A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Methods
2.1. Metabolomic Profile
2.2. Metabolic Studies
2.3. Oral Glucose Tolerance Test
2.4. Hyperinsulinemic-Euglycemic Clamp
2.5. Abdominal Magnetic Resonance Imaging
2.6. Statistical Analyses
3. Results
3.1. Anthropometric and Metabolic Profiles
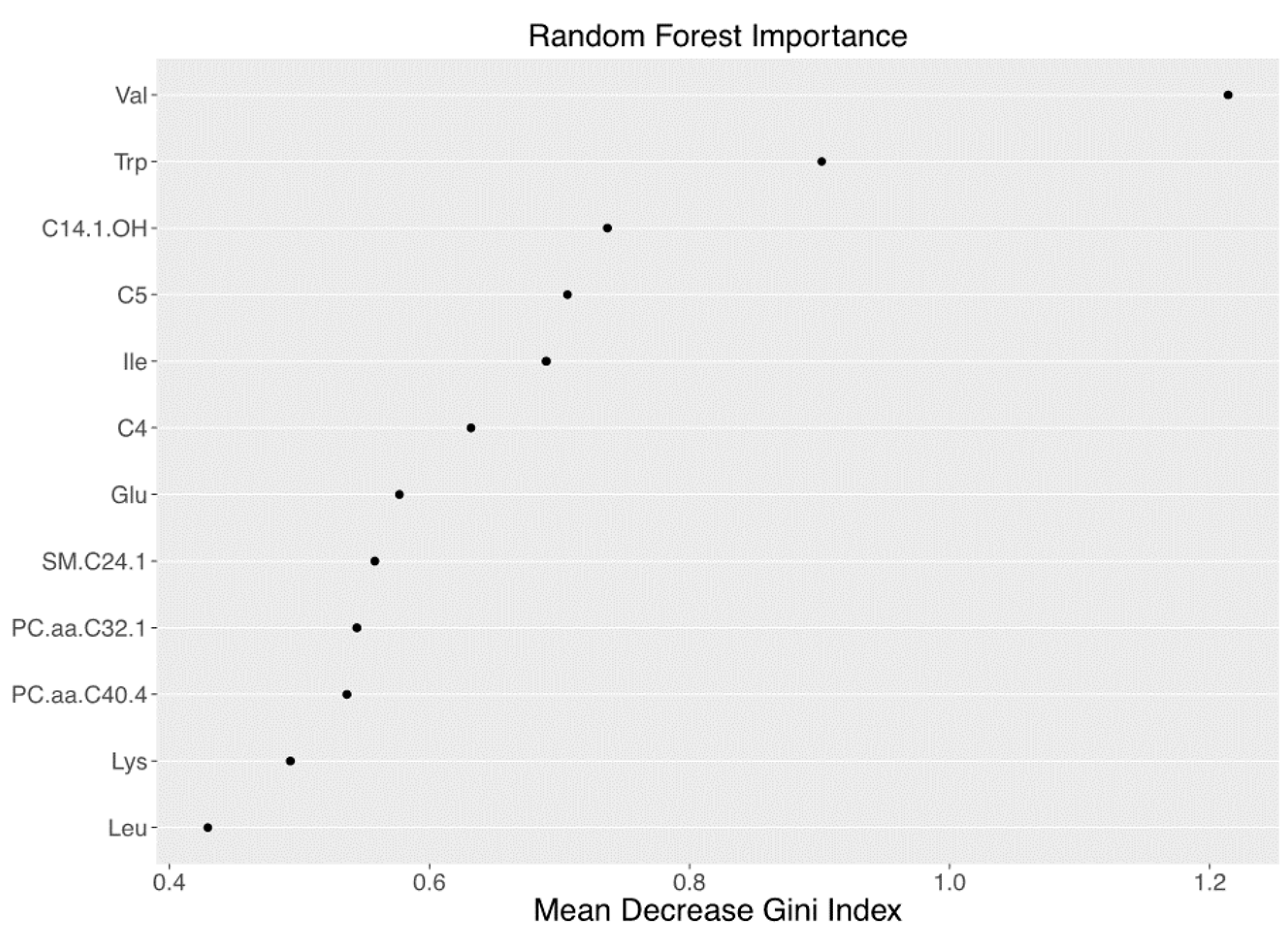
3.2. Metabolomics Signature of Obese Youth with NAFLD
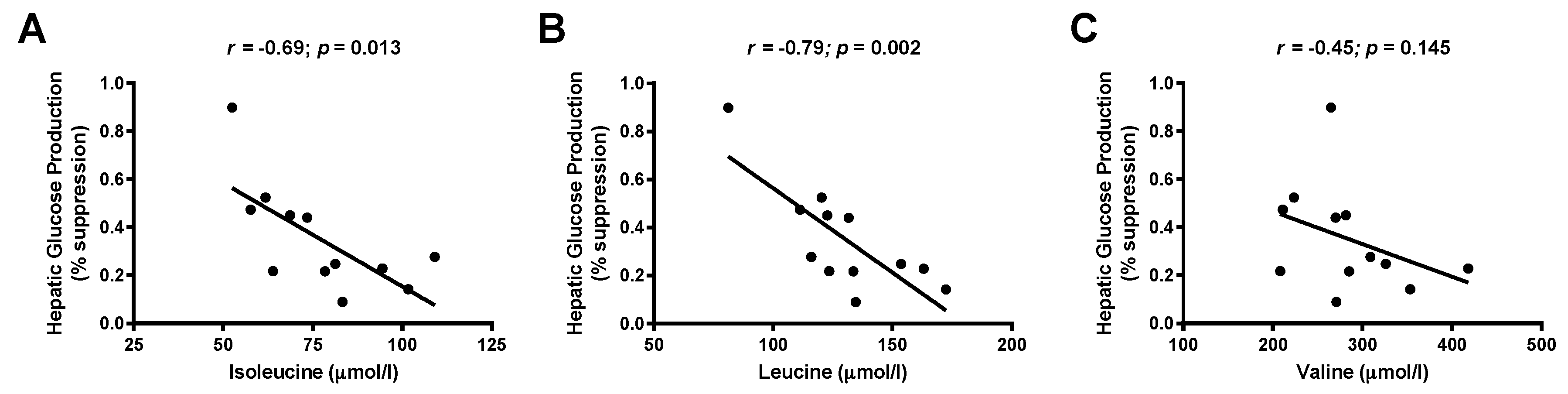
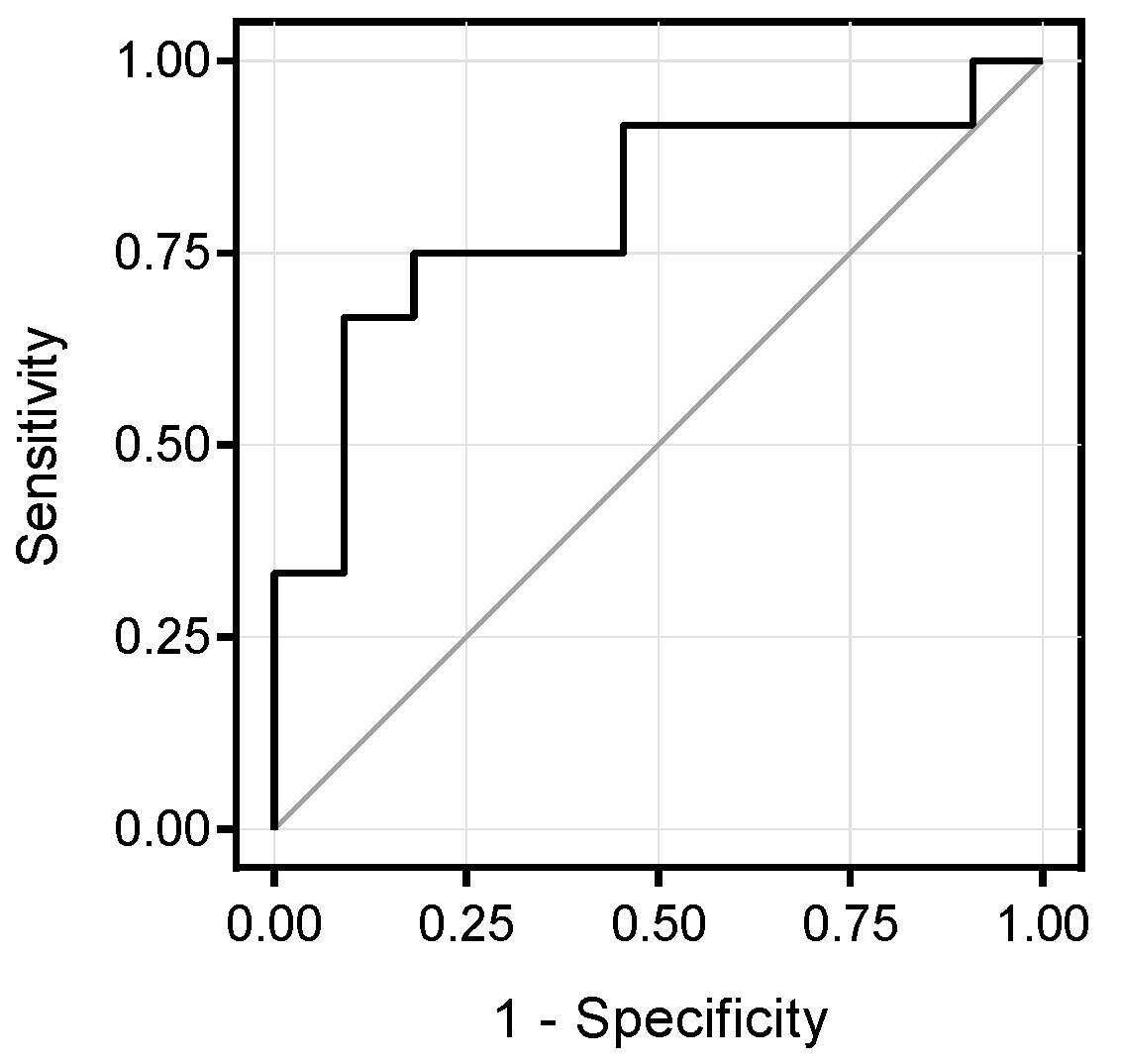
3.3. Associations between Branched-Chain Amino Acids and Hepatic Insulin Sensitivity
3.4. Correlation between Metabolite Concentration and Changes of Hepatic Fat Content over Time
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwimmer, J.B.; McGreal, N.; Deutsch, R.; Finegold, M.J.; Lavine, J.E. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005, 115, e561–e565. [Google Scholar] [CrossRef] [PubMed]
- Mencin, A.A.; Lavine, J.E. Advances in pediatric nonalcoholic fatty liver disease. Pediatr. Clin. N. Am. 2011, 58, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, E.; Cali, A.M.; Weiss, R.; Santoro, N.; Pierpont, B.; Northrup, V.; Caprio, S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 2010, 33, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, E.; Northrup, V.; Weiss, R.; Santoro, N.; Pierpont, B.; Savoye, M.; O’Malley, G.; Caprio, S. Ethnic differences in lipoprotein subclasses in obese adolescents: Importance of liver and intraabdominal fat accretion. Am. J. Clin. Nutr. 2010, 92, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Caprio, S. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in obese adolescents: A looming marker of cardiac dysfunction. Hepatology 2014, 59, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Ishihara, T.; Mifuji-Moroka, R.; Fujita, N.; Kobayashi, Y.; Hasegawa, H.; Iwata, K.; Kaito, M.; Takei, Y. Elevation of branched-chain amino acid levels in diabetes and nafl and changes with antidiabetic drug treatment. Obes. Res. Clin. Pract. 