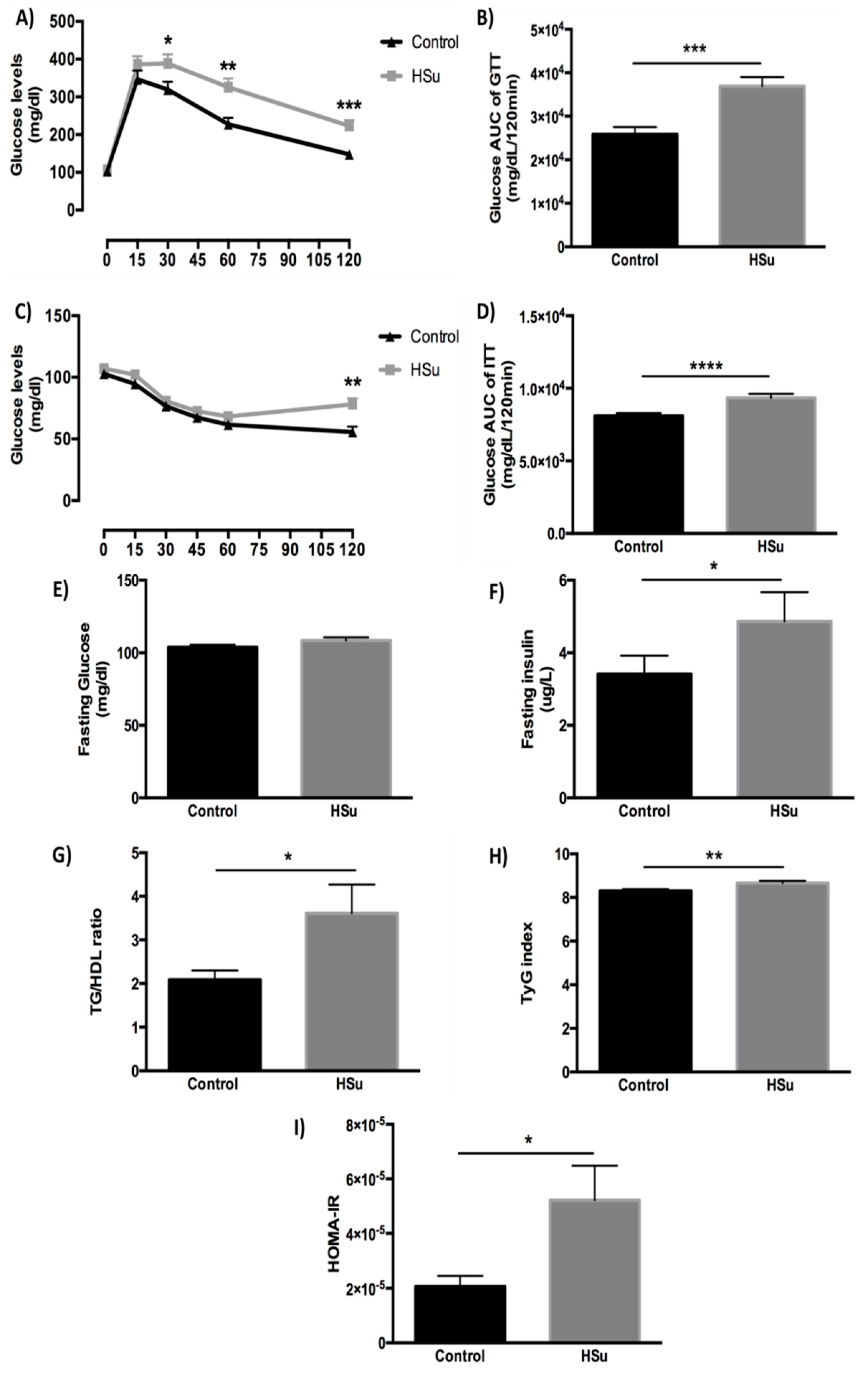
3.3. HSu Diet Impairs Glucose Tolerance and Causes Insulin Resistance
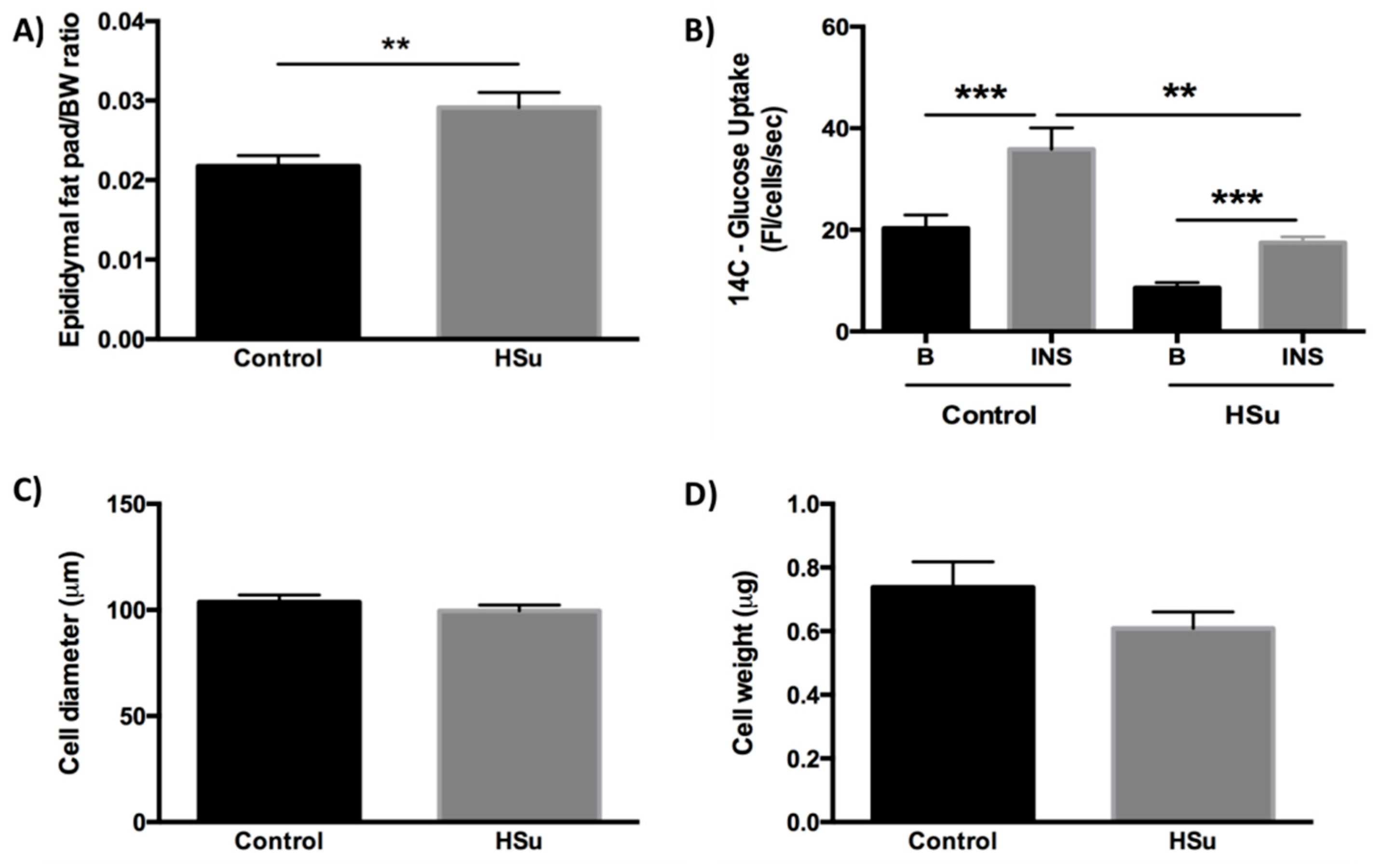
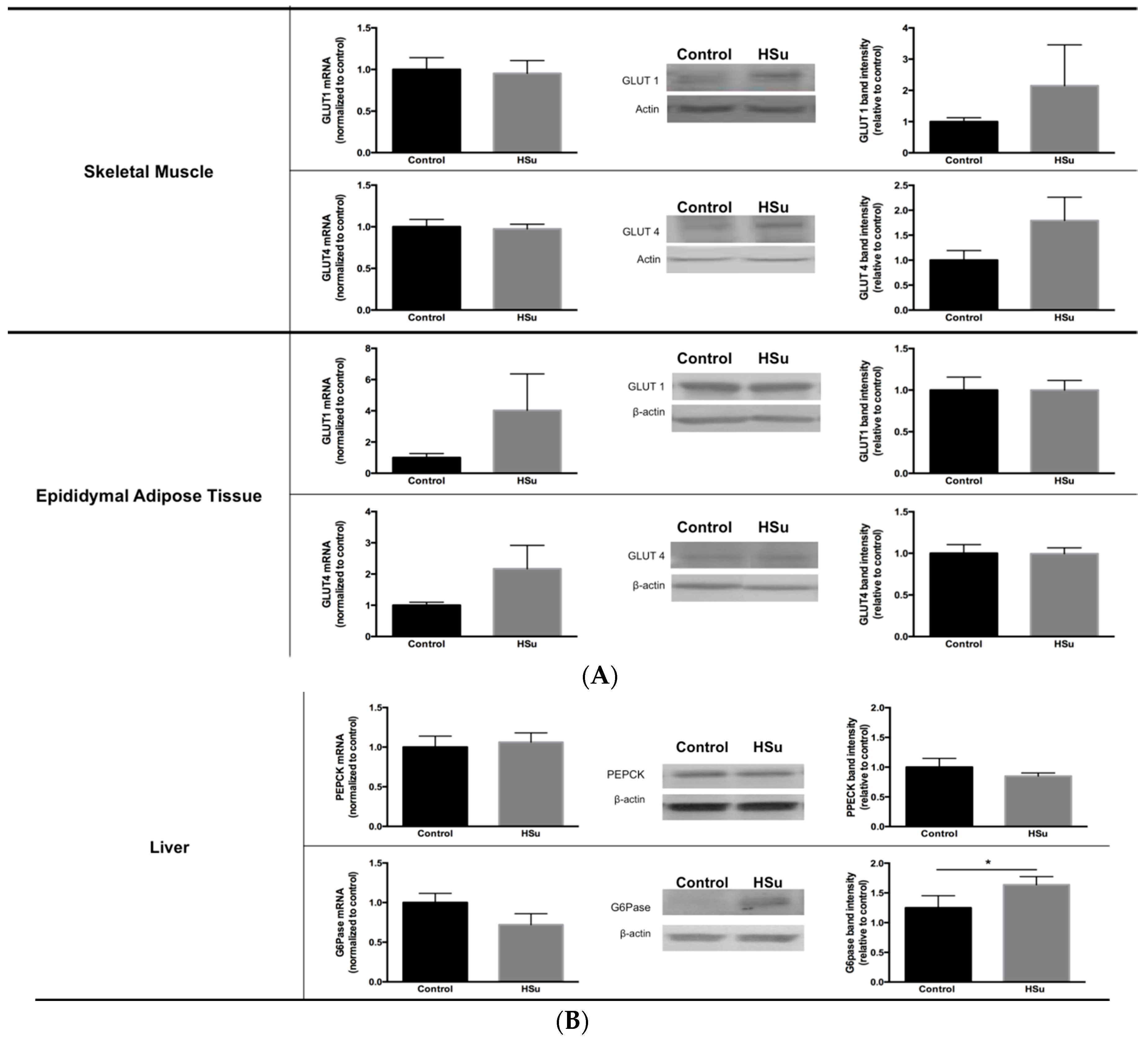
3.4. HSu Diet Increases Fat Mass While the Insulin-Stimulated Glucose Uptake in Adipocytes Is Impaired
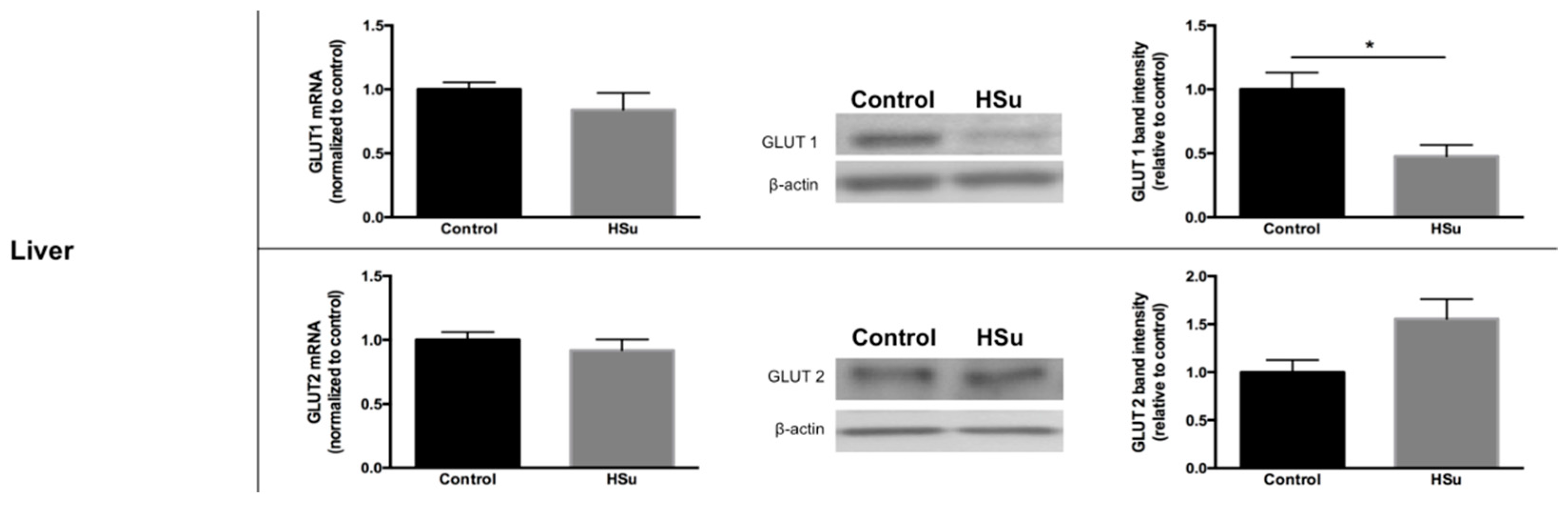
3.5. HSu Diet Decreased GLUT1 but Increased G6Pase Levels in Liver
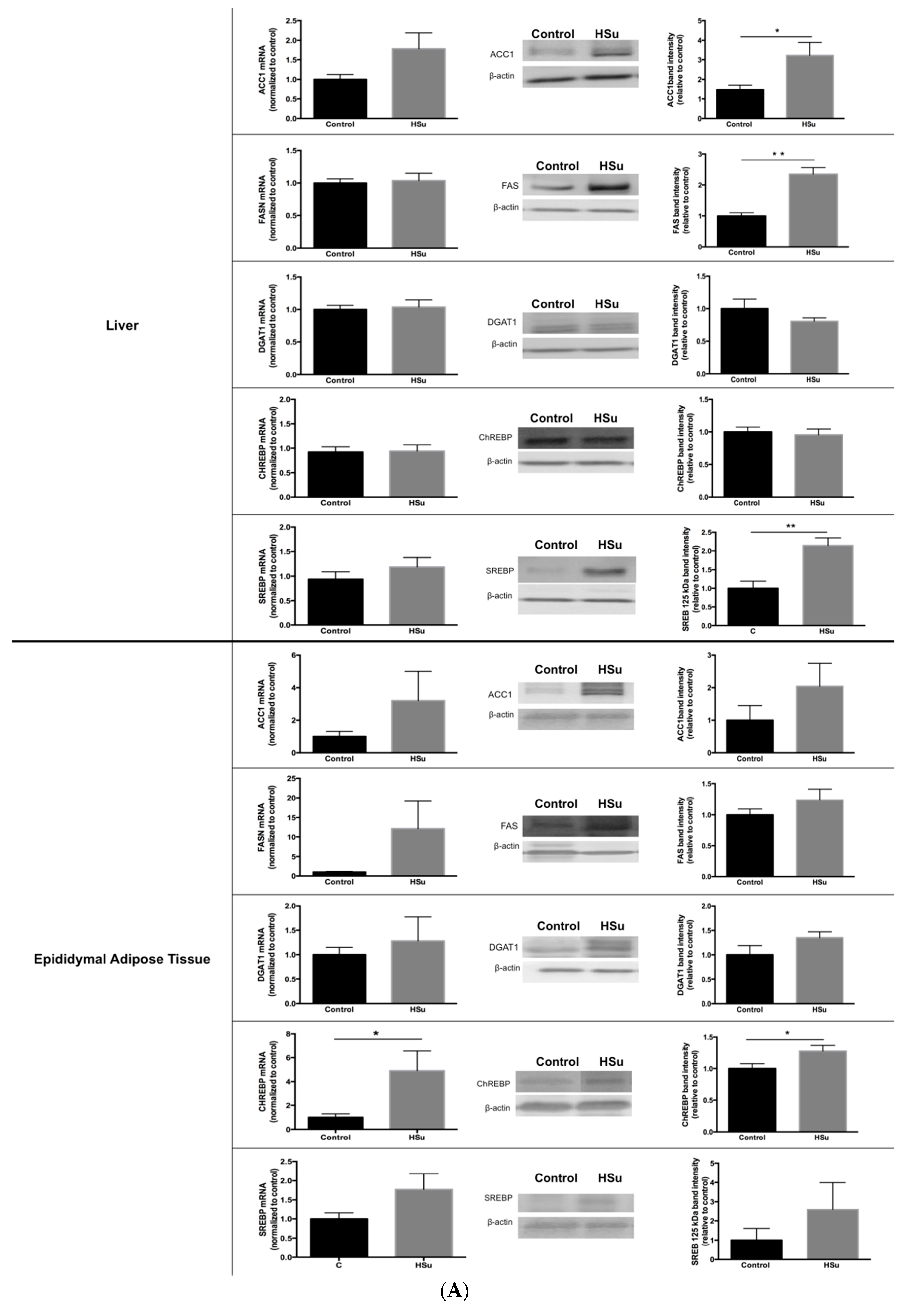
3.6. HSu Diet Increases Hepatic Lipid Biosynthesis, without Alterations in Lipolysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lau, D.C.; Teoh, H. Current and emerging pharmacotherapies for weight management in prediabetes and diabetes. Can. J. Diabetes 2015, 39 (Suppl. S5), S134–S141. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence from Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Parhofer, K.G. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Erion, D.M.; Park, H.J.; Lee, H.Y. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co-morbidities. BMB Rep. 2016, 49, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Van Raalte, D.H.; van der Zijl, N.J.; Diamant, M. Pancreatic steatosis in humans: Cause or marker of lipotoxicity? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Rachek, L.I. Free fatty acids and skeletal muscle insulin resistance. Prog. Mol. Biol. Transl. Sci. 2014, 121, 267–292. [Google Scholar] [PubMed]
