Genome-Wide Association Study of Dietary Pattern Scores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric Measurements and Biochemical Profiling
2.3. Dietary Assessment and Food Pattern Derivation
2.4. Genome-Wide Genotyping and Quality Control
2.5. Gene Expression Analyses
2.6. Functional Analyses
2.7. Statistical Analysis
3. Results
3.1. Subjects’ Description
3.2. Dietary Scores and CVD Risk Factors
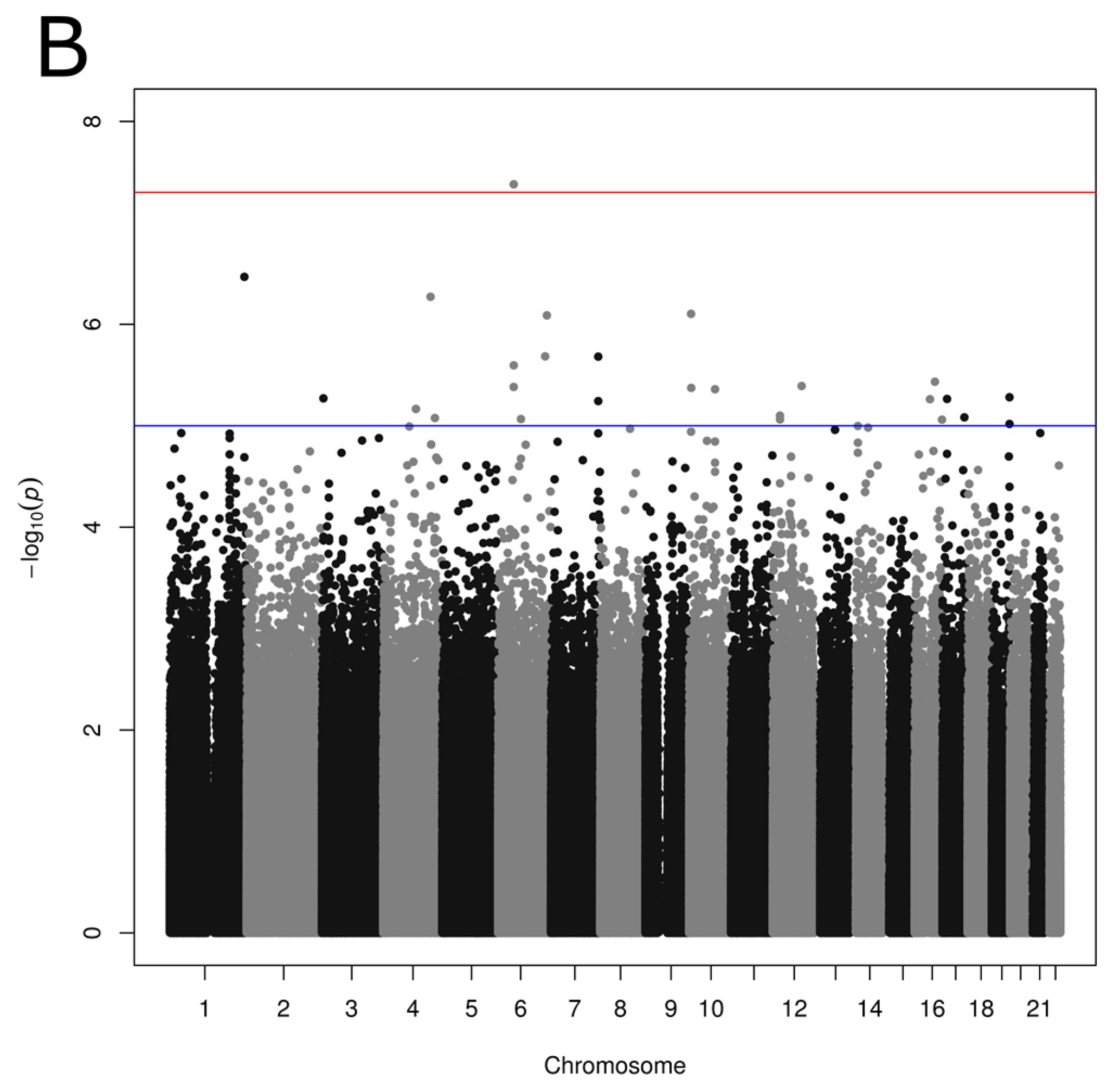
3.3. Association between SNPs and Reported Dietary Patterns
3.4. Impact of SNPs on CVD Risk Factors
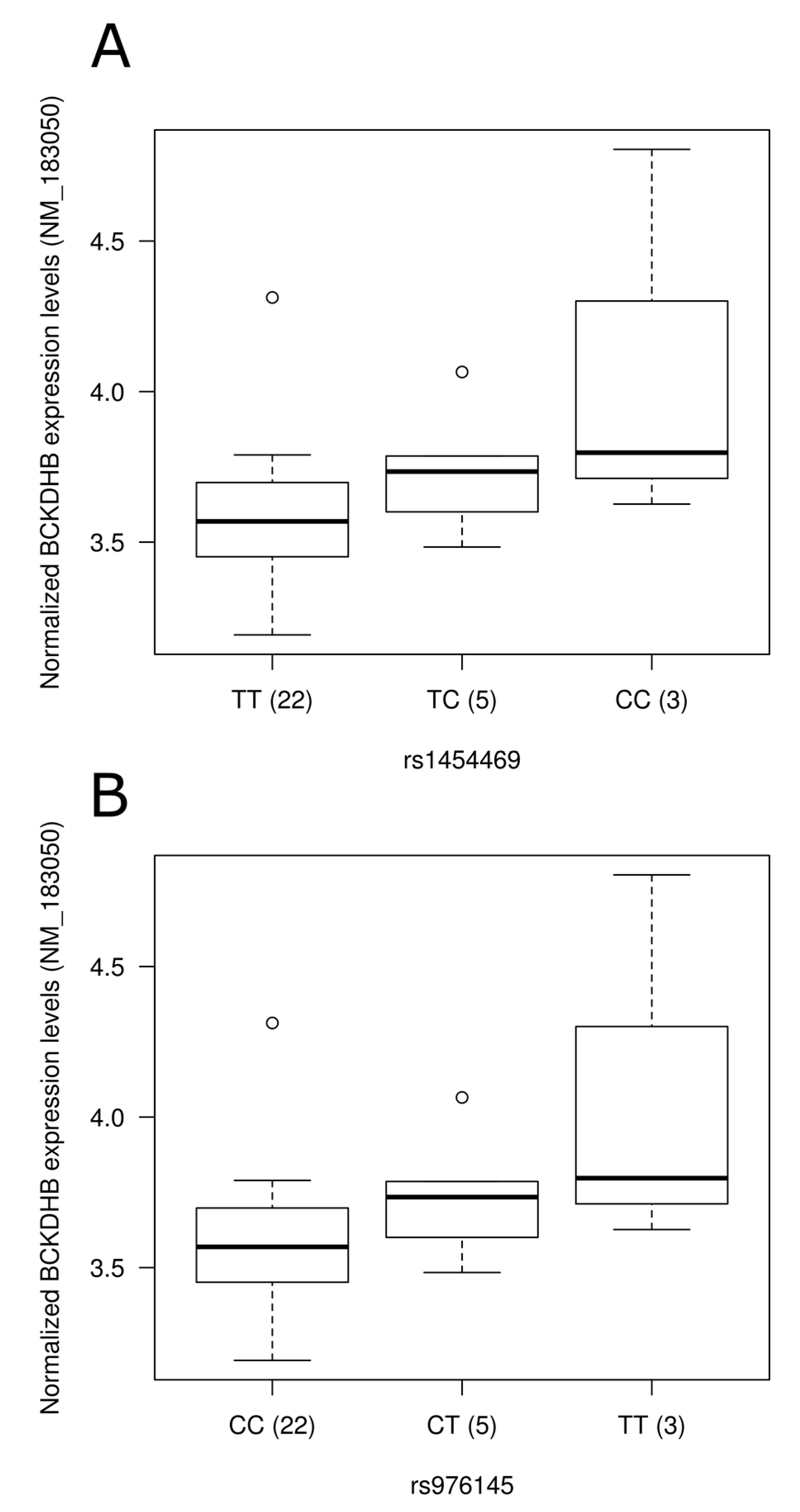
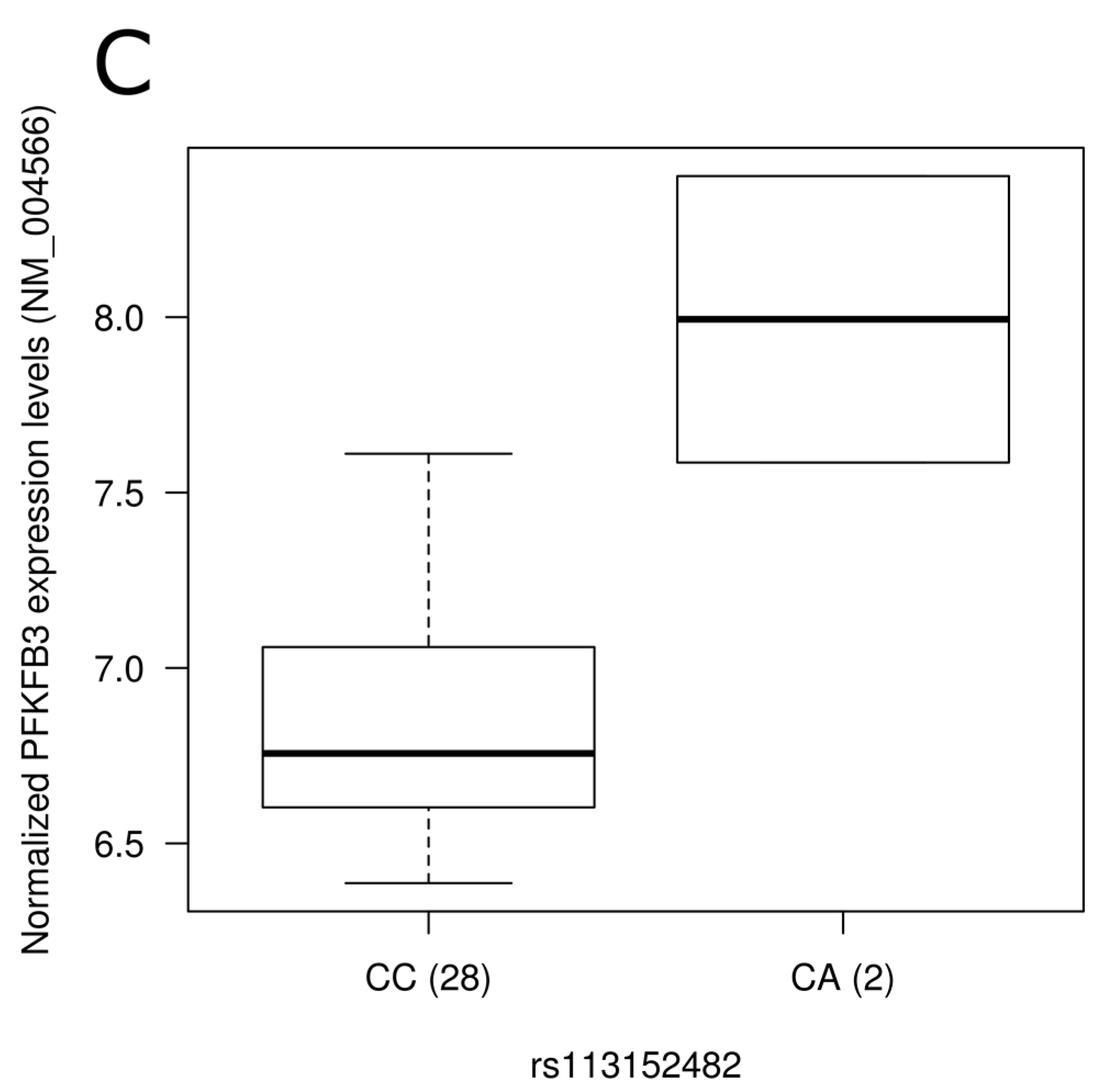
3.5. Impact of SNPs on Gene Expression Level
3.6. Functional Analysis of SNPs
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; McCaffrey, T.A.; Rennie, K.L. Childhood obesity prevention studies: Lessons learned and to be learned. Public Health Nutr. 2006, 9, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.C.; Lindeman, A.K.; Wallace, J.; Niederpruem, M. Diet composition, energy intake, and exercise in relation to body fat in men and women. Am. J. Clin. Nutr. 1990, 52, 426–430. [Google Scholar] [PubMed]
