Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice
Abstract
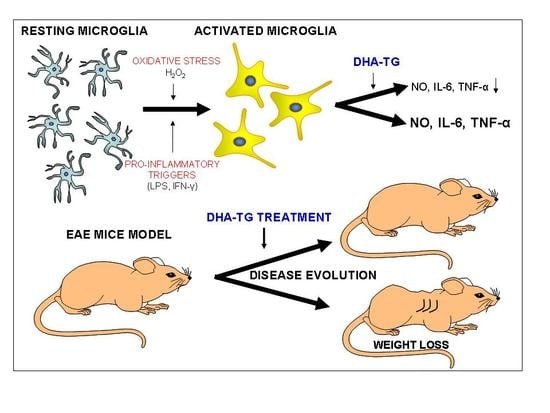
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. Nitrite and Cytokine Quantification
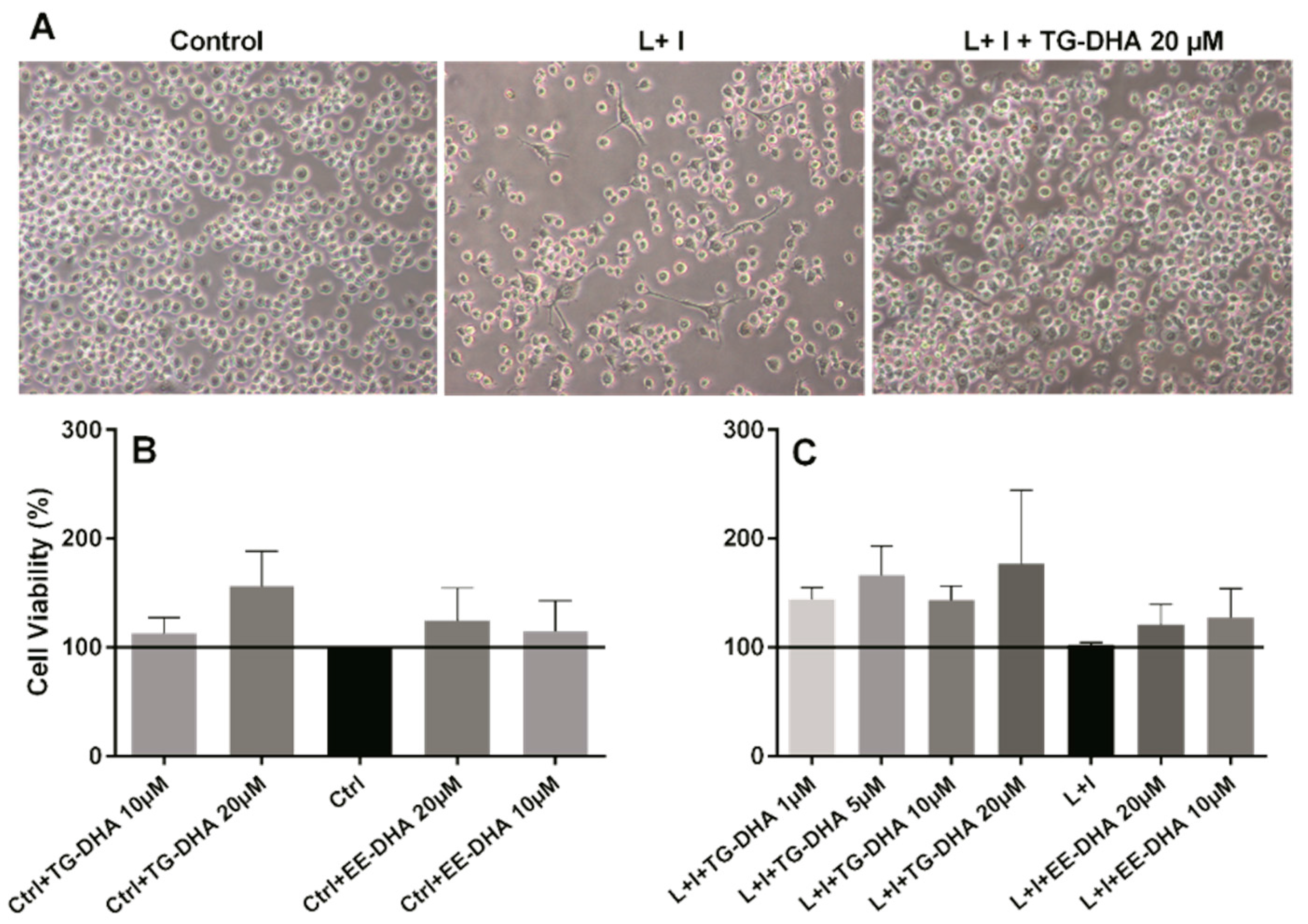
2.4. Cell Viability: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) Reduction Method
2.5. Mice
2.6. EAE Induction and Treatment
2.7. Mouse Splenocytes and CD4+ T-Cells Preparation
2.8. Proliferative Response of Lymphocytes
2.9. Determination of Tissue Fatty Acids Profile
2.10. Statistical Analysis
3. Results
3.1. DHA Increased BV-2 Cells Viability
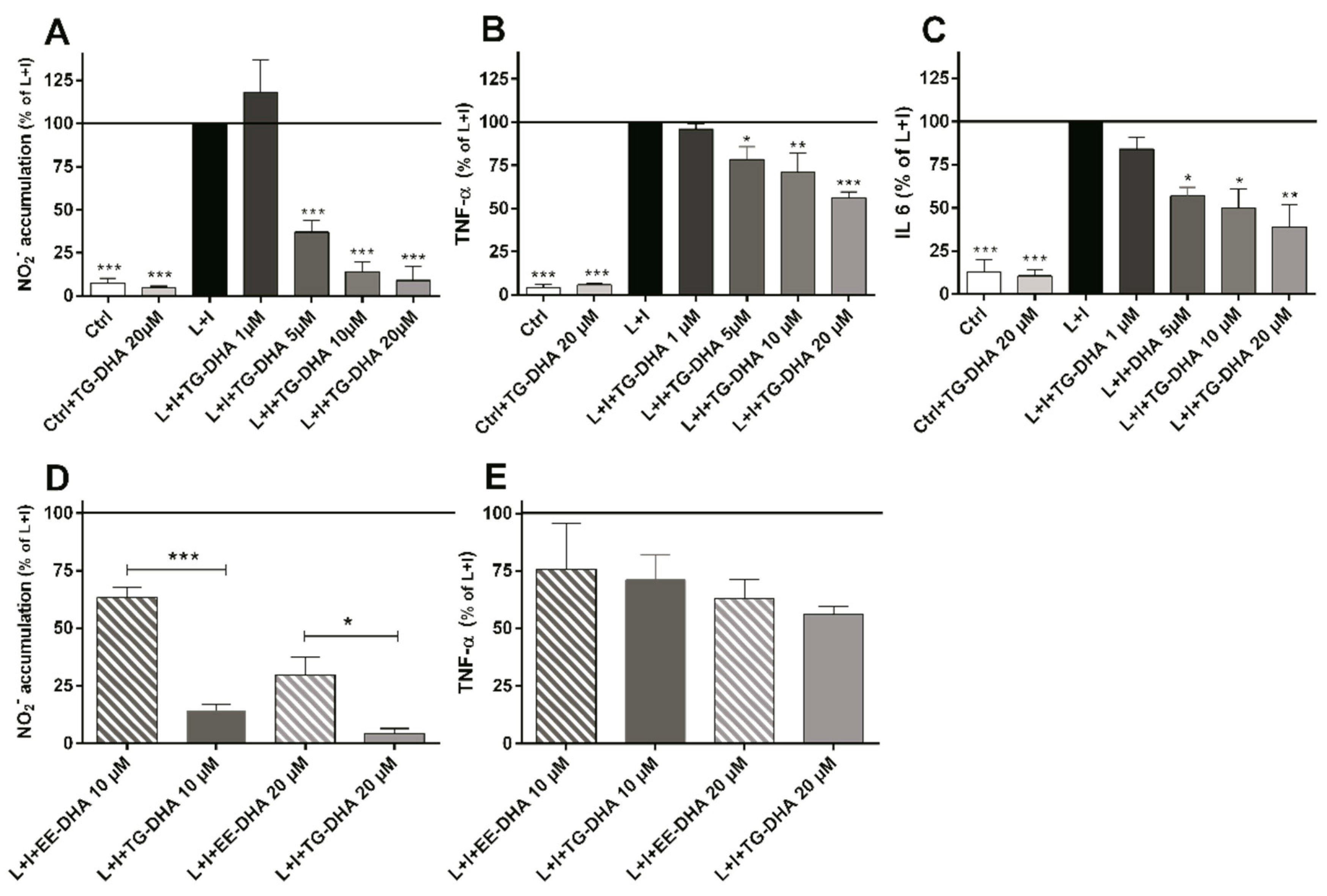
3.2. DHA Attenuated NO Production and Suppressed Induction of Inflammatory Cytokines in LPS-Stimulated BV-2 Microglia Cells
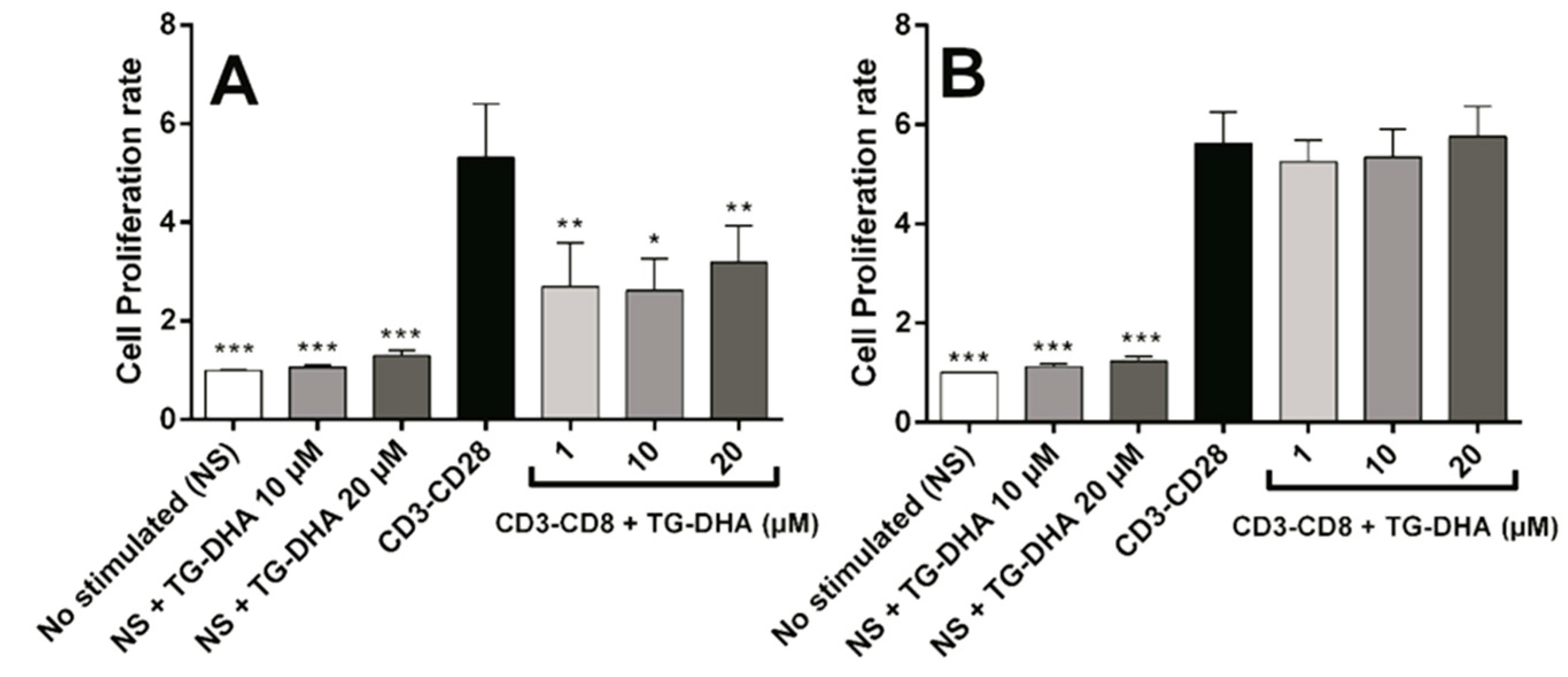
3.3. Effects of DHA on Splenocyte Viability and Proliferation after Lymphocyte Stimulation
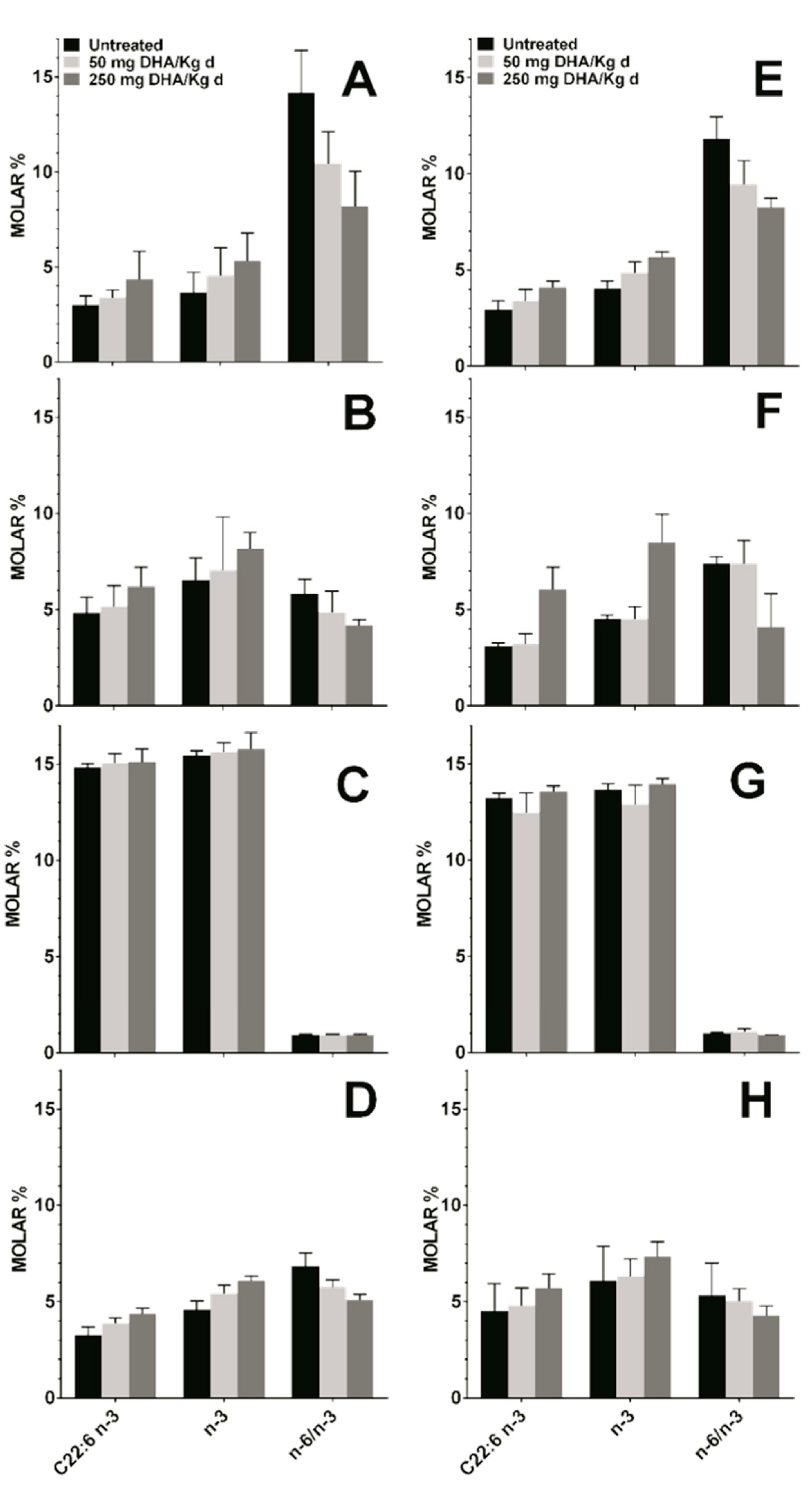
3.4. Effect DHA Dietary Supplementation on Fatty Acid Profile in Mice Tissues
3.5. Dietary DHA Showed a Beneficial Effect on EAE Clinical Course
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Matyszak, M.K.; Denis-Donini, S.; Citterio, S.; Longhi, R.; Granucci, F.; Ricciardi-Castagnoli, P. Microglia induce myelin basic protein-specific t cell anergy or t cell activation, according to their state of activation. Eur. J. Immunol. 1999, 29, 3063–3076. