Effects of Carbohydrate and Glutamine Supplementation on Oral Mucosa Immunity after Strenuous Exercise at High Altitude: A Double-Blind Randomized Trial
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Participants
2.3. Intervention
- Group Hypoxia (Exercise + Altitude + Placebo): Participants consumed glutamine placebo supplements during the six days prior to the test (10 g corn starch + 10 g lactose), taken in the evening. During test day, they performed an exercise session at 70% of VO2peak at a simulated altitude of 4500 m and were given a carbohydrate placebo supplement (Crystal Light®—Kraft Foods, Inc. strawberry, Chicago, IL, USA), 200 mL every 20 min during exercise and during recovery for two hours.
- Group Hypoxia + CHO (Exercise + Altitude + Carbohydrate): Participants consumed glutamine placebo supplements during the six days prior to the test (10 g corn starch + 10 g lactose) taken in the evening. During test day, they performed an exercise session at 70% of VO2peak at a simulated altitude of 4500 m, and were given carbohydrate supplements (Maltodextrin strawberry flavor—Probiótica®—Laboratories, Embu das Artes, São Paulo, Brazil), 200 mL at a concentration of 8% every 20 min during exercise and during recovery for two hours.
- Group Hypoxia + GLN (Exercise + Altitude + Carbohydrate + Glutamine): Participants consumed 20 g of glutamine (Probiótica®—Laboratories, Embu das Artes, São Paulo, Brazil) in the six days prior to the test, between 8:00–10:00 p.m. During test day, they performed an exercise session at 70% of VO2peak at a simulated altitude of 4500 m, and were given carbohydrate supplements (Maltodextrin strawberry flavor—Probiótica®—Laboratories, Embu das Artes, São Paulo, Brazil), 200 mL at a concentration of 8% every 20 minutes during exercise and during recovery for two hours.
Determination of VO2peak