- Jacobs, D.R.; Tapsell, L.C. Food synergy: The key to a healthy diet. Proc. Nutr. Soc. 2013, 72, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zarraga, I.G.; Schwarz, E.R. Impact of dietary patterns and interventions on cardiovascular health. Circulation 2006, 114, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Pavela, G.; Lavie, C.J. The Inadmissibility of What We Eat in America and NHANES Dietary Data in Nutrition and Obesity Research and the Scientific Formulation of National Dietary Guidelines. Mayo Clin. Proc. 2015, 90, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Davy, B.M.; Estabrooks, P.A. The Validity of Self-reported Dietary Intake Data: Focus on the “What We Eat In America” Component of the National Health and Nutrition Examination Survey Research Initiative. Mayo Clin. Proc. 2015, 90, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Shimazu, T.; Ishihara, J.; Takachi, R.; Mizoue, T.; Inoue, M.; Tsugane, S. Reproducibility and validity of dietary patterns assessed by a food frequency questionnaire used in the 5-year follow-up survey of the Japan Public Health Center-Based Prospective Study. J. Epidemiol. 2012, 22, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Schulze, M.B.; Franco, O.H.; van Dam, R.M.; Mantzoros, C.S.; Hu, F.B. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008, 118, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Fung, T.T.; van Dam, R.M.; Rimm, E.B.; Rosner, B.; Hu, F.B. Dietary patterns during adolescence and risk of type 2 diabetes in middle-aged women. Diabetes Care 2012, 35, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Paradis, A.M.; Godin, G.; Perusse, L.; Vohl, M.C. Associations between dietary patterns and obesity phenotypes. Int. J. Obes. (Lond.) 2009, 33, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wilmot, K.A.; Ghasemzadeh, N.; Molloy, D.L.; Burkman, G.; Mekonnen, G.; Gongora, M.C.; Quyyumi, A.A.; Sperling, L.S. Mediterranean Dietary Patterns and Cardiovascular Health. Annu. Rev. Nutr. 2015, 35, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Monforte, M.; Flores-Mateo, G.; Sanchez, E. Dietary patterns and CVD: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2015, 114, 1341–1359. [Google Scholar] [CrossRef] [PubMed]
- Sherzai, A.; Heim, L.T.; Boothby, C.; Sherzai, A.D. Stroke, food groups, and dietary patterns: A systematic review. Nutr. Rev. 2012, 70, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [PubMed]
- Bauer, F.; Elbers, C.C.; Adan, R.A.; Loos, R.J.; Onland-Moret, N.C.; Grobbee, D.E.; van Vliet-Ostaptchouk, J.V.; Wijmenga, C.; van der Schouw, Y.T. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am. J. Clin. Nutr. 2009, 90, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Hellstrand, S.; Gullberg, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in the fat mass and obesity-associated gene (FTO) in association with food preferences in healthy adults. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.L.; Aarts, E.; d’Oleire, U.F.; Dang, L.C.; Greer, S.M.; Jagust, W.J.; D’Esposito, M. Genotype status of the dopamine-related catechol-O-methyltransferase (COMT) gene corresponds with desirability of “unhealthy” foods. Appetite 2015, 92, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. One (small) step towards precision nutrition by use of metabolomics. Lancet Diabetes Endocrinol. 2017, 5, 154–155. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee; Departments of Agriculture and Health and Human Services: Washington, DC, USA, 2015.
