Comparable Performance Characteristics of Plasma Thiamine and Erythrocyte Thiamine Diphosphate in Response to Thiamine Fortification in Rural Cambodian Women
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Date and Blood Collection
2.3. Biochemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Performance of the LC-MS/MS Assay for Plasma Thiamine and TMP
3.2. Population Characteristics and Concentration of Thiamine Forms at Baseline
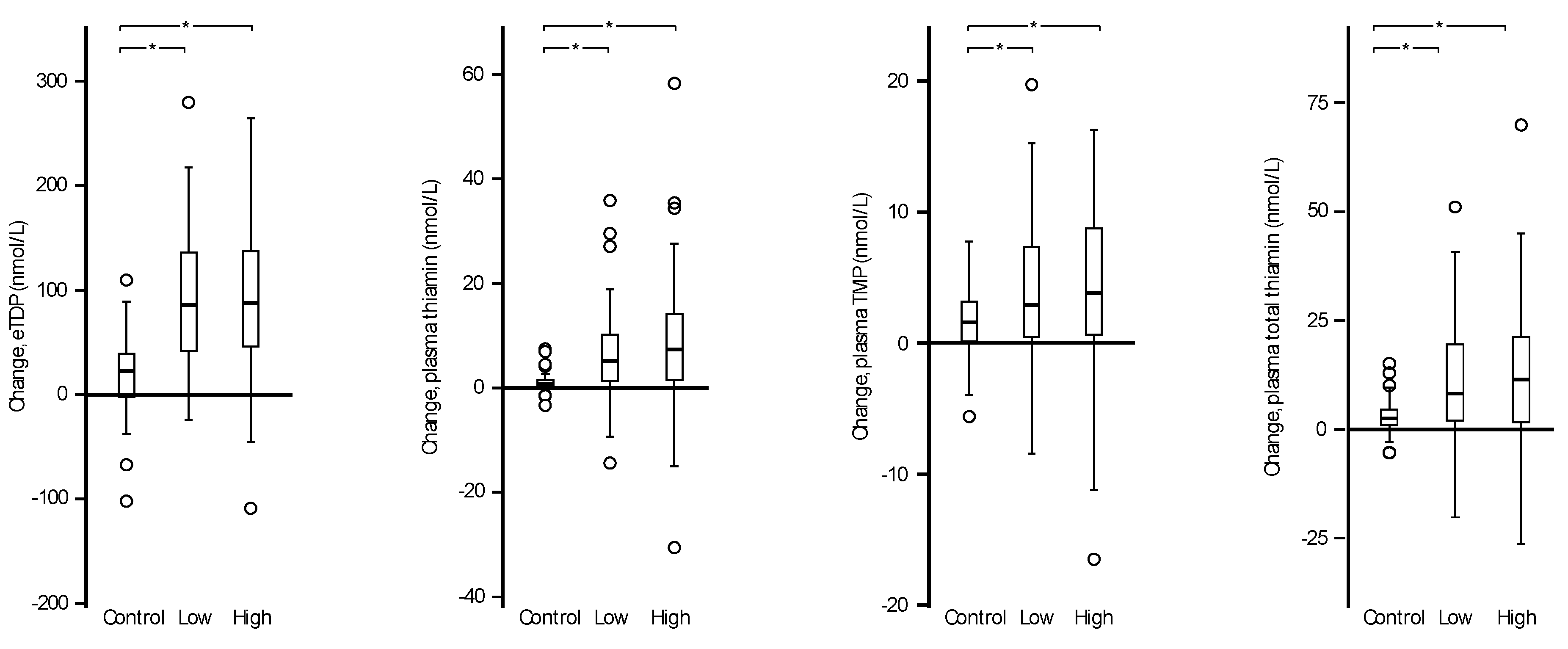
3.3. Responses to Intervention
4. Discussion
4.1. Strengths and Limitations
4.2. Erythrocyte and Plasma Thiamine Forms as Markers of Thiamine Status
4.3. Thiamine Forms and Compartments
5. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
Abbreviations
| ETK | erythrocyte transketolase |
| eThDP | erythrocyte thiamine diphosphate |
| TMP | thiamine monophosphate |
| HPLC-FLD | high performance liquid chromatography with fluorescence detection |
| LC-MS/MS | high performance liquid chromatography-tandem mass spectrometry |
| TTP | thiamine triphosphate |
| AThTP | adenosine thiamine triphosphate |
| AC | activity coefficient |
| ESI | electrospray ionization |
| ANOVA | analysis of variance |
| GLM | general linear model |
| THTR-1 | thiamine transporter-1 |
| RFC-1 | reduced folate carrier-1 |
| TPK1 | thiamine pyrophosphokinase-1 |
| TPPase | thiamine pyrophosphatase |
References
- Bettendorff, L.; Lakaye, B.; Kohn, G.; Wins, P. Thiamine triphosphate: A ubiquitous molecule in search of a physiological role. Metab. Brain Dis. 2014, 29, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Loop diuretic therapy, thiamine balance, and heart failure. Congest. Heart Fail. 2007, 13, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Wooley, J.A. Characteristics of thiamin and its relevance to the management of heart failure. Nutr. Clin. Pract. 2008, 23, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Makarchikov, A.F.; Lakaye, B.; Gulyai, I.E.; Czerniecki, J.; Coumans, B.; Wins, P.; Grisar, T.; Bettendorff, L. Thiamine triphosphate and thiamine triphosphatase activities: From bacteria to mammals. Cell. Mol. Life Sci. 2003, 60, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D. Thiamin(e): The spark of life. Subcell. Biochem. 2012, 56, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, T.; Holmes, C.P.; Ostry, A. An analysis of the development of Canadian food fortification policies: The case of vitamin B. Health Promot. Int. 2005, 20, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Wiemer, K.L.; Dary, O.; Keen, C.L.; King, J.C.; Miller, K.B.; Philbert, M.A.; Tarasuk, V.; Taylor, C.L.; Gaine, P.C.; et al. Fortification and health: Challenges and opportunities. Adv. Nutr. 2015, 6, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Allen, L; De Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; Nations WHOaFaAOotU, Ed.; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2006. [Google Scholar]
- Luxemburger, C.; White, N.J.; ter Kuile, F.; Singh, H.M.; Allier-Frachon, I.; Ohn, M.; Chongsuphajaisiddhi, T.; Nosten, F. Beri-beri: The major cause of infant mortality in Karen refugees. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 251–255. [Google Scholar] [CrossRef]