- Drew, B.G.; Rye, K.A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diabetes Rep. 2010, 10, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Soares, E.; Fernandes, J.; Viana, S.; Carvalho, E.; Pereira, F.C.; Reis, F. Early cardiac changes in a rat model of prediabetes: Brain natriuretic peptide overexpression seems to be the best marker. Cardiovasc. Diabetol. 2013, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.; Prediger, R.D.; Nunes, S.; Castro, A.A.; Viana, S.D.; Lemos, C.; De Souza, C.M.; Agostinho, P.; Cunha, R.A.; Carvalho, E.; et al. Spatial memory impairments in a prediabetic rat model. Neuroscience 2013, 250, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins, F.; Jones, J.G.; Carvalho, R. 2H2O incorporation into hepatic acetyl-CoA and de novo lipogenesis as measured by Krebs cycle-mediated 2H-enrichment of glutamate and glutamine. Magn. Reson. Med. 2011, 66, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.C.; Lee, Y.J.; Park, J.H.; Kim, H.Y.; Yoon, J.J.; Ahn, Y.M.; Tan, R.; Park, M.C.; Cha, J.D.; Choi, K.M.; et al. Fermented red ginseng potentiates improvement of metabolic dysfunction in metabolic syndrome rat models. Nutrients 2016, 8, E369. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Palming, J.; Rizell, M.; Aureliano, M.; Carvalho, E.; Svensson, M.K.; Eriksson, J.W. mTOR inhibition with rapamycin causes impaired insulin signalling and glucose uptake in human subcutaneous and omental adipocytes. Mol. Cell. Endocrinol. 2012, 355, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Palming, J.; Rizell, M.; Aureliano, M.; Carvalho, E.; Svensson, M.K.; Eriksson, J.W. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol. Cell. Endocrinol. 