- Hebert, J.R.; Hurley, T.G.; Steck, S.E.; Miller, D.R.; Tabung, F.K.; Peterson, K.E.; Kushi, L.H.; Frongillo, E.A. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv. Nutr. 2014, 5, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Paradis, A.M.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Associations between dietary patterns and gene expression profiles of healthy men and women: A cross-sectional study. Nutr. J. 2013, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Thifault, E.; Cormier, H.; Bouchard-Mercier, A.; Rudkowska, I.; Paradis, A.M.; Garneau, V.; Ouellette, C.; Lemieux, S.; Couture, P.; Vohl, M.C. Effects of age, sex, body mass index and APOE genotype on cardiovascular biomarker response to an n-3 polyunsaturated fatty acid supplementation. J. Nutrigenet Nutrigenom. 2013, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.; Nadeau, G.; Lapointe, A.; Lamarche, B.; Lemieux, S. Validity and reproducibility of an interviewer-administered food frequency questionnaire for healthy French-Canadian men and women. Nutr. J. 2004, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Rudkowska, I.; Guenard, F.; Julien, P.; Couture, P.; Lemieux, S.; Barbier, O.; Calder, P.C.; Minihane, A.M.; Vohl, M.C. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid supplementation. J. Lipid Res. 2014, 55, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.; Roche, A.; Martorel, R. The Airlie (VA) Consensus Conference; Human Kinetics Publishers: Champaign, IL, USA, 1988; pp. 39–80. [Google Scholar]
- Padwal, R.S.; Hemmelgarn, B.R.; Khan, N.A.; Grover, S.; McKay, D.W.; Wilson, T.; Penner, B.; Burgess, E.; McAlister, F.A.; Bolli, P.; et al. The 2009 Canadian Hypertension Education Program recommendations for the management of hypertension: Part 1—Blood pressure measurement, diagnosis and assessment of risk. Can. J. Cardiol. 2009, 25, 279–286. [Google Scholar] [CrossRef]
- McNamara, J.R.; Schaefer, E.J. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin. Chim. Acta 1987, 166, 1–8. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Desbuquois, B.; Aurbach, G.D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J. Clin. Endocrinol. Metab. 1971, 33, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Richterich, R.; Dauwalder, H. [Determination of plasma glucose by hexokinase-glucose-6-phosphate dehydrogenase method]. Schweiz. Med. Wochenschr. 1971, 101, 615–618. [Google Scholar] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Rudkowska, I.; Paradis, A.M.; Thifault, E.; Julien, P.; Tchernof, A.; Couture, P.; Lemieux, S.; Barbier, O.; Vohl, M.C. Transcriptomic and metabolomic signatures of an n-3 polyunsaturated fatty acids supplementation in a normolipidemic/normocholesterolemic Caucasian population. J. Nutr. Biochem. 2013, 24, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Blazejczyk, M.; Miron, M.; Nadon, R. FlexArray: A Statistical Data Analysis Software for Gene Expression Microarrays; Canadian Bioinformatics Help Desk (CBHD) Newsletter: Montreal, QC, Canada, 2007. [Google Scholar]
- McLaren, W.; Pritchard, B.; Rios, D.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Chollier, M.; Hufton, A.; Heinig, M.; O’Keeffe, S.; Masri, N.E.; Roider, H.G.; Manke, T.; Vingron, M. Transcription factor binding predictions using TRAP for the analysis of ChIP-seq data and regulatory SNPs. Nat. Protoc. 2011, 6, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Gillanders, E.M.; Holmes, T.N.; Bailey-Wilson, J.E. Establishing an adjusted p-value threshold to control the family-wide type 1 error in genome wide association studies. BMC Genom. 2008, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, K.K.; Liu, W.; Chase, G.A.; Tsai, Y.Y.; Fallin, M.D. Comparison of type I error for multiple test corrections in large single-nucleotide polymorphism studies using principal components versus haplotype blocking algorithms. BMC Genet. 2005, 6 (Suppl. 1), S78. [Google Scholar] [CrossRef] [PubMed]