- Coats, D.; Shelton-Dodge, K.; Ou, K.; Khun, V.; Seab, S.; Sok, K.; Prou, C.; Tortorelli, S.; Moyer, T.P.; Cooper, L.E.; et al. Thiamine deficiency in Cambodian infants with and without beriberi. J. Pediatr. 2012, 161, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Doung-ngern, P.; Kesornsukhon, S.; Kanlayanaphotporn, J.; Wanadurongwan, S.; Songchitsomboon, S. Beriberi outbreak among commercial fishermen, Thailand 2005. Southeast Asian J. Trop. Med. Public Health 2007, 38, 130–135. [Google Scholar] [PubMed]
- Khounnorath, S.; Chamberlain, K.; Taylor, A.M.; Soukaloun, D.; Mayxay, M.; Lee, S.J.; Phengdy, B.; Luangxay, K.; Sisouk, K.; Soumphonphakdy, B.; et al. Clinically unapparent infantile thiamin deficiency in Vientiane, Laos. PLoS Negl. Trop. Dis. 2011, 5, e969. [Google Scholar] [CrossRef] [PubMed]
- Latt, N.; Dore, G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern. Med. J. 2014, 44, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.P.; Jamieson, C.P.; Madira, W.; Gatward, N.M.; Palmer, R.L. Prevalence of thiamin deficiency in anorexia nervosa. Int. J. Eat. Disord. 2000, 28, 451–454. [Google Scholar] [CrossRef]
- Bleggi-Torres, L.F.; de Medeiros, B.C.; Werner, B.; Neto, J.Z.; Loddo, G.; Pasquini, R.; de Medeiros, C.R. Neuropathological findings after bone marrow transplantation: An autopsy study of 180 cases. Bone Marrow Transpl. 2000, 25, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F.; Gaudreau, C.; Vincelette, J.; Bourgault, A.M.; Lamothe, F.; Nutini, A.M. Thiamine deficiency and Wernicke’s encephalopathy in AIDS. Metab. Brain Dis. 1991, 6, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Togay-Isikay, C.; Yigit, A.; Mutluer, N. Wernicke’s encephalopathy due to hyperemesis gravidarum: An under-recognised condition. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Davidson, H.; Cooney, J.; St JO’Reilly, D. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: Comparison with erythrocyte transketolase activation assay. Clin. Chem. 2000, 46, 704–710. [Google Scholar] [PubMed]
- Schrijver, J.; Speek, A.J.; Klosse, J.A.; van Rijn, H.J.; Schreurs, W.H. A reliable semiautomated method for the determination of total thiamine in whole blood by the thiochrome method with high-performance liquid chromatography. Ann. Clin. Biochem. 1982, 19 Pt 1, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lynch, P.L.; Young, I.S. Determination of thiamine by high-performance liquid chromatography. J. Chromatogr. A 2000, 881, 267–284. [Google Scholar] [CrossRef]
- Tallaksen, C.M.; Bohmer, T.; Bell, H.; Karlsen, J. Concomitant determination of thiamin and its phosphate esters in human blood and serum by high-performance liquid chromatography. J. Chromatogr. 1991, 564, 127–136. [Google Scholar] [CrossRef]
- Herve, C.; Beyne, P.; Letteron, P.; Delacoux, E. Comparison of erythrocyte transketolase activity with thiamine and thiamine phosphate ester levels in chronic alcoholic patients. Clin. Chim. Acta 1995, 234, 91–100. [Google Scholar] [CrossRef]
- Tallaksen, C.M.; Bohmer, T.; Karlsen, J.; Bell, H. Determination of thiamin and its phosphate esters in human blood, plasma, and urine. Methods Enzymol. 1997, 279, 67–74. [Google Scholar] [PubMed]