2013, 365, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Burgeiro, A.; Fuhrmann, A.; Cherian, S.; Espinoza, D.; Jarak, I.; Carvalho, R.A.; Loureiro, M.; Patrício, M.; Antunes, M.; Carvalho, E. Glucose uptake and lipid metabolism are impaired in epicardial adipose tissue from heart failure patients with or without diabetes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E550–E564. [Google Scholar] [CrossRef] [PubMed]
- Ritze, Y.M.; Bardos, G.; D’Haese, J.G.; Ernst, B.; Thurnheer, M.; Schultes, B.; Bischoff, S.C. Effect of high sugar intake on glucose transporter and weight regulating hormones in mice and humans. PLoS ONE 2014, 9, e101702. [Google Scholar] [CrossRef] [PubMed]
- Lirio, L.M.; Forechi, L.; Zanardo, T.C.; Batista, H.M.; Meira, E.F.; Nogueira, B.V.; Mill, J.G.; Baldo, M.P. Chronic fructose intake accelerates non-alcoholic fatty liver disease in the presence of essential hypertension. J. Diabetes Complicat. 2016, 30, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Breinager, L.; Kyrillou, E.; Lacuna, K.; Rocha, R.; Sclafani, A. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiol. Behav. 2010, 101, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Galgani, J.; Ravussin, E. Energy metabolism, fuel selection and body weight regulation. Int. J. Obes. 2008, 32 (Suppl. S7), S109–S119. [Google Scholar] [CrossRef] [PubMed]
- Chaumontet, C.; Even, P.C.; Schwarz, J.; Simonin-Foucault, A.; Piedcoq, J.; Fromentin, G.; Azzout-Marniche, D.; Tomé, D. High dietary protein decreases fat deposition induced by high-fat and high-sucrose diet in rats. Br. J. Nutr. 2015, 114, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Borsheim, E.; Bui, Q.U.; Tissier, S.; Cree, M.G.; Rønsen, O.; Morio, B.; Ferrando, A.A.; Kobayashi, H.; Newcomer, B.R.; Wolfe, R.R. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition 2009, 25, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendia, L.E.; Rodriguez-Moran, M.; Guerrero-Romero, F. The hypertriglyceridemia is associated with isolated impaired glucose tolerance in subjects without insulin resistance. Endocr. Res. 2015, 40, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Ram, J.; Snehalatha, C.; Nanditha, A.; Selvam, S.; Shetty, S.A.; Godsland, I.F.; Johnston, D.G.; Ramachandran, A. Hypertriglyceridaemic waist phenotype as a simple predictive marker of incident diabetes in Asian-Indian men with prediabetes. Diabet. Med. 2014, 31, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.E.; Vale, C.; Walker, M.; Alberti, K.G.; Mathers, J.C. Dietary carbohydrates and insulin sensitivity: A review of the evidence and clinical implications. Am. J. Clin. Nutr. 1997, 66, 1072–1085. [Google Scholar] [PubMed]
- Giorelli Gde, V.; Matos, L.N.; Saado, A.; Soibelman, V.L.; Dias, C.B. No association between 25-hydroxyvitamin D levels and prediabetes in Brazilian patients. A cross-sectional study. Sao Paulo Med. J. 2015, 133, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.T.; Hagberg, J.M. Aerobic training effects on glucose tolerance in prediabetic and normoglycemic humans. Med. Sci. Sports Exerc. 2011, 43, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, F.; Raffa, M.; Patrone, E.; Castelluccio, A. Glucose metabolism and chronic renal insufficiency. Arch. Ital. Urol. Androl. 1994, 66, 51–56. [Google Scholar] [PubMed]
- Pagliassotti, M.J.; Prach, P.A.; Koppenhafer, T.A.; Pan, D.A. Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am. J. Physiol. 1996, 271, R1319–R1326. [Google Scholar] [PubMed]
