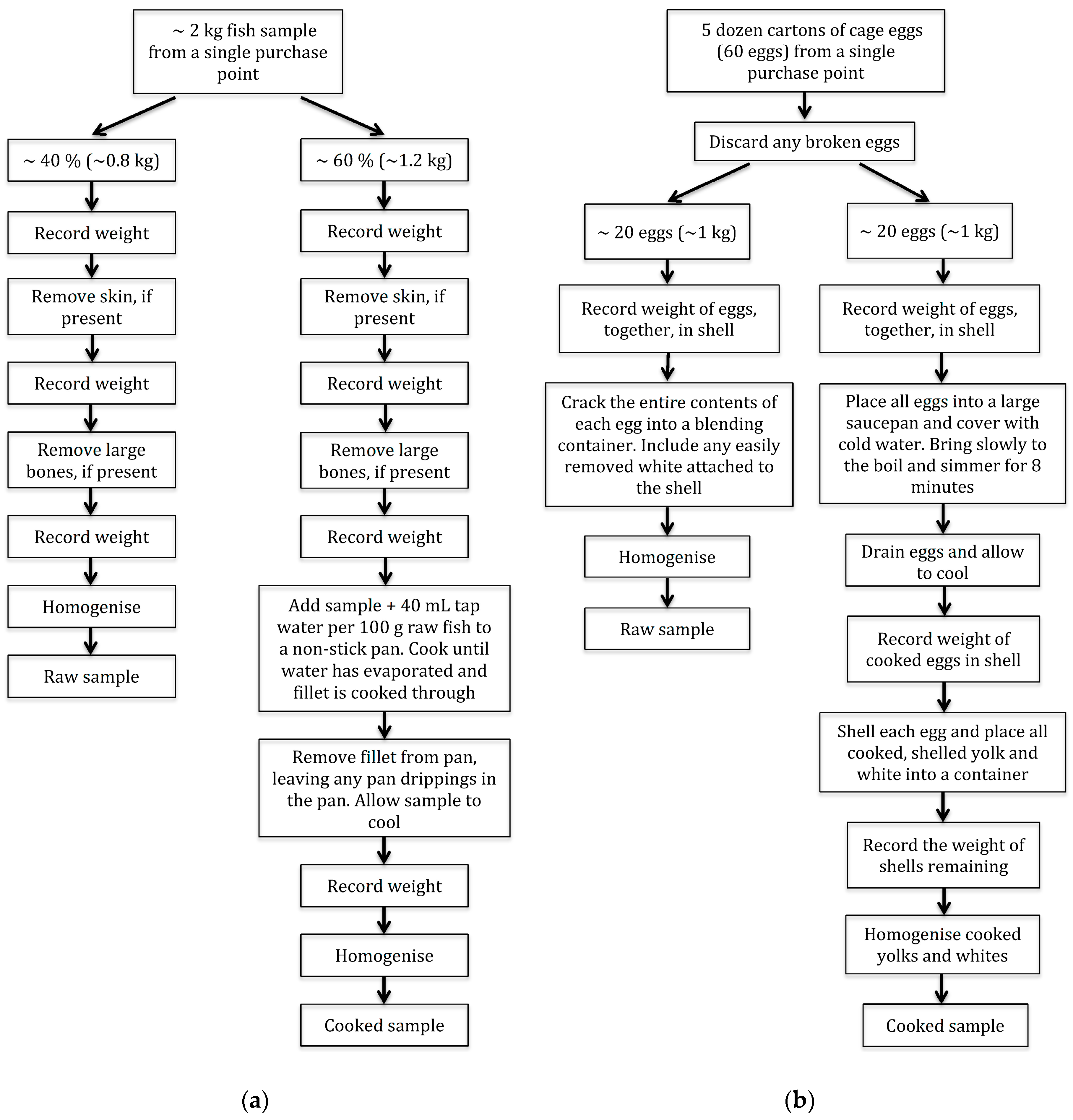
1. Introduction
The 2011–2013 Australian Health Survey revealed that 23% of Australian adults were vitamin D deficient (serum 25-hydroxyvitamin D (25(OH)D) concentrations <50 nmol/L) [
1]. Sunlight exposure offers the greatest potential source of vitamin D via the endogenous, two-step process of ultraviolet-B (UVB) photolytic and thermal conversion of cutaneous 7-dehydrocholesterol to produce vitamin D
3 [
2]. However, the effectiveness of sun exposure as a source of vitamin D
3 varies with geographical location, season, skin pigmentation, lifestyle and environmental factors [
3]. Furthermore, sunlight exposure is often limited due to sun-safety concerns. As such, the maintenance of vitamin D sufficiency in some people relies on dietary vitamin D, which is found naturally in few foods and generally in low concentrations. Fish, meat, eggs and dairy are sources of vitamin D
3, while vitamin D
2 is found in mushrooms. The hydroxylated form of vitamin D
3, 25(OH)D
3, is also obtained from animal sources, and may be up to five times more bioactive than vitamin D
3 [
4,
5]. It is recognised that omitting the 25(OH)D
3 content of animal products from food composition databases is very likely to lead to underestimates of true vitamin D intake [
6]. Hence, the accurate determination of the total vitamin D content of fish, meat, eggs and dairy requires measurement of both vitamin D
3 and 25(OH)D
3.