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific th1/th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of microglia in cns inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.C.; Manzano, R.; Atencia-Cibreiro, G.; Olivan, S.; Munoz, M.J.; Zaragoza, P.; Cordero-Vazquez, P.; Esteban-Perez, J.; Garcia-Redondo, A.; Osta, R. Genetic biomarkers for als disease in transgenic sod1(g93a) mice. PLoS ONE 2012, 7, e32632. [Google Scholar] [CrossRef] [PubMed]
- Sanagi, T.; Yuasa, S.; Nakamura, Y.; Suzuki, E.; Aoki, M.; Warita, H.; Itoyama, Y.; Uchino, S.; Kohsaka, S.; Ohsawa, K. Appearance of phagocytic microglia adjacent to motoneurons in spinal cord tissue from a presymptomatic transgenic rat model of amyotrophic lateral sclerosis. J. Neurosci. Res. 2010, 88, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Kim, J.H.; Greenamyre, J.T. Selective microglial activation in the rat rotenone model of parkinson’s disease. Neurosci. Lett. 2003, 341, 87–90. [Google Scholar] [CrossRef]
- Tada, S.; Okuno, T.; Yasui, T.; Nakatsuji, Y.; Sugimoto, T.; Kikutani, H.; Sakoda, S. Deleterious effects of lymphocytes at the early stage of neurodegeneration in an animal model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2011, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.J.; Talbot, K. Transgenics, toxicity and therapeutics in rodent models of mutant sod1-mediated familial als. Prog. Neurobiol. 2008, 85, 94–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tsao, Y.Y.; Leung, Y.M.; Su, K.P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in bv-2 microglia: Implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.P.; Richards, M.H.; Miller, S.D. Mouse models of multiple sclerosis: Experimental autoimmune encephalomyelitis and theiler’s virus-induced demyelinating disease. Methods Mol. Biol. 2012, 900, 381–401. [Google Scholar] [PubMed]
