Clinical Identification of Geriatric Patients with Hypovitaminosis D: The ‘Vitamin D Status Predictor for Geriatrics’ Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Vitamin D Status Predictor
2.3. Serum 25(OH)D Measure
2.4. Statistical Analysis
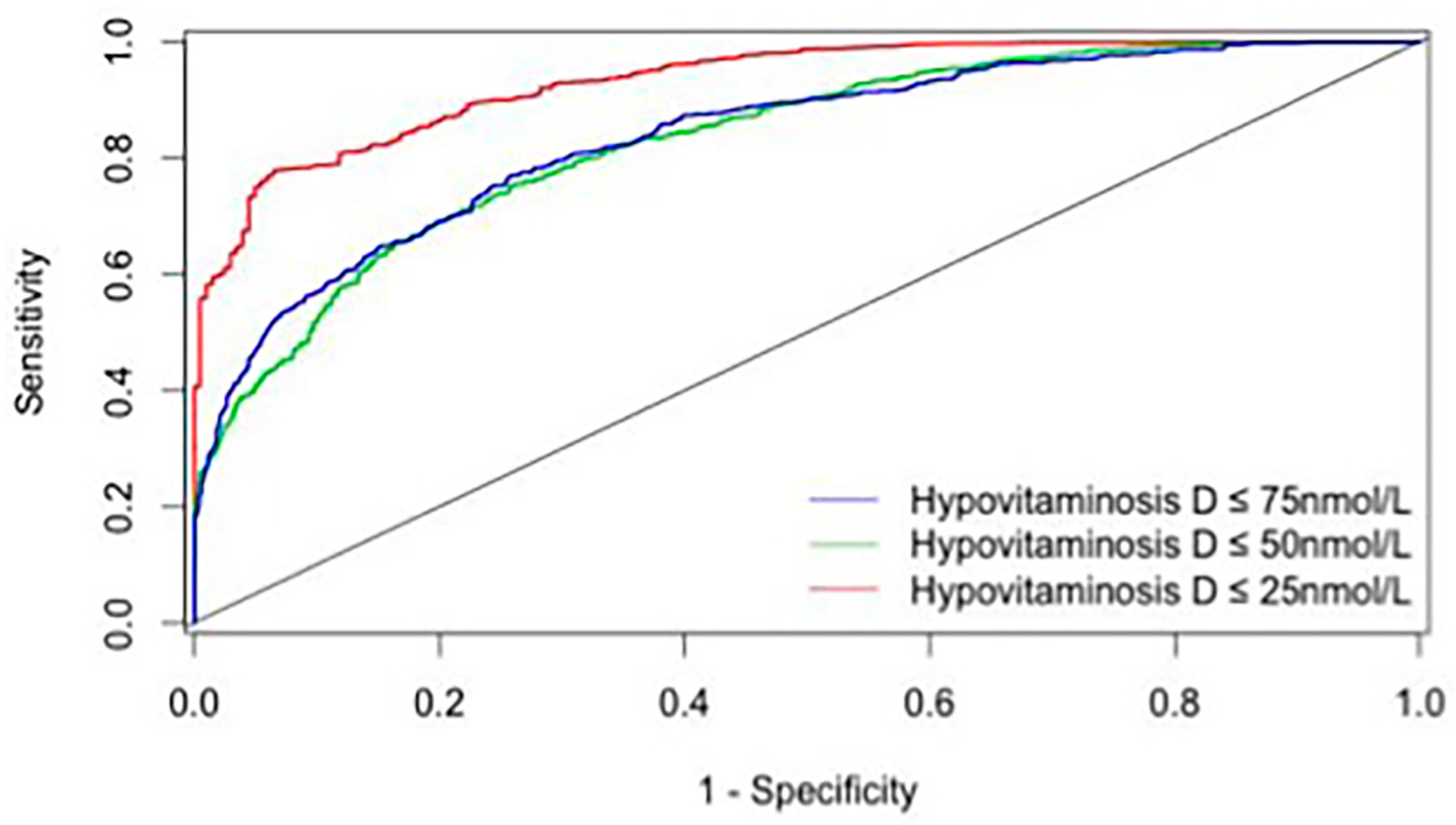
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grübler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Karras, S.N.; Bischoff-Ferrari, H.A.; Annweiler, C.; Boucher, B.J.; Juzeniene, A.; Garland, C.F.; Holick, M.F. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects? Dermatoendocrinology 2016, 8, e1187349. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Duval, G.; Launay, C.P. Estimating vitamin D status and the choice of supplementation dose. JAMA Intern. Med. 2016, 176, 865. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Weber, T.; Colón-Emeric, C. Comparison of cost-effectiveness of vitamin D screening with that of universal supplementation in preventing falls in community-dwelling older adults. J. Am. Geriatr. Soc. 2013, 61, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, R.Y.; Brouwers, J.R.; Geusens, P.P.; Lems, W.F.; van den Bergh, J.P. Calcium and vitamin D supplementation: State of the art for daily practice. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef] [PubMed]
- French National Authority for Health. Clinical Utility of the Measurement of Vitamin D. Saint-Denis: Haute Autorité de Santé (HAS). 2013. Available online: http://www.has-sante.fr/portail/jcms/c_1356838/fr/utilite-clinique-du-dosage-de-la-vitamine-d-rapport-d-evaluation (accessed on 3 May 2017).