In Australia, dietary correction of vitamin D deficiency is complicated by lack of locally relevant vitamin D food composition data [
7]. Since variations in nutritional content of produce may occur with geographical location, climate, feed fortification and animal production practices [
8], it is inappropriate to rely on international vitamin D composition data. Furthermore, international data may not represent species and types of foods commonly consumed in Australia [
9]. Since 2013, vitamin D
3 and 25(OH)D
3 have been measured in a limited number of Australian meat [
10], egg [
8] and seafood [
11] samples, based on convenience or purposive sampling. We build on these studies by presenting new data for the vitamin D
3 and 25(OH)D
3 content of white fish and eggs purchased from retail outlets across five cities in Australia.
4. Discussion
In our study, the vitamin D
3 and 25(OH)D
3 content varied widely between fish samples, which could reflect variation in production practices, feed type and differences within species that may occur due to location, season, and water clarity [
26,
27]. Barramundi (
Lates calcarifer) contained the highest VitDE of the species analysed in our study, although the content was less than in Australian farmed barramundi (10.7 μg/100 g) analysed by LC-IT-MS in 2014 by Padula and colleagues [
11]. The farmed barramundi sample analysed by Padula and colleagues was sourced directly from the point of production, which may have minimised any vitamin D or 25(OH)D
3 deterioration through storage, whilst fortification of farmed fish feed or aforementioned variations in location or time of year may also have contributed to the higher VitDE in that study [
11]. In our study, samples of king dory (Melbourne), hoki (Adelaide) and basa (Perth) showed higher fat and VitDE content (per 100 g) after cooking, compared with raw. This outcome has also been demonstrated in pork, and can be attributed to moisture loss [
28]. VitDE in white fish in our study was greater than in international samples, with the exception of the halibut analysed in the USA. While the same environmental, geographical and production effects that cause variation within Australian samples may play a role, sensitivity of the analysis method and accurate determination of 25(OH)D
3 may also result in substantial differences in VitDE.
We found no overall difference in VitDE between free-range and cage eggs; however, considerable variation was seen in samples from different cities. Vitamin D content may vary in eggs according to feed fortification and exposure of hens to UV-B radiation [
29,
30]. Layer feed fortification with vitamin D
3 is common practice in Australia with usual amounts of approximately 75 μg of vitamin D
3 per kilogram of feed [
31]. Kühn and colleagues found that free-range farming was effective in raising the vitamin D content of eggs, but only when hens had adequate access to suitable outdoor areas [
30]. This finding was reflected in a recently published study that showed greater vitamin D
3 concentrations in both free-range and organic egg yolks, and higher 25(OH)D
3 content in organic egg yolk, compared with egg yolks from hens kept indoors [
32]. Authors suggested that sunlight exposure was a likely influence. A previous study in Australia found that egg yolk contained 38% more vitamin D
3 and 300% more 25(OH)D
3 when hen feed was fortified with both vitamin D
3 and 25OH)D
3 [
8]. Feed fortification with only vitamin D
3 also increases the content of both vitamin D
3 and 25(OH)D
3 in eggs [
33]. Our study included free-range eggs from a low-stocking density farm (130 hens/ha)—these eggs had higher vitamin D
3 (but not 25(OH)D
3) content than eggs from farms with higher stocking densities. Further research, involving sampling eggs from farms using various stocking densities where feed composition is known, would be required to investigate whether any differences in the vitamin D
3 and 25(OH)D
3 content of eggs is due to production practices (e.g., sunlight exposure) or feed fortification.