- Kroenke, M.A.; Carlson, T.J.; Andjelkovic, A.V.; Segal, B.M. Il-12- and il-23-modulated t cells induce distinct types of eae based on histology, cns chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008, 205, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.R.; Golumbek, P.T.; Sim, J.; Dorsey, D.; Russell, J.H. Regional cns responses to ifn-gamma determine lesion localization patterns during eae pathogenesis. J. Exp. Med. 2008, 205, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Swanborg, R.H. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin. Immunol. Immunopathol. 1995, 77, 4–13. [Google Scholar] [CrossRef]
- Yednock, T.A.; Cannon, C.; Fritz, L.C.; Sanchez-Madrid, F.; Steinman, L.; Karin, N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992, 356, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, N.; Lassmann, S.; Li, Z.; Odoardi, F.; Ritter, T.; Ziemssen, T.; Klinkert, W.E.; Ellwart, J.W.; Bradl, M.; Krivacic, K.; et al. The activation status of neuroantigen-specific t cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 2004, 199, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Cartier, N.; Lewis, C.A.; Zhang, R.; Rossi, F.M. The role of microglia in human disease: Therapeutic tool or target? Acta Neuropathol. 2014, 128, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential roles of m1 and m2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013, 14, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Storer, P.D.; Xu, J.; Chavis, J.; Drew, P.D. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: Implications for multiple sclerosis. J. Neuroimmunol. 2005, 161, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, M. Peroxisome proliferator-activated receptor-gamma in amyotrophic lateral sclerosis and huntington’s disease. PPAR Res. 2008, 2008, 418765. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, M.; Kipiani, K.; Chen, J.; Calingasan, N.Y.; Beal, M.F. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2005, 191, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through ppar(gamma): Rxr heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Pettit, L.K.; Varsanyi, C.; Tadros, J.; Vassiliou, E. Modulating the inflammatory properties of activated microglia with docosahexaenoic acid and aspirin. Lipids Health Dis. 2013, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Ajmone-Cat, M.A.; Salvatori, M.I.; De Simone, R.; Mancini, M.; Biagioni, S.; Bernardo, A.; Cacci, E.; Minghetti, L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J. Neurosci. Res. 2012, 90, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Dongmo, B.M.; Avallone, R. Supplementation of omega 3 fatty acids improves oxidative stress in activated bv2 microglial cell line. Int. J. Food Sci. Nutr. 2015, 66, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Trepanier, M.O.; Bazinet, R.P. N-3 polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects-a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Virgili, N.; Espinosa-Parrilla, J.F.; Mancera, P.; Pasten-Zamorano, A.; Gimeno-Bayon, J.; Rodriguez, M.J.; Mahy, N.; Pugliese, M. Oral administration of the katp channel opener diazoxide ameliorates disease progression in a murine model of multiple sclerosis. J. Neuroinflamm. 