- Annweiler, C.; Kabeshova, A.; Legeay, M.; Fantino, B.; Beauchet, O. Derivation and validation of a clinical diagnostic tool for the identification of older community-dwellers with hypovitaminosis D. J. Am. Med. Dir. Assoc. 2015, 16, e8–e19. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Pochic, S.; Fantino, B.; Legrand, E.; Bataille, R.; Montero-Odasso, M.; Beauchet, O. Serum vitamin D concentration and short-term mortality among geriatric inpatients in acute care settings. Adv. Ther. 2010, 27, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé. Stratégie de Prise en Charge en cas de Dénutrition Protéinoénergétique chez la Personne âgée. 2007. Available online: https://www.has-sante.fr/portail/jcms/c_546549/fr/strategie-de-prise-en-charge-en-cas-de-denutrition-proteino-energetique-chez-la-personne-agee (accessed on 3 May 2017).
- Beauchet, O.; Dubost, V.; Revel Delhom, C.; Berrut, G.; Belmin, J.; French Society of Geriatrics and Gerontology. How to manage recurrent falls in clinical practice: Guidelines of the French Society of Geriatrics and Gerontology. J. Nutr. Health Aging 2011, 15, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi, F.; Launay, C.P.; Annweiler, C.; Beauchet, O. Fear of falling and gait variability in older adults: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2015, 16, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Herbert, R.; Lewis, S.; Mahendran, R.; Platt, J.; Bhattacharyya, B. Screening for depression among acutely ill geriatric inpatients with a short Geriatric Depression Scale. Age Ageing 1997, 26, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2010, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, A. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comput. Biol. Med. 2000, 30, 127–134. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Cheriet, S.; Cavalier, E. Distinctive aspects of laboratory testing to evaluate mineral and bone metabolism in patients with chronic kidney disease. Jt. Bone Spine 2012, 79, S99–S103. [Google Scholar] [CrossRef]
- Chevallereau, G.; Gleyses, X.; Roussel, L.; Hamdan, S.; Beauchet, O.; Annweiler, C. Proposal and validation of a quick question to rate the influence of diet in geriatric epidemiological studies on vitamin D. Int. J. Vitam. Nutr. Res. 2013, 83, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, S.; Roussel, L.; Gleyses, X.; Chevallereau, G.; Schott, A.M.; Beauchet, O.; Annweiler, C. Detection of hypovitaminosis D in older adults: A classification tree analysis. J. Am. Geriatr. Soc. 2014, 62, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Hélard, L.; Montero-Odasso, M.; de Decker, L.; Berrut, G.; Annweiler, C. Hypovitaminosis D in geriatric inpatients: A marker of severity of chronic diseases. Aging Clin. Exp. Res. 2012, 24, 188–192. [Google Scholar] [PubMed]
- Beauchet, O.; Launay, C.P.; Maunoury, F.; de Decker, L.; Fantino, B.; Annweiler, C. Association between vitamin D deficiency and long hospital stay in geriatric acute care unit: Results from a pilot cohort study. Aging Clin. Exp. Res. 2013, 25, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.D. Accuracy of 25-hydroxyvitamin D assays: Confronting the issues. Curr. Drug Targets 2001, 12, 19–28. [Google Scholar] [CrossRef]