2015, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Franken, H.; Dammeier, S.; Rosenbaum, L.; Kantartzis, K.; Peter, A.; Zell, A.; Adam, P.; Li, J.; Xu, G.; et al. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes Care 2013, 36, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gallego, E.; Guirro, M.; Riera-Borrull, M.; Hernandez-Aguilera, A.; Marine-Casado, R.; Fernandez-Arroyo, S.; Beltran-Debon, R.; Sabench, F.; Hernandez, M.; del Castillo, D.; et al. Mapping of the circulating metabolome reveals alpha-ketoglutarate as a predictor of morbid obesity-associated non-alcoholic fatty liver disease. Int. J. Obes. (Lond.) 2015, 39, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wiklund, P.; Autio, R.; Borra, R.; Ojanen, X.; Xu, L.; Tormakangas, T.; Alen, M. Adipose tissue dysfunction and altered systemic amino acid metabolism are associated with non-alcoholic fatty liver disease. PLoS ONE 2015, 10, e0138889. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Zhai, G.; Macgregor, A.; Prehn, C.; Romisch-Margl, W.; Suhre, K.; Adamski, J.; Cassidy, A.; Illig, T.; Spector, T.D.; et al. Targeted metabolomics profiles are strongly correlated with nutritional patterns in women. Metabolomics 2013, 9, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Goring, H.; Cole, S.A.; Comuzzie, A.G. Global metabolomic profiling targeting childhood obesity in the hispanic population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Prinsen, H.; Giannini, C.; de Graaf, R.; Juchem, C.; Li, F.; Caprio, S.; Santoro, N.; Herzog, R.I. Elevated alpha-hydroxybutyrate and BCAA levels predict deterioration of glycemic control in adolescents. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Newbern, D.; Gumus Balikcioglu, P.; Balikcioglu, M.; Bain, J.; Muehlbauer, M.; Stevens, R.; Ilkayeva, O.; Dolinsky, D.; Armstrong, S.; Irizarry, K.; et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: Metabolomic profiling and principal components analysis. J. Clin. Endocrinol. Metab. 2014, 99, 4730–4739. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A.; et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Yu, Z.; Kleber, M.; Singmann, P.; Holzapfel, C.; He, Y.; Mittelstrass, K.; Polonikov, A.; Prehn, C.; Romisch-Margl, W.; et al. Childhood obesity is associated with changes in the serum metabolite profile. Obes. Facts 2012, 5, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gang, X.; Liu, Y.; Sun, C.; Han, Q.; Wang, G. Using metabolomic profiles as biomarkers for insulin resistance in childhood obesity: A systematic review. J. Diabetes Res. 2016, 2016, 8160545. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Michaliszyn, S.F.; de las Heras, J.; Bacha, F.; Lee, S.; Chace, D.H.; DeJesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: Evidence for enhanced mitochondrial oxidation. Diabetes Care 2012, 35, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Michaliszyn, S.F.; Sjaarda, L.A.; Mihalik, S.J.; Lee, S.; Bacha, F.; Chace, D.H.; de Jesus, V.R.; Vockley, J.; Arslanian, S.A. Metabolomic profiling of amino acids and beta-cell function relative to insulin sensitivity in youth. J. Clin. Endocrinol. Metab. 2012, 97, E2119–E2124. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Di Sessa, A.; Caprio, S.; Chalasani, N.; Liu, W.; Liang, T.; Graf, J.; Herzog, R.I.; Johnson, C.D.; Umano, G.R.; et al. Oxidized derivatives of linoleic acid in pediatric metabolic syndrome: Is their pathogenic role modulated by the genetic background and the gut microbiota? Antioxid. Redox Signal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 1), S5–S20. [Google Scholar]
- Matsuda, M.; De Fronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Yeckel, C.W.; Weiss, R.; Dziura, J.; Taksali, S.E.; Dufour, S.; Burgert, T.S.; Tamborlane, W.V.; Caprio, S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J. Clin. Endocrinol. Metab. 2004, 89, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dufour, S.; Taksali, S.E.; Tamborlane, W.V.; Petersen, K.F.; Bonadonna, R.C.; Boselli, L.; Barbetta, G.; Allen, K.; Rife, F.; et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003, 362, 951–957. [Google Scholar] [CrossRef]