- Sudmant, P.H.; Rausch, T.; Gardner, E.J.; Handsaker, R.E.; Abyzov, A.; Huddleston, J.; Zhang, Y.; Ye, K.; Jun, G.; Hsi-Yang, F.M.; et al. An integrated map of structural variation in 2,504 human genomes. Nature 2015, 526, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Saleheen, D.; Been, L.F.; Garavito, M.L.; Braun, T.; Bjonnes, A.; Young, R.; Ho, W.K.; Rasheed, A.; Frossard, P.; et al. Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in Sikhs of Punjabi origin from India. Diabetes 2013, 62, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Rask-Andersen, M.; Almen, M.S.; Lind, L.; Schioth, H.B. Association of the LINGO2-related SNP rs10968576 with body mass in a cohort of elderly Swedes. Mol. Genet. Genom. 2015, 290, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Comuzzie, A.G.; Cole, S.A.; Laston, S.L.; Voruganti, V.S.; Haack, K.; Gibbs, R.A.; Butte, N.F. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS ONE 2012, 7, e51954. [Google Scholar] [CrossRef] [PubMed]
- Athanasiu, L.; Mattingsdal, M.; Kahler, A.K.; Brown, A.; Gustafsson, O.; Agartz, I.; Giegling, I.; Muglia, P.; Cichon, S.; Rietschel, M.; et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J. Psychiatr. Res. 2010, 44, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Fanous, A.H.; Zhou, B.; Aggen, S.H.; Bergen, S.E.; Amdur, R.L.; Duan, J.; Sanders, A.R.; Shi, J.; Mowry, B.J.; Olincy, A.; et al. Genome-wide association study of clinical dimensions of schizophrenia: Polygenic effect on disorganized symptoms. Am. J. Psychiatry 2012, 169, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Liu, X.F.; Aragam, N. A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophr. Res. 2010, 124, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Wang, J.C.; Wetherill, L.; Le, N.; Bertelsen, S.; Hinrichs, A.L.; Budde, J.; Agrawal, A.; Almasy, L.; Bucholz, K.; et al. Genome-wide survival analysis of age at onset of alcohol dependence in extended high-risk COGA families. Drug Alcohol. Depend. 2014, 142, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, F.; Zhang, H.; Zhang, X.Y.; Wang, F.; Li, C.S.; Lu, L.; Hong, J.; Lu, L.; Krystal, J.; et al. Genome-wide search for replicable risk gene regions in alcohol and nicotine co-dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Schutte, B.C.; Mitros, J.P.; Bartlett, J.A.; Walters, J.D.; Jia, H.P.; Welsh, M.J.; Casavant, T.L.; McCray, P.B., Jr. Discovery of five conserved beta -defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA 2002, 99, 2129–2133. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Hoffmann, K.; Spranger, J.; Klipstein-Grobusch, K.; Mohlig, M.; Pfeiffer, A.F.; Boeing, H. A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam Study cohort. Diabetologia 2005, 48, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rimm, E.B.; Spiegelman, D.; Rifai, N.; Tofler, G.H.; Willett, W.C.; Hu, F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [PubMed]
- Gruner, M.; Grubbs, J.; McDonagh, A.; Valdes, D.; Winbush, A.; van der Linden, A.M. Cell-Autonomous and Non-Cell-Autonomous Regulation of a Feeding State-Dependent Chemoreceptor Gene via MEF-2 and bHLH Transcription Factors. PLoS Genet. 2016, 12, e1006237. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.; Eschrich, K. Splice isoforms of ubiquitous 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in human brain. Brain Res. Mol. Brain Res. 2001, 87, 190–195. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; Xu, H.; Woo, S.L.; Halim, V.; Morgan, C.; Wu, C. A role for inducible 6-phosphofructo-2-kinase in the control of neuronal glycolysis. J. Nutr. Biochem. 