2011, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.L. Toxicology and safety of dha. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of grp78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, D.J.; Abbey, M. Effects of n-3 fatty acids on growth and survival of j774 macrophages. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Paolisso, G.; Casamassimi, A.; Al-Omran, M.; Barbieri, M.; Sommese, L.; Infante, T.; Ignarro, L.J. Effects of nitric oxide on cell proliferation: Novel insights. J. Am. Coll. Cardiol. 2013, 62, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Arrington, J.L.; Chapkin, R.S.; Switzer, K.C.; Morris, J.S.; McMurray, D.N. Dietary n-3 polyunsaturated fatty acids modulate purified murine t-cell subset activation. Clin. Exp. Immunol. 2001, 125, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Manzoni Jacintho, T.; Gotho, H.; Gidlund, M.; Garcia Marques, C.; Torrinhas, R.; Mirtes Sales, M.; Linetzky Waitzberg, D. Anti-inflammatory effect of parenteral fish oil lipid emulsion on human activated mononuclear leukocytes. Nutr. Hosp. 2009, 24, 288–296. [Google Scholar] [PubMed]
- Schley, P.D.; Jijon, H.B.; Robinson, L.E.; Field, C.J. Mechanisms of omega-3 fatty acid-induced growth inhibition in mda-mb-231 human breast cancer cells. Breast Cancer Res. Treat. 