2.4. Altitude Simulation
2.5. Sessions of Exercise and Recovery
2.6. Hemoglobin O2 Saturation (SaO2%)
2.7. Saliva Collection
2.8. Determinants in Saliva
2.9. Statistical Analysis
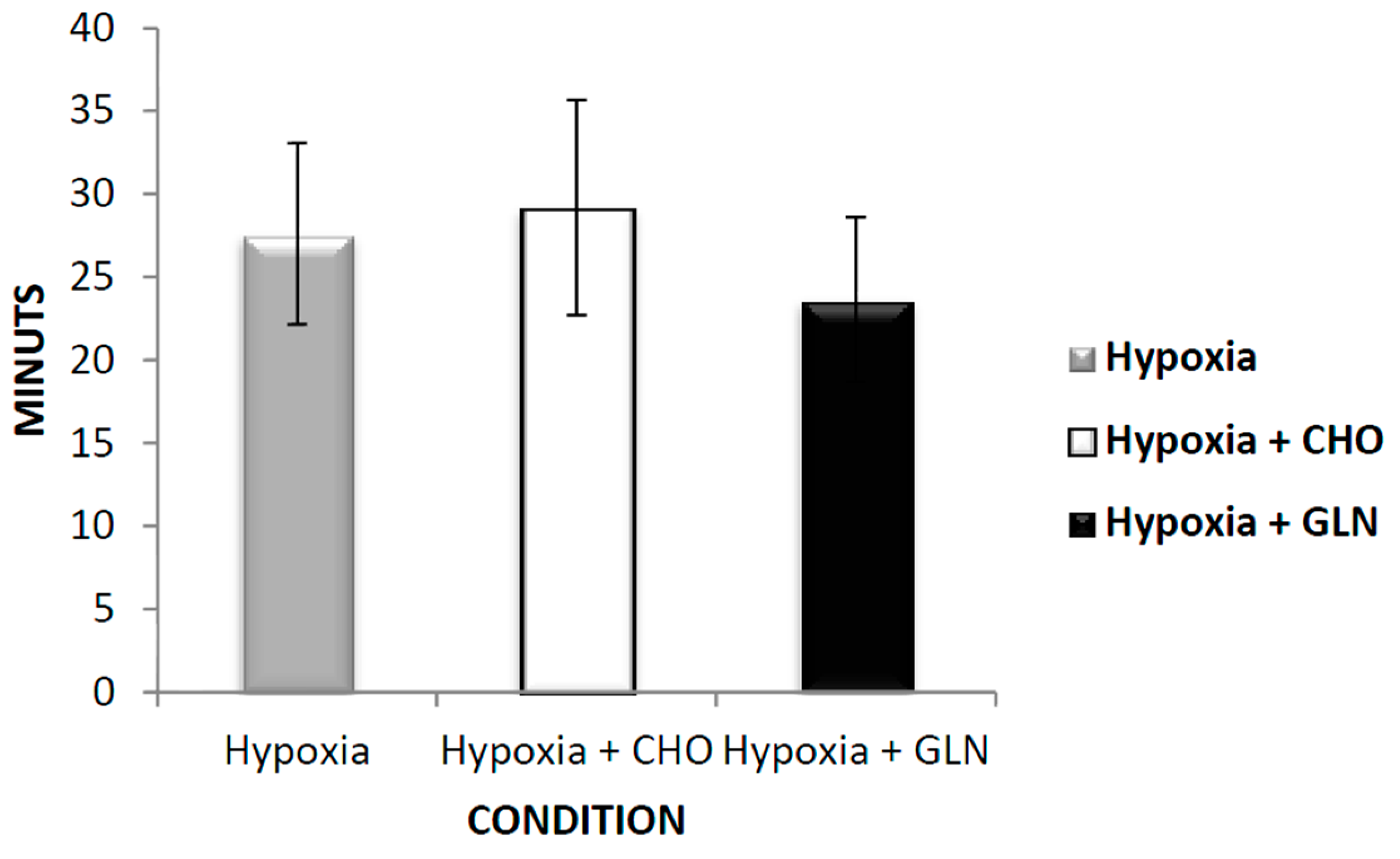
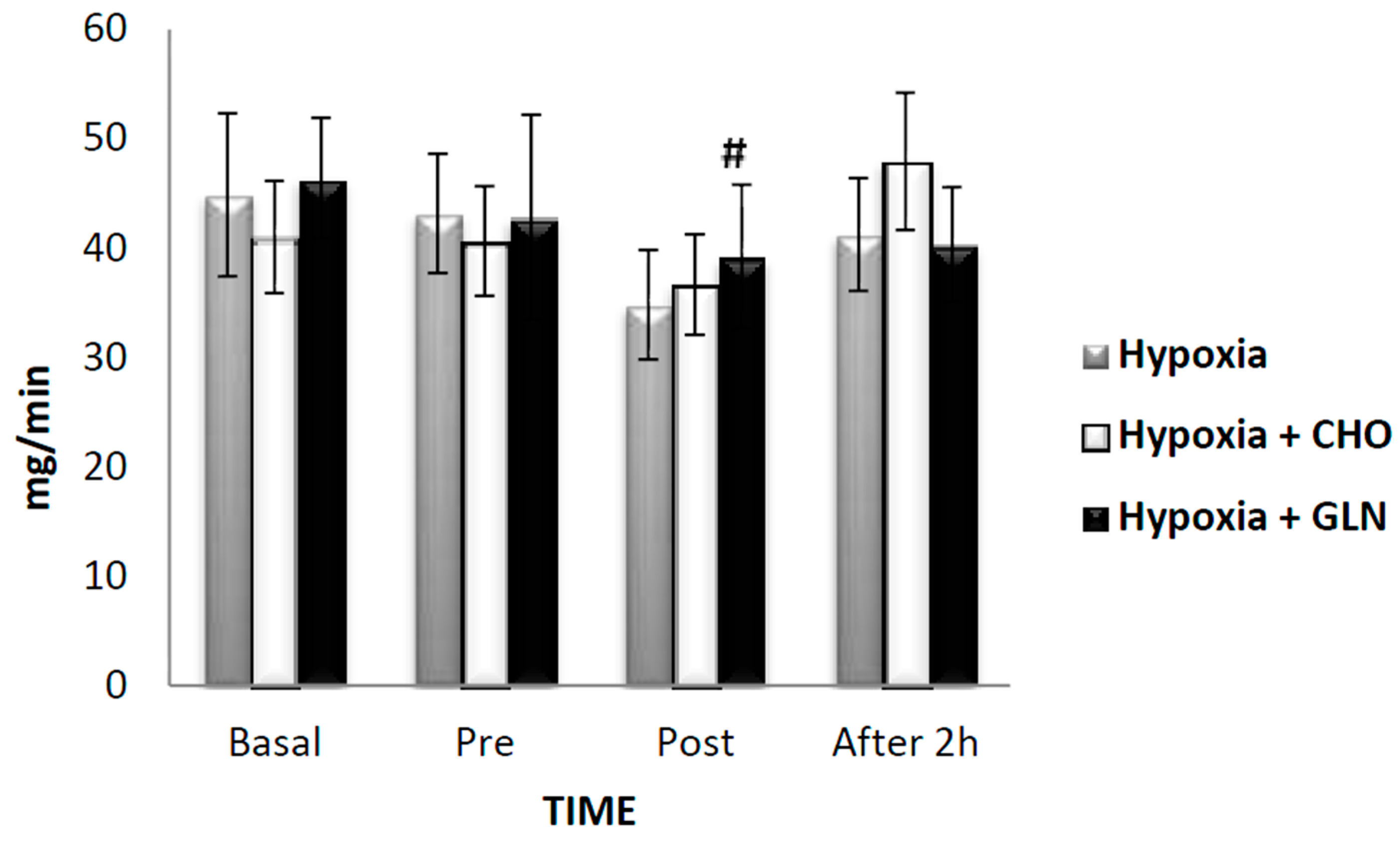
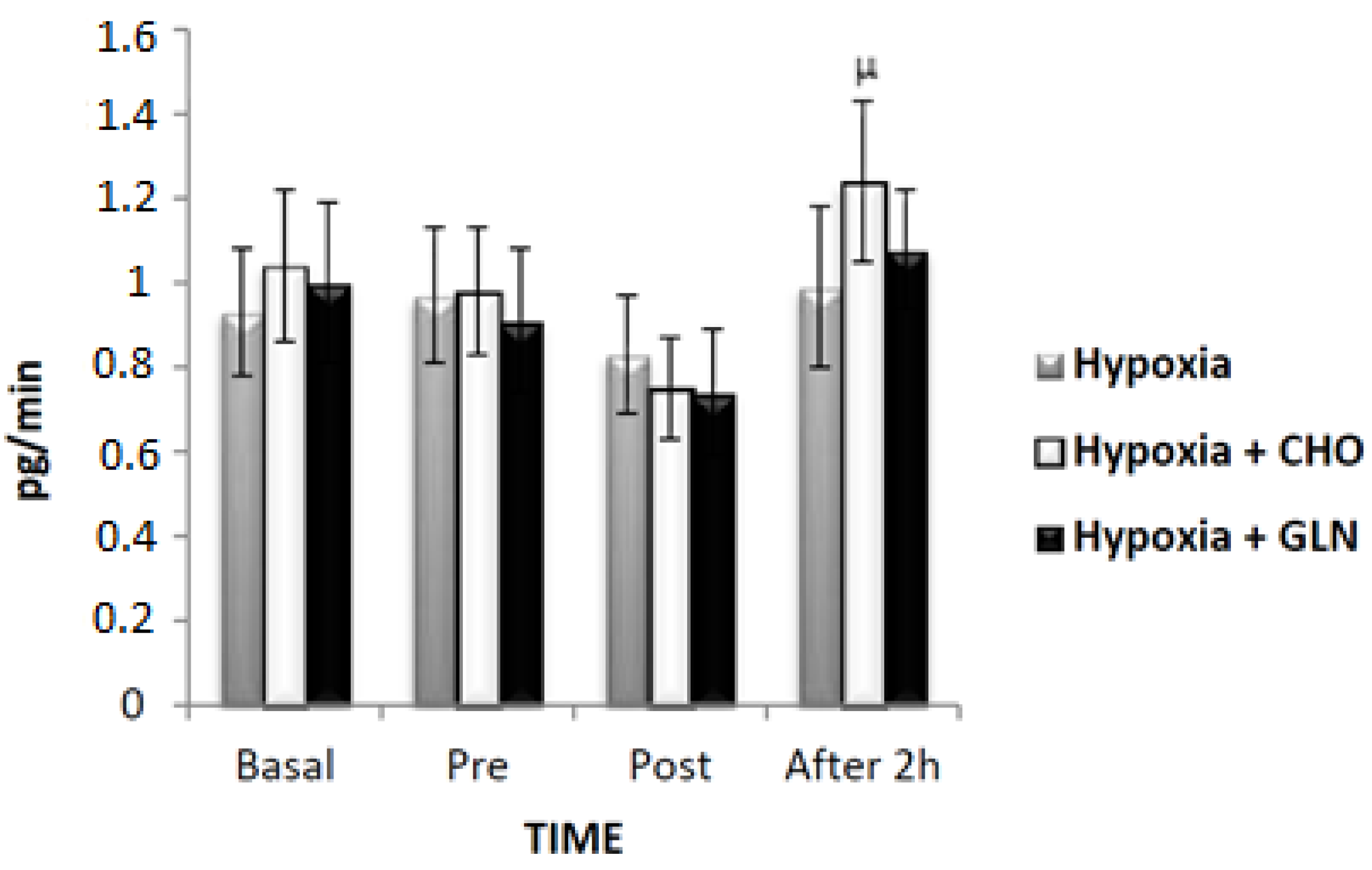
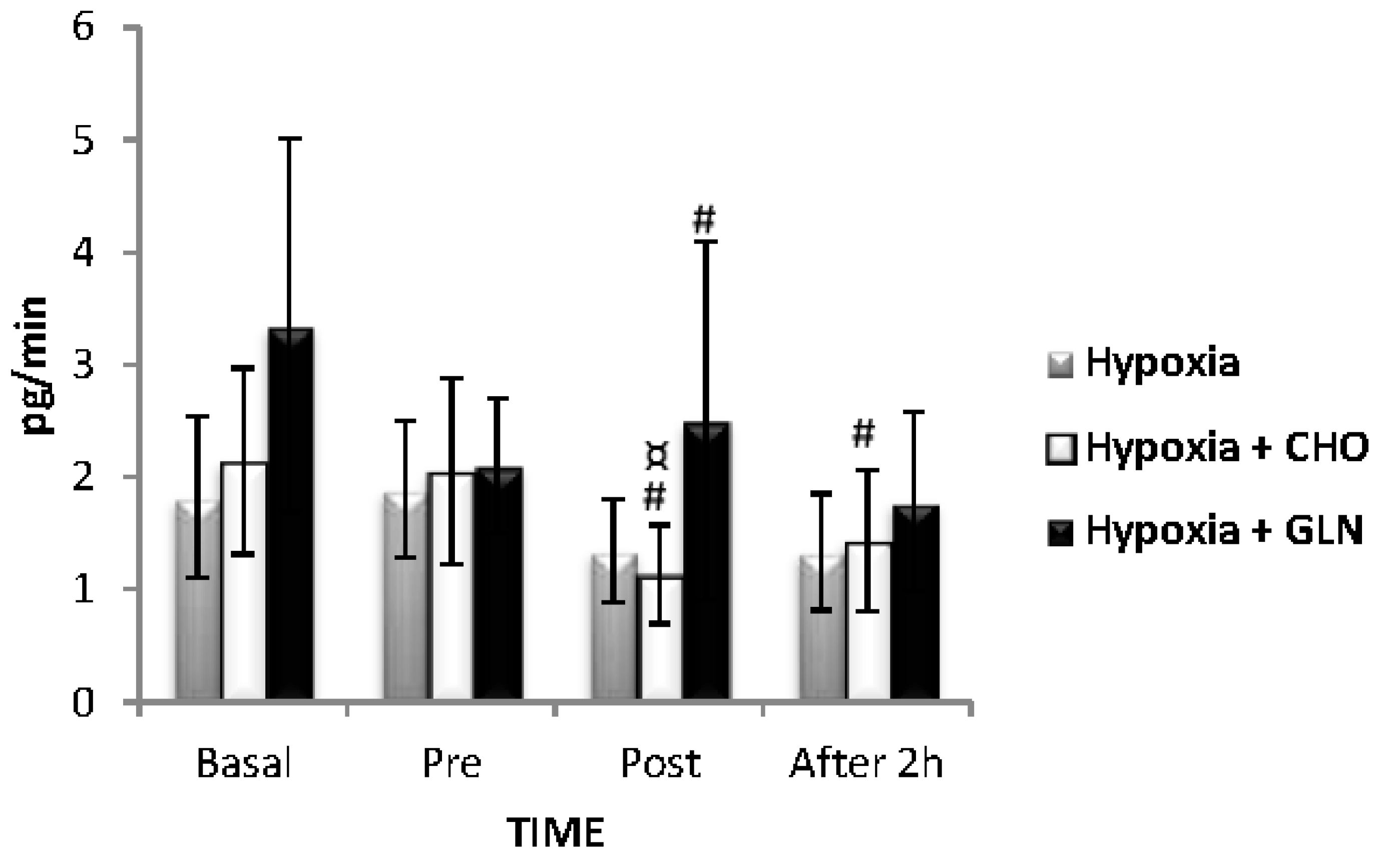
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Born, D.P.; Faiss, R.; Willis, S.J.; Strahler, J.; Millet, G.P.; Holmberg, H.C.; Sperlich, B. Circadian variation of salivary immunoglobin A, alpha-amylase activity and mood in response to repeated double-poling sprints in hypoxia. Eur. J. Appl. Physiol. 2016, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Yoshikawa, T.; Ueda, S.-Y.; Katsura, Y.; Orita, K.; Fujimoto, S. Effects of acute prolonged strenuous exercise on the salivary stress markers and inflammatory cytokines. J. Phys. Fit. Sports Med. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Slavish, D.C.; Graham-Engeland, J.E.; Smyth, J.M.; Engeland, C.G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immunity 2015, 44, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Ganju, L.; Singh, S.B. Hypoxia modulates innate immune factors: A review. Int. Immunopharmacol. 2015, 28, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Tschöp, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschöp, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.A.; Jacobs, K.A.; Subudhi, A.W.; Fattor, J.A.; Rock, P.B.; Muza, S.R.; Cymerman, A.; Friedlander, A.L. Cytokine responses at high altitude: Effects of exercise and antioxidants at 4300 m. Med. Sci. Sports Exerc. 2006, 38, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, M.; Eckle, T.; Eltzschig, H.K. The hypoxia-inflammation link and potential drug targets. Curr. Opin. Anaesthesiol. 2011, 24, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.E.; Celeste Simon, M. Hypoxia-inducible factors: Crosstalk between inflammation and metabolism. Semin. Cell Dev. Biol. 2012, 23, 389–394. [Google Scholar] [CrossRef] [PubMed]