2013, 24, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, W.R.; Gu, X.; Liu, Y.; Bottino, R.; Crabtree, G.R.; Kim, S.K. Neonatal beta cell development in mice and humans is regulated by calcineurin/NFAT. Dev. Cell 2012, 23, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kipanyula, M.J.; Kimaro, W.H.; Seke Etet, P.F. The Emerging Roles of the Calcineurin-Nuclear Factor of Activated T-Lymphocytes Pathway in Nervous System Functions and Diseases. J. Aging Res. 2016, 2016, 5081021. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.C.; Borenstein-Auerbach, N.; McGlynn, K.; Kunnathodi, F.; Shahbazov, R.; Syed, I.; Kanak, M.; Takita, M.; Levy, M.F.; Naziruddin, B. NFAT targets signaling molecules to gene promoters in pancreatic beta-cells. Mol. Endocrinol. 2015, 29, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.B.; Lavine, J.A.; Suhonen, J.I.; Krautkramer, K.A.; Rabaglia, M.E.; Sperger, J.M.; Fernandez, L.A.; Yandell, B.S.; Keller, M.P.; Wang, I.M.; et al. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Mol. Endocrinol. 2010, 24, 1822–1834. [Google Scholar] [CrossRef] [PubMed]
- Evanson, N.K.; Tasker, J.G.; Hill, M.N.; Hillard, C.J.; Herman, J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010, 151, 4811–4819. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.J.; Couto, L.; Cohen, V.; Lalazar, Y.; Makotkine, I.; Williams, N.; Yehuda, R.; Goldstein, R.Z.; Geer, E.B. Glucocorticoid Regulation of Food-Choice Behavior in Humans: Evidence from Cushing’s Syndrome. Front. Neurosci. 2016, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Ladyman, S.R.; Grattan, D.R. JAK-STAT and feeding. JAKSTAT 2013, 2, e23675. [Google Scholar] [CrossRef] [PubMed]
- Furigo, I.C.; Ramos-Lobo, A.M.; Frazao, R.; Donato, J., Jr. Brain STAT5 signaling and behavioral control. Mol. Cell. Endocrinol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Dougkas, A.; Yaqoob, P.; Givens, D.I.; Reynolds, C.K.; Minihane, A.M. The impact of obesity-related SNP on appetite and energy intake. Br. J. Nutr. 2013, 110, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.; Charbonnier, L.; van, M.F.; van der Laan, L.N.; Spetter, M.S. Food-induced brain responses and eating behaviour. Proc. Nutr. Soc. 2012, 71, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.J.; Glimcher, P.W. Comparing apples and oranges: Using reward-specific and reward-general subjective value representation in the brain. J. Neurosci. 2011, 31, 14693–14707. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, B.L.; Lissner, L.; Osler, M. Do we eat less fat, or just report so? Int. J. Obes. Relat. Metab. Disord. 2000, 24, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, H.L.; Ozanne, S.E. Programming of cardiovascular disease across the life-course. J. Mol. Cell. Cardiol. 2015, 83, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Guenard, F.; Deshaies, Y.; Cianflone, K.; Kral, J.G.; Marceau, P.; Vohl, M.C. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc. Natl. Acad. Sci. USA 2013, 110, 11439–11444. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P. Physical Activity, Sedentary Behaviours, and Cardiovascular Health: When Will Cardiorespiratory Fitness Become a Vital Sign? Can. J. Cardiol. 2016, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All | Men | Women |
|---|---|---|---|
| Number | 141 | 68 | 73 |
| Age (years) | 31.6 ± 8.8 | 31.1 ± 8.0 | 32.0 ± 9.6 |
| BMI (kg/m2) | 28.4 ± 3.8 | 28.1 ± 3.7 | 28.7 ± 3.8 |
| Waist girth (cm) | 94.5 ± 11.0 | 96.3 ± 11.2 | 92.9 ± 10.7 |
| Lipid profile | |||
| Total-C (mmol/L) | 4.90 ± 0.97 | 4.88 ± 1.03 | 4.92 ± 0.92 |
| LDL-C (mmol/L) | 2.88 ± 0.88 | 3.01 ± 0.95 | 2.75 ± 0.80 |
| HDL-C (mmol/L) | 1.42 ± 0.38 b | 1.25 ± 0.29 | 1.58 ± 0.39 |
| TG (mmol/L) | 1.32 ± 0.68 | 1.37 ± 0.72 | 1.27 ± 0.65 |
| Total-C/HDL-C | 3.66 ± 1.10 b | 4.10 ± 1.16 | 3.25 ± 0.88 |
| Blood pressure (mm Hg) | |||
| SBP | 113.0 ± 12.3 b | 118.5 ± 12.9 | 107.8 ± 9.1 |