- Weiss, R.; Caprio, S.; Trombetta, M.; Taksali, S.E.; Tamborlane, W.V.; Bonadonna, R. Beta-cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005, 54, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Cali, A.M.; De Oliveira, A.M.; Kim, H.; Chen, S.; Reyes-Mugica, M.; Escalera, S.; Dziura, J.; Taksali, S.E.; Kursawe, R.; Shaw, M.; et al. Glucose dysregulation and hepatic steatosis in obese adolescents: Is there a link? Hepatology 2009, 49, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.H.; Gardner, K.G.; Potter, C.J.; Schmalbrock, P.; Smith, M.A. Introduction of fast mr imaging in the assessment of hepatic steatosis. Magn. Reson. Imaging 1997, 15, 287–293. [Google Scholar] [CrossRef]
- Mascaro, J.; Asner, G.P.; Knapp, D.E.; Kennedy-Bowdoin, T.; Martin, R.E.; Anderson, C.; Higgins, M.; Chadwick, K.D. A tale of two “forests”: Random forest machine learning aids tropical forest carbon mapping. PLoS ONE 2014, 9, e85993. [Google Scholar] [CrossRef] [PubMed]
- Lanz, C.; Patterson, A.D.; Slavik, J.; Krausz, K.W.; Ledermann, M.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography-mass spectrometry combined with random forests machine learning algorithm. Radiat. Res. 2009, 172, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Zhao, H.; Zhou, H.H. False discovery rate control with groups. J. Am. Stat. Assoc. 2010, 105, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Feldstein, A.E.; Enoksson, E.; Pierpont, B.; Kursawe, R.; Kim, G.; Caprio, S. The association between hepatic fat content and liver injury in obese children and adolescents: Effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care 2013, 36, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’yasova, D.; Chen, Y.I.; Haffner, S.M.; Hanley, A.J. Branched-chain amino acids and insulin metabolism: The insulin resistance atherosclerosis study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Banton, S.; Tran, V.T.; Konomi, J.V.; Li, S.; Jones, D.P.; Vos, M.B. Amino acid metabolism is altered in adolescents with nonalcoholic fatty liver disease—An untargeted, high resolution metabolomics study. J. Pediatr. 