- McNamee, E.N.; Korns Johnson, D.; Homann, D.; Clambey, E.T. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 2013, 55, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Ganju, L. Influence of high altitude exposure on the immune system: A review. Immunol. Investig. 2010, 39, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.; Bishop, N.C.; Spielmann, G.; Pistillo, M.; Reed, J.; Ograjsek, T.; Park, Y.; Mehta, S.K.; Pierson, D.L.; Simpson, R.J. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur. J. Appl. Physiol. 2015, 115, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Teixeira, A.; Lira, F.; Tufik, S.; Mello, M.; Santos, R. Moderate acute exercise (70% VO2peak) induces TGF-β, α-amylase and IgA in saliva during recovery. Oral Dis. 2014, 20, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Papacosta, E.; Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 2011, 14, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S. Altitude, exercise and immune function. Exerc. Immunol. Rev. 2005, 11, 6–16. [Google Scholar] [PubMed]
- Shephard, R.J. Immune changes induced by exercise in an adverse environment. Can. J. Physiol. Pharmacol. 1998, 76, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S. Physiological responses to exercise at altitude: An update. Sports Med. 2008, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Poortmans, J.R.; Newsholme, E.A. Does glutamine have a role in reducing infections in athletes? Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Newsholme, E.A. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 1997, 13, 738–742. [Google Scholar] [CrossRef]