- Dutta, K.; Podolin, D.A.; Davidson, M.B.; Davidoff, A.J. Cardiomyocyte dysfunction in sucrose-fed rats is associated with insulin resistance. Diabetes 2001, 50, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor, N.S.; Lee, S.; Bacha, F.; Tfayli, H.; Arslanian, S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: Comparison with the hyperinsulinemic-euglycemic clamp. Pediatr. Diabetes 2016, 17, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dufour, S.; Taksali, S.E.; Tamborlane, W.V.; Petersen, K.F.; Bonadonna, R.C.; Boselli, L.; Barbetta, G.; Allen, K.; Rife, F.; et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003, 362, 951–957. [Google Scholar] [CrossRef]
- Neeland, I.J.; Turer, A.T.; Ayers, C.R.; Powell-Wiley, T.M.; Vega, G.L.; Farzaneh-Far, R.; Grundy, S.M.; Khera, A.; McGuire, D.K.; de Lemos, J.A. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012, 308, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jo, K.; Kim, K.J.; Lee, Y.H.; Han, E.; Yoon, H.J.; Wang, H.J.; Kang, E.S.; Yun, M. Visceral adiposity is associated with altered myocardial glucose uptake measured by (18)FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.; Kahn, B.B.; Lodish, H.F. Expression of the low Km GLUT-1 glucose transporter is turned on in perivenous hepatocytes of insulin-deficient diabetic rats. Endocrinology 1991, 129, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Tobin, V.; Le Gall, M.; Fioramonti, X.; Stolarczyk, E.; Blazquez, A.G.; Klein, C.; Prigent, M.; Serradas, P.; Cuif, M.H.; Magnan, C.; et al. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes 2008, 57, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Rondinone, C.; Smith, U. Insulin resistance in fat cells from obese Zucker rats—Evidence for an impaired activation and translocation of protein kinase B and glucose transporter 4. Mol. Cell. Biochem. 2000, 206, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Barosa, C.; Jones, J.; Dube, S.; Carter, R.; Basu, A.; Rizza, R.A. Pathogenesis of prediabetes: Role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J. Clin. Endocrinol. Metab. 2013, 98, E409–E417. [Google Scholar] [CrossRef] [PubMed]
- Dinneen, S.F. The postprandial state: Mechanisms of glucose intolerance. Diabet. Med. 1997, 14 (Suppl. S3), S19–S24. [Google Scholar] [CrossRef]
- Ryu, M.H.; Cha, Y.S. The effects of a high-fat or high-sucrose diet on serum lipid profiles, hepatic acyl-CoA synthetase, carnitine palmitoyltransferase-I, and the acetyl-CoA carboxylase mRNA levels in rats. J. Biochem. Mol. Biol. 2003, 36, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Bruckdorfer, K.R.; Khan, I.H.; Yudkin, J. Fatty acid synthetase activity in the liver and adipose tissue of rats fed with various carbohydrates. Biochem. J. 1972, 129, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Agheli, N.; Kabir, M.; Berni-Canani, S.; Petitjean, E.; Boussairi, A.; Luo, J.; Bornet, F.; Slama, G.; Rizkalla, S.W. Plasma lipids and fatty acid synthase activity are regulated by short-chain fructo-oligosaccharides in sucrose-fed insulin-resistant rats. J. Nutr. 1998, 128, 1283–1288. [Google Scholar] [PubMed]