2005, 92, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Marino, A.; Cuzzocrea, S. N-3 fatty acids: Role in neurogenesis and neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the nrf-2/ho-1 pathway in 3t3-l1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Shahar, S.; Rajab, N.; Yusoff, N.A.; Jamal, R.A.; Then, S.M. The role of long chain omega-3 polyunsaturated fatty acids in reducing lipid peroxidation among elderly patients with mild cognitive impairment: A case-control study. J. Nutr. Biochem. 2013, 24, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chang, C.D.; Chen, P.H.; Su, J.R.; Chen, C.C.; Chaung, H.C. Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res. 2012, 1451, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Huang, Y.; Kong, A.N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Neuroprotectin d1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and alzheimer’s disease. J. Lipid Res. 2009, 50 (Suppl. S400–S405). [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lim, J.W.; Kim, H. Inhibitory mechanism of omega-3 fatty acids in pancreatic inflammation and apoptosis. Ann. N. Y. Acad. Sci. 2009, 1171, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.A.; Harvey, K.A.; Ruzmetov, N.; Miller, S.J.; Zaloga, G.P. N-3 fatty acids prevent whereas trans-fatty acids induce vascular inflammation and sudden cardiac death. Br. J. Nutr. 2009, 102, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Akhtar Khan, N. Polyunsaturated fatty acids in the modulation of t-cell signalling. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, Y.; Liu, Y.; Zhang, J.; Kishimoto, C.; Ma, A.; Liu, Z. Peroxisome proliferator-activated receptor-gamma ligands ameliorate experimental autoimmune myocarditis associated with inhibition of self-sensitive t cells. J. Cardiovasc. Pharmacol. 2004, 43, 868–875. [Google Scholar] [CrossRef] [PubMed]