- Wong, S.H.; Williams, C.; Adams, N. Effects of ingesting a large volume of carbohydrate electrolyte solution on rehydration during recovery and subsequent exercise capacity. Int. J. Sports Nutr. 2000, 10, 375–393. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Smith, L.L.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Kaminsky, D.E.; Shute, M. Cytokine changes after a marathon race. J. Appl. Physiol. 2001, 91, 109–114. [Google Scholar] [PubMed]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar] [PubMed]
- Bailey, N.; Clark, M.; Nordlund, M.; Shelton, M.; Farver, K. New paradigm in nutrition support: Using evidence to drive practice. Crit. Care Nurs. Q. 2012, 35, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.W.; Crowe, M.; Blank, S.E. Chronic glutamine supplementation increases nasal but not salivary IgA during 9 days of interval training. J. Appl. Physiol. 2004, 97, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Caris, A.V.; Lira, F.S.; de Mello, M.T.; Oyama, L.M.; dos Santos, R.V. Carbohydrate and glutamine supplementation modulates the Th1/Th2 balance after exercise performed at a simulated altitude of 4500 m. Nutrition 2014, 30, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Immunonutrition support for athletes. J. Nutr. Rev. 2008, 66, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.A.; Kenefick, R.W.; Koch, A.J. Influence of carbohydrate ingestion on salivary immunoglobulin A following resistance exercise. J. Int. Soc. Sports Nutr. 2013, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.C.; Blannin, A.K.; Armstrong, E.; Rickman, M.; Gleeson, M. Carbohydrate and fluid intake affect the saliva flow rate and IgA response to cycling. Med. Sci. Sports Exerc. 2000, 32, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Service. Sample Size Calculator.Disponivel em. Available online: http://www.nss.gov.au/nss/home.nsf/pages/Sample+Size+Calculator+Description?OpenDocument (accessed on 2 April 2016).