2016, 172, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Pietilainen, K.H.; Naukkarinen, J.; Rissanen, A.; Saharinen, J.; Ellonen, P.; Keranen, H.; Suomalainen, A.; Gotz, A.; Suortti, T.; Yki-Jarvinen, H.; et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: Pathways behind acquired obesity. PLoS Med. 2008, 5, e51. [Google Scholar] [CrossRef] [PubMed]
- Burrill, J.S.; Long, E.K.; Reilly, B.; Deng, Y.; Armitage, I.M.; Scherer, P.E.; Bernlohr, D.A. Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes. Mol. Endocrinol. 2015, 29, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000, 130, 1016S–1022S. [Google Scholar] [PubMed]
- Verrey, F. System l: Heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003, 445, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.D.; Wang, H.; Huang, W.; Kekuda, R.; Rajan, D.P.; Leibach, F.H.; Ganapathy, V. Human LAT1, a subunit of system L amino acid transporter: Molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999, 255, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Kalavalapalli, S.; Bril, F.; Garrett, T.J.; Nautiyal, M.; Mathew, J.T.; Williams, C.M.; Cusi, K. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E311–E319. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef] [PubMed]

| HFF < 5.5% (n = 48) | HFF ≥ 5.5% (n = 30) | p | |
|---|---|---|---|
| CLINICAL FEATURES | |||
| Age (years) | 13.6 ± 3.0 | 12.8 ± 2.8 | 0.30 |
| Gender (M/F) | 17/31 (35%/65%) | 21/9 (70%/30%) | 0.003 |
| Race (Caucasian/African American/Hispanic/Asian) | 15/19/13/1 (31%/40%/27%/2%) | 11/3/16/0 (37%/10%/53%/0%) | 0.02 |
| Glucose tolerance (NGT/IGT) | 41/7 (85%/15%) | 20/10 (67%/33%) | 0.10 |
| BMI (kg/m2) | 34.1 ± 7.2 | 33.6 ± 5.1 | 0.64 |
| BMI z-score | 2.2 ± 0.3 | 2.3 ± 0.2 | 0.24 |