- Lu, J.; Frank, E.L. Rapid HPLC measurement of thiamine and its phosphate esters in whole blood. Clin. Chem. 2008, 54, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Puts, J.; de Groot, M.; Haex, M.; Jakobs, B. Simultaneous Determination of Underivatized Vitamin B1 and B6 in Whole Blood by Reversed Phase Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. PLoS ONE 2015, 10, e0132018. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Chen, L.T.; Gold, M.; Boyce, H.W., Jr. Plasma and erythrocyte thiamin concentrations in geriatric outpatients. J. Am. Coll. Nutr. 1996, 15, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Warnock, L.G.; Prudhomme, C.R.; Wagner, C. The determination of thiamin pyrophosphate in blood and other tissues, and its correlation with erythrocyte transketolase activity. J. Nutr. 1978, 108, 421–427. [Google Scholar] [PubMed]
- Tallaksen, C.M.; Bohmer, T.; Bell, H. Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol. Clin. Exp. Res. 1992, 16, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.L. Thiamin in Clinical Practice. JPEN J. Parenter. Enter. Nutr. 2015, 39, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, K.C.; Karakochuk, C.D.; Kroeun, H.; Sokhoing, L.; Chan, B.B.; Borath, M.; Sophonneary, P.; Moore, K.; Tong, J.K.; McLean, J.; et al. Household Consumption of Thiamin-Fortified Fish Sauce Increases Erythrocyte Thiamin Concentrations among Rural Cambodian Women and Their Children Younger Than 5 Years of Age: A Randomized Controlled Efficacy Trial. J. Pediatr. 2017, 181, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; Hustad, S.; Ueland, P.M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Helmers, K. Rice in the Cambodian Economy: Past and Present; Nesbitt, H.J., Ed.; Rice Production in Cambodia, International Rice Research Institute: Manila, Philippines, 1997; pp. 1–14. [Google Scholar]
- Whitfield, K.C.; Karakochuk, C.D.; Liu, Y.; McCann, A.; Talukder, A.; Kroeun, H.; Ward, M.; McNulty, H.; Lynd, L.D.; Kitts, D.D.; et al. Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. J. Nutr. 2015, 145, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Coats, D.; Frank, E.L.; Reid, J.M.; Ou, K.; Chea, M.; Khin, M.; Preou, C.; Enders, F.T.; Fischer, P.R.; Topazian, M. Thiamine pharmacokinetics in Cambodian mothers and their breastfed infants. Am. J. Clin. Nutr. 2013, 98, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Icke, G.C.; Thom, J.; Riley, W.J. Intestinal absorption of thiamin in man compared with folate and pyridoxal and its subsequent urinary excretion. J. Nutr. Sci. Vitaminol. (Tokyo) 1984, 30, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Tallaksen, C.M.; Sande, A.; Bohmer, T.; Bell, H.; Karlsen, J. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur. J. Clin. Pharmacol. 1993, 44, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bemeur, C.B.R. Thiamin. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C.C.B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 317–324. [Google Scholar]
- Ariaey-Nejad, M.R.; Balaghi, M.; Baker, E.M.; Sauberlich, H.E. Thiamin metabolism in man. Am. J. Clin. Nutr. 1970, 23, 764–778. [Google Scholar] [PubMed]