| DBP | 68.5 ± 8.4 | 68.7 ± 8.6 | 68.3 ± 8.3 |
| Fasting glucose (mmol/L) | 5.00 ± 0.46 | 5.06 ± 0.47 | 4.94 ± 0.44 |
| Insulin (pmol/L) | 93.2 ± 87.5 | 100.6 ± 119.2 | 86.4 ± 39.6 |
| Self-reported diet scores | |||
| Prudent | −0.022 ± 1.012 a | −0.207 ± 1.041 | 0.150 ± 0.960 |
| High/low scores (>0) | 70/71 | 29/39 | 41/32 |
| Western | −0.009 ± 0.980 a | 0.202 ± 1.087 | −0.207 ± 0.829 |
| High/low score (>0) | 70/712 | 44/24 | 26/47 |
| SNP ID a | rs Number | Gene | Associated Pattern | Total-C | LDL-C | HDL-C | Total-C/HDL-C | SBP | Fasting Glucose | Insulin |
|---|---|---|---|---|---|---|---|---|---|---|
| kgp4289407 | rs114123656 | LINC01246 b | Prudent | --- | --- | --- | --- | --- | --- | 0.02 |
| kgp6444538 | rs115510004 | LOC645949 b | Prudent | --- | --- | 0.008 | --- | --- | --- | --- |
| rs10097298 | rs10097298 | LOC100130298 b | Prudent | --- | --- | --- | --- | --- | 0.03 | --- |
| kgp2826446 | rs76838052 | C10orf142 b | Prudent | --- | 0.02 | --- | --- | --- | --- | --- |
| kgp9480999 | rs74842138 | GDF10 b | Prudent | 0.04 | --- | --- | --- | 0.03 | --- | --- |
| rs7144547 | rs7144547 | STON2 | Prudent | --- | --- | 0.004 | 0.03 | --- | --- | --- |
| rs163269 | rs163269 | ACSM1 | Prudent | --- | --- | --- | --- | --- | --- | 0.02 |
| rs6499924 | rs6499924 | CNGB1 | Prudent | --- | --- | --- | --- | 0.04 | --- | 0.0005 |
| kgp5504930 | rs13042507 | CTCFL b | Prudent | --- | --- | --- | --- | --- | 0.02 | --- |
| kgp6972810 | rs73180793 | PCK1 b | Prudent | --- | --- | 0.02 | --- | --- | 0.02 | --- |
| kgp12008054 | rs6070157 | PCK1 | Prudent | --- | --- | 0.02 | --- | --- | 0.02 | --- |
| kgp10614850 | rs11552145 | PCK1 | Prudent | --- | --- | 0.04 | --- | --- | 0.01 | --- |
| kgp9374426 | rs116812750 | RGS7 | Western | 0.02 | 0.009 | --- | --- | 0.01 | --- | --- |
| kgp8978882 | rs112040989 | LOC101929468 b | Western | 0.03 | --- | --- | --- | 0.02 | --- | --- |
| kgp9399667 | rs112764838 | TET2 b | Western | 0.03 | --- | --- | --- | 0.02 | --- | --- |
| kgp9469075 | rs72736220 | LOC100996286 b | Western | --- | --- | --- | --- | 0.03 | --- | --- |
| kgp29240591 | rs148696004 | TLL1 b | Western | --- | --- | --- | --- | --- | 0.01 | --- |
| kgp9282379 | rs200247 | TFAP2D b | Western | --- | --- | --- | --- | 0.04 | --- | --- |
| kgp9033598 | rs79041188 | ESR1 | Western | --- | --- | --- | --- | 0.05 | --- | --- |
| kgp26148321 | rs141382233 | ARID1B b | Western | 0.02 | 0.01 | --- | --- | --- | --- | --- |
| kgp4441528 | rs2535974 | ACTR3B b | Western | --- | --- | --- | --- | 0.008 | --- | --- |
| kgp1054774 | rs113152482 | PFKFB3 | Western | 0.03 | --- | --- | --- | --- | --- | --- |
| rs1348307 | rs1348307 | LINC00706 b | Western | --- | --- | --- | --- | --- | 0.006 | 0.0008 |
| rs7911681 | rs7911681 | NRG3 | Western | --- | --- | --- | --- | --- | 0.03 | --- |
| kgp6498073 | rs112633616 | LOC101928441 b | Western | --- | 0.03 | --- | --- | --- | --- | --- |
| kgp27660318 | rs140957346 | EEA1 | Western | --- | --- | --- | --- | --- | 0.03 | --- |
| kgp25610618 | rs140552175 | LOC101928880 | Western | --- | 0.05 | --- | --- | 0.02 | 0.002 | --- |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guénard, F.; Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.-C. Genome-Wide Association Study of Dietary Pattern Scores. Nutrients 2017, 9, 649. https://doi.org/10.3390/nu9070649
Guénard F, Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl M-C. Genome-Wide Association Study of Dietary Pattern Scores. Nutrients. 2017; 9(7):649. https://doi.org/10.3390/nu9070649
Chicago/Turabian StyleGuénard, Frédéric, Annie Bouchard-Mercier, Iwona Rudkowska, Simone Lemieux, Patrick Couture, and Marie-Claude Vohl. 2017. "Genome-Wide Association Study of Dietary Pattern Scores" Nutrients 9, no. 7: 649. https://doi.org/10.3390/nu9070649