| Body Fat (%) | 44.27 ± 7.17 | 45.35 ± 7.53 | 0.34 |
| GLUCOSE METABOLISM | |||
| Fasting glucose (mg/dL) | 91 ± 7 | 94 ± 6 | 0.15 |
| Fasting insulin (µU/mL) | 25 ± 11.3 | 45.4 ± 23.9 | <0.001 |
| 2 h glucose (mg/dL) | 114 ± 22 | 128 ± 28 | 0.01 |
| Hemoglobin A1C (%) | 5.4 ± 0.2 | 5.5 ± 0.3 | 0.31 |
| WBISI | 2.12 ± 0.89 | 1.2 ± 0.8 | <0.001 |
| IGI | 4.1 ± 2.8 | 5.93 ± 5.6 | 0.08 |
| DI | 8.0 ± 6.4 | 6.7 ± 7.1 | 0.50 |
| LIPID PROFILE | |||
| Total Cholesterol (mg/dL) | 148 ± 26 | 159 ± 36 | 0.19 |
| HDL Cholesterol (mg/dL) | 45 ± 9 | 42.47 ± 10 | 0.23 |
| LDL Cholesterol (mg/dL) | 87 ± 23 | 92 ± 30 | 0.55 |
| Triglycerides (mg/dL) | 78 ± 38 | 127 ± 80 | 0.002 |
| LIVER FUNCTION | |||
| Alanine Transaminase (U/L) | 16.5 ± 6.9 | 40.2 ± 28.5 | <0.001 |
| Aspartate Transaminase (U/L) | 19.7 ± 4.3 | 30.3 ± 15.0 | 0.001 |
| BODY FAT COMPOSITION | |||
| Visceral (cm2) | 55.6 ± 28.6 | 79.7 ± 24.8 | <0.001 |
| Deep Subcutaneous (cm2) | 188.6 ± 141.6 | 177.1 ± 57.1 | 0.40 |
| Subcutaneous (cm2) | 552.1 ± 224.5 | 527.9 ± 167.4 | 0.90 |
| Superficial Subcutaneous (cm2) | 168.1 ± 70.3 | 129.8 ± 52.8 | 0.002 |
| Deep/Superficial Subcutaneous | 1.1 ± 0.4 | 1.5 ± 0.5 | 0.02 |
| Hepatic Fat Fraction (%) | 1.1 ± 1.6 | 18.3± 10.2 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goffredo, M.; Santoro, N.; Tricò, D.; Giannini, C.; D’Adamo, E.; Zhao, H.; Peng, G.; Yu, X.; Lam, T.T.; Pierpont, B.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 642. https://doi.org/10.3390/nu9070642
Goffredo M, Santoro N, Tricò D, Giannini C, D’Adamo E, Zhao H, Peng G, Yu X, Lam TT, Pierpont B, et al. A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease. Nutrients. 2017; 9(7):642. https://doi.org/10.3390/nu9070642
Chicago/Turabian StyleGoffredo, Martina, Nicola Santoro, Domenico Tricò, Cosimo Giannini, Ebe D’Adamo, Hongyu Zhao, Gang Peng, Xiaoqing Yu, Tukiet T. Lam, Bridget Pierpont, and et al. 2017. "A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease" Nutrients 9, no. 7: 642. https://doi.org/10.3390/nu9070642