| % of Total Fatty Acids | ||||
|---|---|---|---|---|
| Total Fatty Acids | Plasma | RBC | ||
| Ctl (n = 7) | EAE (n = 7) | Ctl (n = 7) | EAE (n = 7) | |
| SFA | 31.70 ± 0.96 | 31.70 ± 0.49 | 47.53 ± 4.23 | 50.25 ± 6.89 |
| MUFA | 16.97 ± 1.19 | 16.23 ± 1.35 | 13.27 ± 0.57 | 14.54 ± 0.81 |
| PUFA | 51.33 ± 1.12 | 52.07 ± 1.36 | 39.20 ± 4.74 | 35.21 ± 7.60 |
| PUFA n-6 | 47.68 ± 1.36 | 47.60 ± 1.24 | 32.01 ± 4.43 | 29.93 ± 5.75 |
| PUFA n-3 | 3.65 ± 1.08 | 3.87 ± 0.91 | 6.55 ± 1.12 | 5.28 ± 1.93 |
| n-6/n-3 ratio | 14.17 ± 2.22 | 11.07 ± 2.25 | 5.83 ± 0.74 | 6.09 ± 1.29 |
| C22:6 n-3 | 3.01 ± 0.48 | 2.96 ± 0.79 | 4.83 ± 0.83 | 3.39 ± 1.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancera, P.; Wappenhans, B.; Cordobilla, B.; Virgili, N.; Pugliese, M.; Rueda, F.; Espinosa-Parrilla, J.F.; Domingo, J.C. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients 2017, 9, 681. https://doi.org/10.3390/nu9070681
Mancera P, Wappenhans B, Cordobilla B, Virgili N, Pugliese M, Rueda F, Espinosa-Parrilla JF, Domingo JC. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients. 2017; 9(7):681. https://doi.org/10.3390/nu9070681
Chicago/Turabian StyleMancera, Pilar, Blanca Wappenhans, Begoña Cordobilla, Noemí Virgili, Marco Pugliese, Fèlix Rueda, Juan F. Espinosa-Parrilla, and Joan C. Domingo. 2017. "Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice" Nutrients 9, no. 7: 681. https://doi.org/10.3390/nu9070681