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Research Randomizer. Disponível em. Available online: https://www.randomizer.org/ (accessed on 2 February 2016).
- Sassi, A.; Marcora, S.M.; Rampinini, E.; Mognoni, P.; Impellizzeri, F.M. Prediction of time to exhaustion from blood lactate response during submaximal exercise in competitive cyclists. Eur. J. Appl. Physiol. 2006, 97, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Doust, J.H. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J. Sports Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M.P. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extreme Physiol. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Tannheimer, M.; Thomas, A.; Gerngross, H. Oxygen saturation course and altitude symptomatology during an expedition to broad peak (8047 m). Int. J. Sports Med. 2002, 23, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Pomidori, L.; Bonardi, D.; Campigotto, F.; Fasano, V.; Gennari, A.; Valli, G.; Palange, P.; Cogo, A. The hypoxic profile during trekking to the Pyramid Laboratory. High Alt. Med. Biol. 2009, 10, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Golja, P.; Flander, P.; Klemenc, M.; Maver, J.; Princi, T. Carbohydrate ingestion improves oxygen delivery in acute hypoxia. High Alt. Med. Biol. 2008, 9, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Charlot, K.; Pichon, A.; Richalet, J.P.; Chapelot, D. Effects of a high-carbohydrate versus high-protein meal on acute responses to hypoxia at rest and exercise. Eur. J. Appl. Physiol. 2013, 113, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Tani, H.; Masuda, A.; Kobayashi, T.; Nishino, T.; Kimura, H.; Masuyama, S.; Kuriyama, T. Effect of prior O2 breathing on ventilatory response to sustained isocapnic hypoxia in adult humans. J. Appl. Physiol. 1996, 81, 1627–1632. [Google Scholar] [PubMed]