In international data sources, the vitamin D3 content of eggs was generally higher than in our Australian samples. However, since the 25(OH)D3 content of international samples was either very low (UK), unreported (USA) or not adjusted for bioactivity (The Netherlands), the considerably higher amounts of 25(OH)D3 detected in our egg samples led to greater VitDE content overall compared with international data.
The adequate intake (AI) of vitamin D for Australians aged 1–50 years is 5 μg/day, increasing to 10 μg/day for 51–70 year olds, and 15 μg/day for those aged >70 years [
34]. If, as has been suggested, 25(OH)D
3 has five times greater bioactivity than vitamin D
3, one cooked serve (100 g cooked weight) of white fish may supply between 43% and 60% of the AI for 1–50 year olds (depending on the fish species), and one cooked serve (2 large eggs, 120 g) of eggs may provide 100% of the AI for 1–50 year olds. However, it has been acknowledged that the current guidelines for the AI of vitamin D in Australia are out of date [
35]. In the United States, the recommended dietary allowance for vitamin D is 15 µg/day for infants, children and adults aged ≤70 years (including during pregnancy and lactation) and 20 µg/day for adults aged >70 years [
36]. The European Food Safety Authority recently set an AI for vitamin D at 10 μg/day for infants aged 7–11 months and 15 μg/day for children aged 1–17 years and adults [
37].
Both white fish and eggs available in Australia may provide nutritionally useful amounts of vitamin D; however, conveying this information to consumers is hampered by the lack of Australian food composition data. In addition, 25(OH)D
3 is not recognised in the Australia New Zealand Food Standards Code as contributing to the vitamin D content of foods [
38], despite 25(OH)D
3 being used as a fortificant in animal feed, including in layer poultry farming [
8]. The Food Standards Code states that one serve of a food must contain at least 1 µg of vitamin D for a general “source of” claim, or 2.5 µg of vitamin D for a “good source of” claim [
38]. As our study demonstrates, 25(OH)D
3 may contribute substantially to the vitamin D content of foods. Although the potency of 25(OH)D
3 compared with vitamin D
3 is debated, there is general agreement that 25(OH)D
3 is more bioactive than vitamin D
3 [
4,
39,
40,
41,
42,
43]. Therefore, failure to measure 25(OH)D
3 in food may result in considerable underestimation of vitamin D intakes. According to Heaney and colleagues, the finding of 25(OH)D
3 in food provides a possible explanation for the gap between the calculated total basal input of vitamin D (sun exposure plus traditional food sources of vitamin D) and measured serum 25(OH)D concentrations [
44].
A major strength of our study was the measurement of 25(OH)D
3, which is frequently lacking in food composition databases. Accurate determination of 25(OH)D
3 at low levels is crucial, since each amount unmeasured may represent up to a five-fold loss in overall vitamin D content, leading to underestimation of intake and misrepresentation of intake versus requirement. A further strength of this study was the multi-city sampling plan: we sampled from five major Australian cities, which reflect where the majority of Australians are buying food. Financial constraints meant that we were only able to explore a limited selection of white fish species available in Australia. We did not explore the differences between skin-on versus skin-off fish fillets, nor did we compare different cooking methods for fish and eggs. A comparison of wild versus Australian farmed fish may be warranted, since Lu and colleagues showed that farmed salmon in the United States had approximately 25% of the vitamin D content of wild salmon [
45]. Egg yolk and white were not analysed separately, nor were dried versions of any form. All samples were purchased in March, at the end of the Australian summer; hence, the effect of seasonality was not investigated.