- Im, S.S.; Kang, S.Y.; Kim, S.Y.; Kim, H.I.; Kim, J.W.; Kim, K.S.; Ahn, Y.H. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes 2005, 54, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Ducluzeau, P.H.; Perretti, N.; Laville, M.; Andreelli, F.; Vega, N.; Riou, J.P.; Vidal, H. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes 2001, 50, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schön, M.R.; Abumrad, N.A.; Blüher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lampidonis, A.D.; Rogdakis, E.; Voutsinas, G.E.; Stravopodis, D.J. The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene 2011, 477, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. The metabolic signature associated with the Western dietary pattern: A cross-sectional study. Nutr. J. 2013, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Malik, V.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009, 89, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Consumption of sugar-sweetened beverages is positively related to insulin resistance and higher plasma leptin concentrations in men and non overweight women. J. Nutr. 2014, 144, 1099–1105. [Google Scholar] [CrossRef] [PubMed]


 Apolipoprotein; CE—Cholesterol ester; IDL—Intermediate-density protein; LDL—Low-density lipoprotein; LDL—Lipoprotein lipase; TG—Triglycerides; VLDL—Very low-density lipoprotein.
Apolipoprotein; CE—Cholesterol ester; IDL—Intermediate-density protein; LDL—Low-density lipoprotein; LDL—Lipoprotein lipase; TG—Triglycerides; VLDL—Very low-density lipoprotein.
 Apolipoprotein; CE—Cholesterol ester; IDL—Intermediate-density protein; LDL—Low-density lipoprotein; LDL—Lipoprotein lipase; TG—Triglycerides; VLDL—Very low-density lipoprotein.