- Singleton, C.K.; Martin, P.R. Molecular mechanisms of thiamine utilization. Curr. Mol. Med. 2001, 1, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Galvin, R.; Brathen, G.; Ivashynka, A.; Hillbom, M.; Tanasescu, R.; Leone, M.A. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur. J. Neurol. 2010, 17, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Zastre, J.A.; Hanberry, B.S.; Sweet, R.L.; McGinnis, A.C.; Venuti, K.R.; Bartlett, M.G.; Govindarajan, R. Up-regulation of vitamin B1 homeostasis genes in breast cancer. J. Nutr. Biochem. 2013, 24, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Laforenza, U. Thiamine intestinal transport and related issues: Recent aspects. Proc. Soc. Exp. Biol. Med. 2000, 224, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Babaei-Jadidi, R.; Al Ali, H.; Rabbani, N.; Antonysunil, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 2007, 50, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Bettendorff, L. Thiamine homeostasis in neuroblastoma cells. Neurochem. Int. 1995, 26, 295–302. [Google Scholar] [CrossRef]
| n | Geometric Mean (5th, 95th Percentile) | |
|---|---|---|
| Age (years) | 196 | 29.5 (21.0, 42.0) |
| BMI (kg/m2) | 196 | 21.7 (17.7, 28.8) |
| Hemoglobin (g/L) | 196 | 123 (106, 142) |
| eThDP (nmol/L) | 196 | 159 (87, 248) |
| Plasma thiamine (nmol/L) | 196 | 1.6 (0.3, 14.0) |
| Plasma TMP (nmol/L) | 188 | 5.4 (1.8, 17.0) |
| Plasma total thiamine (nmol/L) | 196 | 7.2 (2.2, 28.5) |
| eThDP | Plasma Thiamine | Plasma TMP a | |
|---|---|---|---|
| Plasma thiamine | 0.41 ** | ||
| Plasma TMP a | 0.57 ** | 0.86 ** | |
| Plasma total thiamine | 0.54 ** | 0.90 ** | 0.98 ** |
| Control (n = 66) | Low (n = 66) | High (n = 64) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiamine Form | Before | After | Change a | Before | After | Change a | Before | After | Change a | p b | R2 |
| eThDP (nmol/L) | 148 (89, 232) | 166 (102, 285) | 1.12 | 159 (83, 243) | 245 (137, 391) | 1.54 † | 170 (83, 285) | 259 (126, 402) | 1.52 † | ≤0.001 | 0.39 |
| Plasma thiamine (nmol/L) | 0.7 (0.3, 2.0) | 1.7 (0.8, 4.9) | 2.43 | 2.2 (0.4, 14.5) | 7.8 (1.3, 28.9) | 3.55 † | 2.8 (0.4, 17.9) | 10.5 (1.8, 49.2) | 3.75 † | ≤0.001 | 0.45 |
| Plasma TMP (nmol/L) | 3.3 (1.4, 7.3) | 5.0 (2.6, 11.3) | 1.52 | 6.5 (2.0, 17.1) | 10.6 (4.4, 22.1) | 1.63 † | 7.4 (2.0, 21.7) | 11.7 (5.8, 24.7) | 1.58 † | ≤0.004 | 0.11 |
| Plasma total thiamine (nmol/L) | 4.0 (1.9, 8.6) | 6.8 (3.6, 15.9) | 1.70 | 8.9 (2.8, 28.8) | 19.2 (6.6, 48.1) | 2.16 † | 10.7 (2.4, 46.6) | 23.4 (7.5, 72.5) | 2.19 † | ≤0.001 | 0.39 |
| eThDP | Plasma Thiamine | Plasma TMP | |
|---|---|---|---|
| Plasma thiamine | 0.61 ** | ||
| Plasma TMP | 0.53 ** | 0.83 ** | |
| Plasma total thiamine | 0.60 ** | 0.96 ** | 0.93 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCann, A.; Midttun, Ø.; Whitfield, K.C.; Kroeun, H.; Borath, M.; Sophonneary, P.; Ueland, P.M.; Green, T.J. Comparable Performance Characteristics of Plasma Thiamine and Erythrocyte Thiamine Diphosphate in Response to Thiamine Fortification in Rural Cambodian Women. Nutrients 2017, 9, 676. https://doi.org/10.3390/nu9070676
McCann A, Midttun Ø, Whitfield KC, Kroeun H, Borath M, Sophonneary P, Ueland PM, Green TJ. Comparable Performance Characteristics of Plasma Thiamine and Erythrocyte Thiamine Diphosphate in Response to Thiamine Fortification in Rural Cambodian Women. Nutrients. 2017; 9(7):676. https://doi.org/10.3390/nu9070676
Chicago/Turabian StyleMcCann, Adrian, Øivind Midttun, Kyly C. Whitfield, Hou Kroeun, Mam Borath, Prak Sophonneary, Per Magne Ueland, and Timothy J. Green. 2017. "Comparable Performance Characteristics of Plasma Thiamine and Erythrocyte Thiamine Diphosphate in Response to Thiamine Fortification in Rural Cambodian Women" Nutrients 9, no. 7: 676. https://doi.org/10.3390/nu9070676