- Favano, A.; Santos-Silva, P.R.; Nakano, E.Y.; Pedrinelli, A.; Hernandez, A.J.; Greve, J.M. Peptide glutamine supplementation for tolerance of intermittent exercise in soccer players. Clinics 2008, 63, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Pilardeau, P.; Richalet, J.P.; Bouissou, P.; Vaysse, J.; Larmignat, P.; Boom, A. Saliva flow and composition in humans exposed to acute altitude hypoxia. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 59, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Allgrove, J.E.; Gomes, E.; Hough, J.; Gleeson, M. Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J. Sports Sci. 2008, 26, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, I.S.; Hem, E.; Gleeson, M. Effect of acute exercise and hypoxia on markers of systemic and mucosal immunity. Eur. J. Appl. Physiol. 2016, 116, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.C.; Gleeson, M. Acute and chronic effects of exercise on markers of mucosal immunity. Front. Biosci. 2009, 14, 4444–4456. [Google Scholar] [CrossRef]
- Krzywkowski, K.; Petersen, E.W.; Ostrowski, K.; Link-Amster, H.; Boza, J.; Halkjaer-Kristensen, J.; Klarlund Pedersen, B. Effect of glutamine and protein supplementation on exercise-induced decreases in salivary IgA. J. Appl. Physiol. 2001, 91, 832–838. [Google Scholar] [PubMed]
- Silva, R.P.; Natali, A.J.; Paula, S.O.; Locatelli, J.; Marins, J.C.B. Imunoglobulina A salivar (IgA-s) e exercício: Relevância do controle em atletas e implicações metodológicas. Rev. Bras. Med. Esporte 2009, 15, 459–466. [Google Scholar] [CrossRef]