Apolipoprotein; CE—Cholesterol ester; IDL—Intermediate-density protein; LDL—Low-density lipoprotein; LDL—Lipoprotein lipase; TG—Triglycerides; VLDL—Very low-density lipoprotein.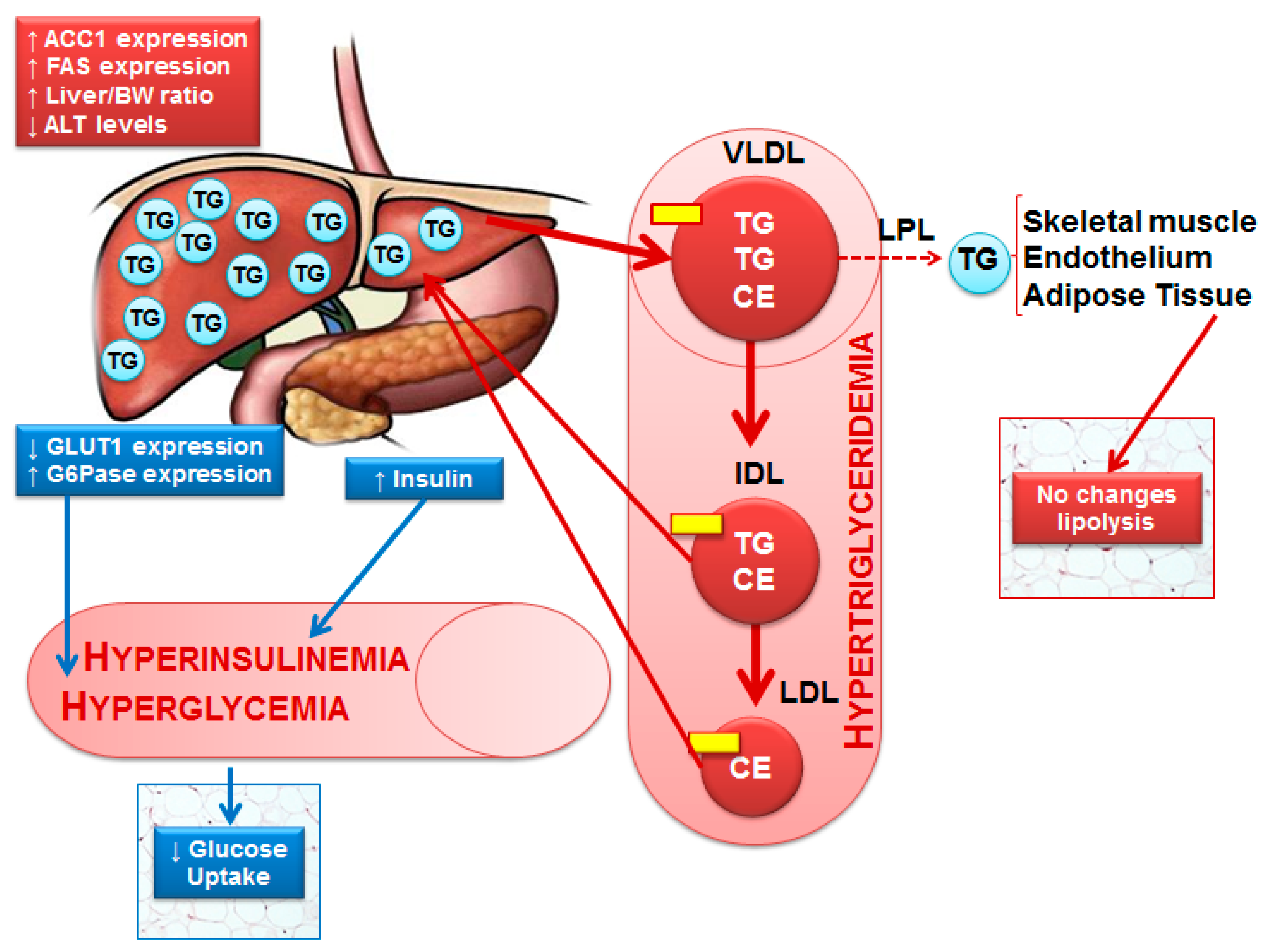
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgeiro, A.; Cerqueira, M.G.; Varela-Rodríguez, B.M.; Nunes, S.; Neto, P.; Pereira, F.C.; Reis, F.; Carvalho, E. Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet. Nutrients 2017, 9, 638. https://doi.org/10.3390/nu9060638
Burgeiro A, Cerqueira MG, Varela-Rodríguez BM, Nunes S, Neto P, Pereira FC, Reis F, Carvalho E. Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet. Nutrients. 2017; 9(6):638. https://doi.org/10.3390/nu9060638
Chicago/Turabian StyleBurgeiro, Ana, Manuela G. Cerqueira, Bárbara M. Varela-Rodríguez, Sara Nunes, Paula Neto, Frederico C. Pereira, Flávio Reis, and Eugénia Carvalho. 2017. "Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet" Nutrients 9, no. 6: 638. https://doi.org/10.3390/nu9060638