| Clinical Characteristics | Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole Sample (n = 199) | Serum 25(OH)D Concentration, nmol/L | |||||||||
| Severe Vitamin D Deficiency ≤ 25 nmol/L (n = 67) | >25 nmol/L (n = 132) | p-Value * | Vitamin D Deficiency ≤ 50 nmol/L (n = 136) | >50 nmol/L (n = 63) | p-Value * | Vitamin D Insufficiency ≤ 75 nmol/L (n = 184) | >75 nmol/L (n = 15) | p-Value * | ||
| Item 1- Female gender | 106 (53.3) | 34 (51) | 72 (55) | 0.61 | 66 (49) | 40 (64) | 0.05 | 94 (51.1) | 12 (80) | 0.03 † |
| Item 2- Age, years (mean ± SD) | 82.0 ± 7.8 | 83.9 ± 7.2 | 81.1 ± 7.9 | 0.01 † | 82.3 ± 8.0 | 81.4 ± 7.4 | 0.41 | 82.1 ± 7.9 | 80.7 ± 7.0 | 0.73 |
| Item 3- Number of drugs daily taken (mean ± SD) | 5.3 ± 3.6 | 6.2 ± 3.8 | 4.8 ± 3.4 | 0.006 † | 5.5 ± 3.7 | 4.8 ± 3.4 | 0.23 | 5.2 ± 3.7 | 5.3 ± 2.4 | 0.62 |
| Item 4- Body mass index, kg/m2 (mean ± SD) | 25.9 ± 4.5 | 25.3 ± 4.3 | 26.2 ± 4.7 | 0.18 | 26.0 ± 4.6 | 25.7 ± 4.4 | 0.65 | 25.8 ± 4.3 | 26.7 ± 7.3 | 0.41 |
| Item 5- Use walking aids | 89 (44.7) | 34 (51) | 55 (42) | 0.22 | 62 (46) | 27 (43) | 0.72 | 82 (44.6) | 7 (47) | 0.88 |
| Item 6- Use psychoactive drugs | 80 (40.2) | 27 (40) | 53 (40) | 0.98 | 50 (37) | 30 (48) | 0.15 | 70 (38.0) | 10 (67) | 0.03 † |
| Item 7- Wearing glasses | 108 (54.3) | 32 (48) | 76 (58) | 0.19 | 67 (49) | 41 (65) | 0.05 | 99 (53.8) | 9 (60) | 0.64 |
| Item 8- Sad mood | 62 (31.2) | 22 (33) | 40 (30) | 0.72 | 39 (29) | 23 (37) | 0.27 | 55 (29.9) | 7 (47) | 0.18 |
| Item 9- Fear of falling | 88 (44.2) | 32 (48) | 56 (42) | 0.47 | 61 (45) | 27 (43) | 0.79 | 80 (43.5) | 8 (53) | 0.46 |
| Item 10- History of falls | 93 (46.7) | 36 (54) | 57 (43) | 0.16 | 61 (45) | 32 (51) | 0.44 | 87 (47.3) | 6 (40) | 0.59 |
| Item 11- Cognitive disorders | 125 (62.8) | 39 (58) | 86 (65) | 0.34 | 78 (57) | 47 (75) | 0.02 † | 112 (60.9) | 13 (87) | 0.05 |
| Item 12- Undernutrition | 22 (11.5) | 10 (15) | 12 (10) | 0.25 | 17 (13) | 5 (8) | 0.31 | 19 (10.7) | 3 (20) | 0.28 |
| Item 13- Polymorbidity | 108 (54.3) | 40 (60) | 68 (52) | 0.27 | 74 (54) | 34 (54) | 0.95 | 100 (54.3) | 8 (53) | 0.94 |
| Item 14- History of vertebral fractures | 8 (4.0) | 3 (5) | 5 (4) | 0.82 | 5 (4) | 3 (5) | 0.72 | 8 (4.3) | 0 (0) | 0.41 |
| Item 15- Living alone | 82 (41.4) | 28 (42) | 54 (41) | 0.94 | 55 (41) | 27 (43) | 0.78 | 76 (41.5) | 6 (40) | 0.91 |
| Item 16- Use anti-osteoporotic drugs | 12 (6.0) | 1 (2) | 11 (8) | 0.06 | 4 (3) | 8 (13) | 0.007 † | 8 (4.3) | 4 (27) | <0.001 † |
| 25-hydroxyvitamin D, nmol/L (mean ± SD) | 40 ± 23 | 16 ± 5 | 52 ± 18 | <0.001 † | 27 ± 13 | 68 ± 11 | <0.001 † | 36 ± 20 | 84 ± 7 | <0.001 † |
| Hypovitaminosis D | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Severe Vitamin D Deficiency 25(OH)D ≤ 25 nmol/L | Vitamin D Deficiency 25(OH)D ≤ 50 nmol/L | Vitamin D Insufficiency 25(OH)D ≤ 75 nmol/L | |||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Item 1- Female gender | 0.86 | 0.48–1.55 | 0.61 | 0.54 | 0.29–0.99 | 0.05 | 0.26 | 0.06–0.85 | 0.04 * |