- Yamaoka, M.; Yamaguchi, S.; Okuyama, M.; Tomoike, H. Anti-inflammatory cytokine profile in human heart failure: Behavior of interleukin-10 in association with tumor necrosis factor-alpha. Jpn. Circ. J. 1999, 63, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.A.; Abdullah, N.; Singh, R.; Sosroseno, W. Effect of acute exercise on the levels of salivary cortisol, tumor necrosis factor-alpha and nitric oxide. J. Oral Sci. 2010, 52, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.C.; Blannin, A.K.; Robson, P.J.; Walsh, N.P.; Gleeson, M. The effects of carbohydrate supplementation on immune responses to a soccer-specific exercise protocol. J. Sports Sci. 1999, 17, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Faquin, W.C.; Schneider, T.J.; Goldberg, M.A. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood 1992, 79, 1987–1994. [Google Scholar] [PubMed]
- Klausen, T.; Olsen, N.V.; Poulsen, T.D.; Richalet, J.P.; Pedersen, B.K. Hypoxemia increases serum interleukin-6 in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 480–482. [Google Scholar] [CrossRef] [PubMed]

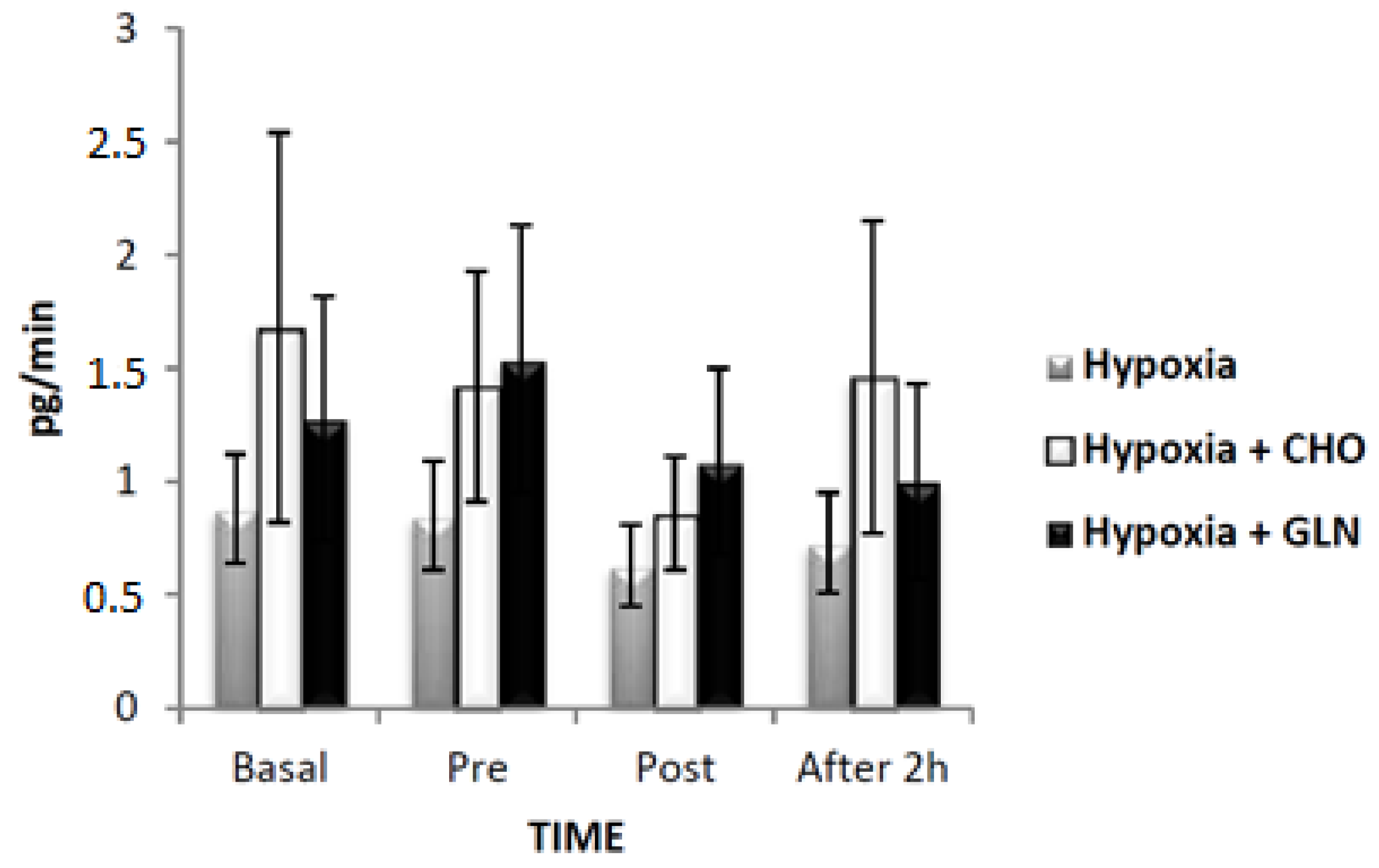
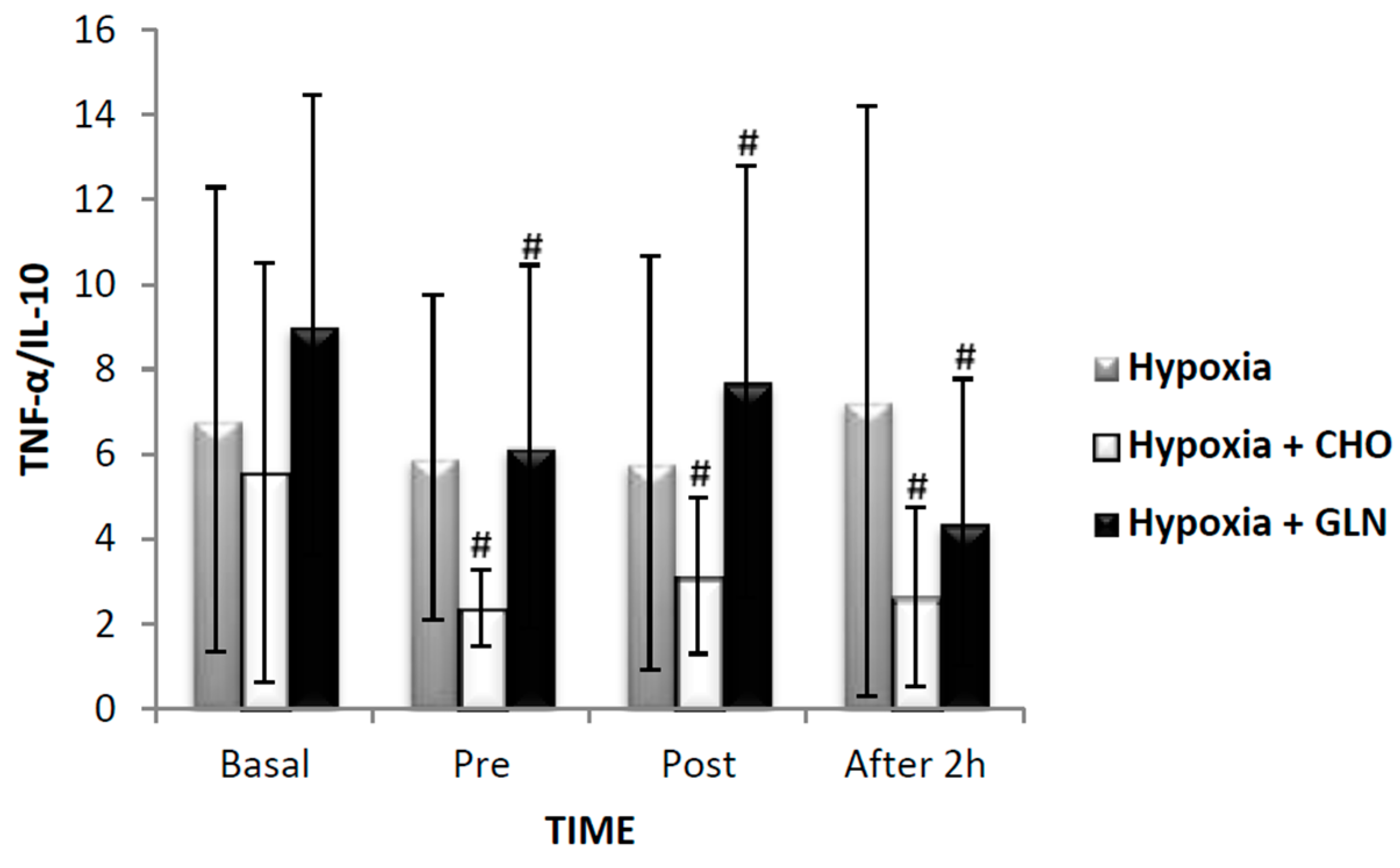
| Condition | ||||
|---|---|---|---|---|
| Hypoxia | Hypoxia + CHO | Hypoxia + GLN | ||
| SaO2% | Basal | 97.13 ± 0.27 | 97.13 ± 0.21 | 96.87 ± 0.24 |
| Pre-exercise | 85.47 ± 1.35 A | 82.40 ± 1.23 A | 84.33 ± 1.01 A | |
| Post-exercise | 79.67 ± 1.37 AB | 81.47 ± 1.02 A | 81.53 ± 1.43 A | |
| 2 h after | 85.40 ± 1.00 AC | 89.33 ± 0.46 ABC | 90.27 ± 0.55 ABC* | |
| Condition | ||||
|---|---|---|---|---|
| Hypoxia | Hypoxia + CHO | Hypoxia + GLN | ||
| SaO2% | Basal | 0.90 ± 0.11 | 0.89 ± 0.12 | 0.89 ± 0.09 |
| Pre-exercise | 0.88 ± 0.11 | 0.81 ± 0.10 | 0.76 ± 0.08 | |
| Post-exercise | 0.75 ± 0.10 | 0.76 ± 0.11 | 0.79 ± 0.13 | |
| 2 h after | 0.92 ± 0.11 | 1.04 ± 0.14 C | 0.85 ± 0.10 | |
| Condition | ||||
|---|---|---|---|---|
| Hypoxia | Hypoxia + CHO | Hypoxia + GLN | ||
| IgA | Basal | 48.40 ± 2.07 | 46.47 ± 1.6 | 49.80 ± 2.27 |
| Pre-exercise | 49.93 ± 2.21 | 50.47 ± 3.30 | 48.40 ± 1.67 | |
| Post-exercise | 47.33 ± 3.34 | 50.80 ± 3.88 | 45.60 ± 2.02 | |
| 2 h after | 45.07 ± 1.25 | 46.73 ± 3.70 | 47.00 ± 2.60 | |
| IL-10 | Basal | 1.11 ± 0.15 | 1.22 ± 0.16 | 1.13 ± 0.17 |
| Pre-exercise | 1.14 ± 0.17 | 1.27 ± 0.19 | 1.25 ± 0.20 | |
| Post-exercise | 1.08 ± 0.12 | 1.01 ± 0.13 | 1.02 ± 0.17 | |
| 2 h after | 1.11 ± 0.16 | 1.22 ± 0.15 | 1.24 ± 0.15 | |
| TNF-α | Basal | 2.06 ± 0.80 | 2.07 ± 0.69 | 3.69 ± 1.79 |
| Pre-exercise | 2.18 ± 0.67 | 2.30 ± 0.80 | 2.74 ± 0.75 | |
| Post-exercise | 2.02 ± 0.98 | 1.54 ± 0.62 AB | 3.06 ± 2.25 | |
| 2 h after | 1.34 ± 0.44 | 1.12 ± 0.39 AB | 2.03 ± 0.73 | |
| IL-6 | Basal | 1.05 ± 0.26 | 1.50 ± 0.68 | 1.42 ± 0.58 |
| Pre-exercise | 0.93 ± 0.22 | 1.57 ± 0.51 | 1.92 ± 0.63 | |
| Post-exercise | 0.97 ± 0.30 | 1.15 ± 0.36 | 1.26 ± 0.48 | |
| 2 h after | 0.82 ± 0.22 | 1.12 ± 0.37 | 1.06 ± 0.37 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caris, A.V.; Da Silva, E.T.; Dos Santos, S.A.; Tufik, S.; Dos Santos, R.V.T. Effects of Carbohydrate and Glutamine Supplementation on Oral Mucosa Immunity after Strenuous Exercise at High Altitude: A Double-Blind Randomized Trial. Nutrients 2017, 9, 692. https://doi.org/10.3390/nu9070692
Caris AV, Da Silva ET, Dos Santos SA, Tufik S, Dos Santos RVT. Effects of Carbohydrate and Glutamine Supplementation on Oral Mucosa Immunity after Strenuous Exercise at High Altitude: A Double-Blind Randomized Trial. Nutrients. 2017; 9(7):692. https://doi.org/10.3390/nu9070692
Chicago/Turabian StyleCaris, Aline Venticinque, Edgar Tavares Da Silva, Samile Amorim Dos Santos, Sergio Tufik, and Ronaldo Vagner Thomatieli Dos Santos. 2017. "Effects of Carbohydrate and Glutamine Supplementation on Oral Mucosa Immunity after Strenuous Exercise at High Altitude: A Double-Blind Randomized Trial" Nutrients 9, no. 7: 692. https://doi.org/10.3390/nu9070692