| Item 2- Age, years | 1.05 | 1.01–1.09 | 0.02 * | 1.02 | 0.98–1.06 | 0.41 | 1.02 | 0.96–1.10 | 0.51 |
| Item 3- Number of drugs daily taken | 1.12 | 1.03–1.22 | 0.007 * | 1.05 | 0.97–1.15 | 0.23 | 0.99 | 0.86–1.16 | 0.93 |
| Item 4- Body mass index, kg/m2 | 0.95 | 0.89–1.02 | 0.15 | 1.02 | 0.96–1.10 | 0.55 | 0.96 | 0.87–1.08 | 0.51 |
| Item 5- Use walking aids | 1.50 | 0.86–2.61 | 0.16 | 1.20 | 0.68–2.14 | 0.54 | 0.98 | 0.37–2.79 | 0.97 |
| Item 6- Use psychoactive drugs | 1.01 | 0.55–1.83 | 0.98 | 0.64 | 0.35–1.17 | 0.15 | 0.31 | 0.09–0.90 | 0.04 * |
| Item 7- Wearing glasses | 0.67 | 0.37–1.22 | 0.19 | 0.52 | 0.28–0.96 | 0.04 * | 0.78 | 0.25–2.24 | 0.64 |
| Item 8- Sad mood | 1.12 | 0.59–2.10 | 0.72 | 0.70 | 0.37–1.33 | 0.27 | 0.49 | 0.17–1.45 | 0.19 |
| Item 9- Fear of falling | 1.24 | 0.69–2.24 | 0.47 | 1.08 | 0.60–1.99 | 0.79 | 0.67 | 0.23–1.95 | 0.46 |
| Item 10- History of falls | 1.53 | 0.85–2.77 | 0.16 | 0.79 | 0.43–1.43 | 0.44 | 1.35 | 0.47–4.16 | 0.59 |
| Item 11- Cognitive disorders | 0.75 | 0.41–1.37 | 0.34 | 0.46 | 0.23–0.87 | 0.02 * | 0.24 | 0.04–0.90 | 0.07 |
| Item 12- Undernutrition | 1.75 | 0.70–4.31 | 0.22 | 1.66 | 0.62–5.24 | 0.34 | 0.46 | 0.13–2.15 | 0.26 |
| Item 13- Polymorbidity | 1.39 | 0.77–2.55 | 0.27 | 1.02 | 0.56–1.85 | 0.95 | 1.04 | 0.35–3.02 | 0.94 |
| Item 14- History of vertebral fractures | 1.19 | 0.24–5.01 | 0.82 | 0.76 | 0.18–3.82 | 0.72 | - | - | 0.99 |
| Item 15- Living alone | 1.04 | 0.57–1.88 | 0.91 | 0.91 | 0.50–1.67 | 0.75 | 1.06 | 0.37–3.26 | 0.92 |
| Item 16- Use anti-osteoporotic drugs | 0.17 | 0.01–0.89 | 0.09 | 0.21 | 0.05–0.69 | 0.01 * | 0.13 | 0.03–0.53 | 0.002 * |
| Hypovitaminosis D | True Positive | False Positive | True Negative | False Negative | Sensitivity, % | Specificity, % | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|---|---|
| Vitamin D insufficiency ≤ 75 nmol/L | 8 | 31 | 153 | 7 | 53.3 | 83.2 | 20.5 | 95.6 |
| Vitamin D deficiency ≤ 50 nmol/L | 105 | 31 | 35 | 28 | 79.0 | 53.3 | 77.2 | 55.6 |
| Severe vitamin D deficiency ≤ 25 nmol/L | 58 | 53 | 79 | 12 | 86.2 | 60.0 | 93.8 | 38.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annweiler, C.; Riou, J.; Alessandri, A.; Gicquel, D.; Henni, S.; Féart, C.; Kabeshova, A. Clinical Identification of Geriatric Patients with Hypovitaminosis D: The ‘Vitamin D Status Predictor for Geriatrics’ Study. Nutrients 2017, 9, 658. https://doi.org/10.3390/nu9070658
Annweiler C, Riou J, Alessandri A, Gicquel D, Henni S, Féart C, Kabeshova A. Clinical Identification of Geriatric Patients with Hypovitaminosis D: The ‘Vitamin D Status Predictor for Geriatrics’ Study. Nutrients. 2017; 9(7):658. https://doi.org/10.3390/nu9070658
Chicago/Turabian StyleAnnweiler, Cédric, Jérémie Riou, Axel Alessandri, David Gicquel, Samir Henni, Catherine Féart, and Anastasiia Kabeshova. 2017. "Clinical Identification of Geriatric Patients with Hypovitaminosis D: The ‘Vitamin D Status Predictor for Geriatrics’ Study" Nutrients 9, no. 7: 658. https://doi.org/10.3390/nu9070658





