Reduction of Asthmatic Parameters by Sea Hare Hydrolysates in a Mouse Model of Allergic Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Chemicals
2.3. Preparation of Sea Hare Hydrolysates (SHH)
2.4. Experimental Animals
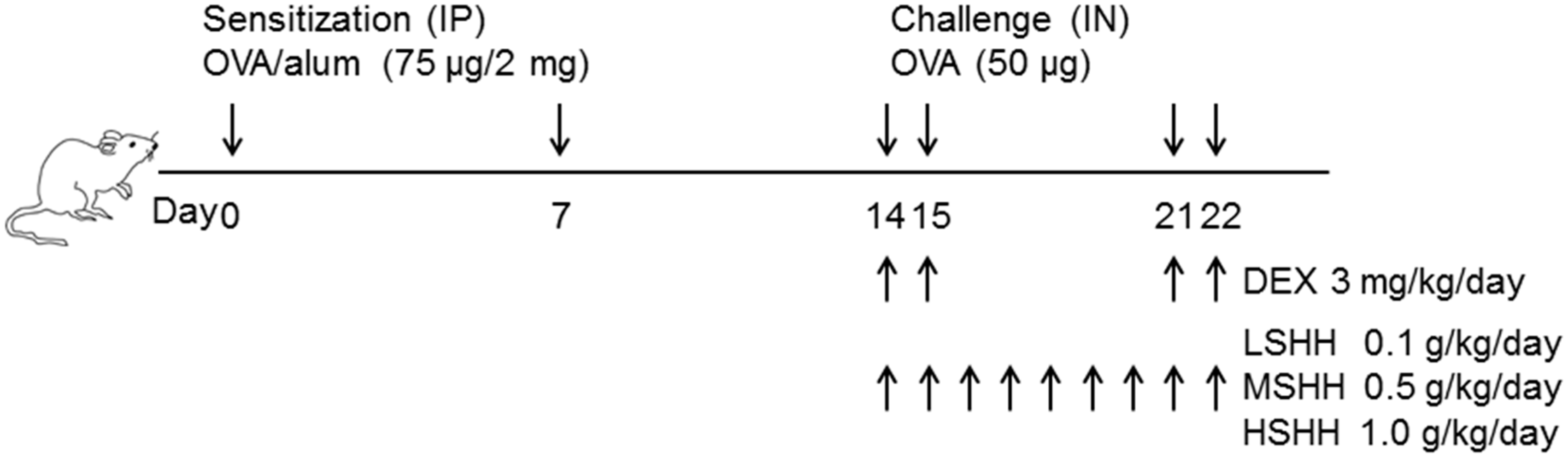
2.5. Experimental Asthma Models and Intervention
2.6. Collection of Bronchoalveolar Lavage Fluid (BALF) and Differential Cell Count
2.7. Hematoxylin and Eosin (H&E) and Periodic Acid–Schiff (PAS) Stains
2.8. Measurement of Total and OVA-Specific Immunoglobulin E (IgE) in Serum
2.9. Immunoassay for T Helper cell Type 2 (Th2) Cytokines, Leukotrienes, and Histamine in BALF
2.10. Culture of Asthmatic Bronchial Smooth Muscle Cells (ABSMCs)
2.11. Cell Viability Assay
2.12. Measurement of Intracellular Ca2+ Levels
2.13. Cell Contraction Assay
2.14. Statistics
3. Results
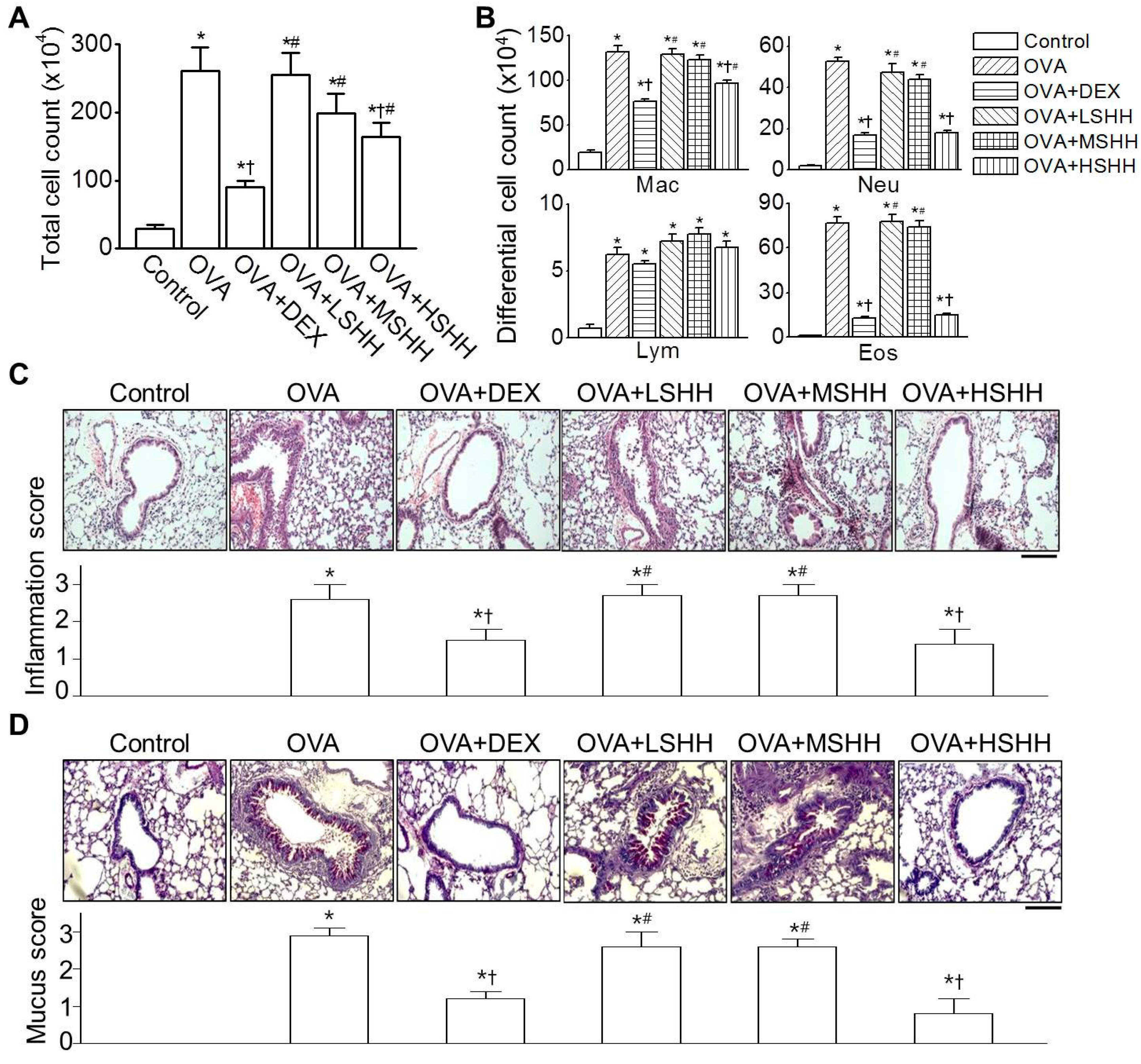
3.1. Alleviation of Ovalbumin (OVA)-Induced Airway Inflammation by High-Dose SHH (HSHH)
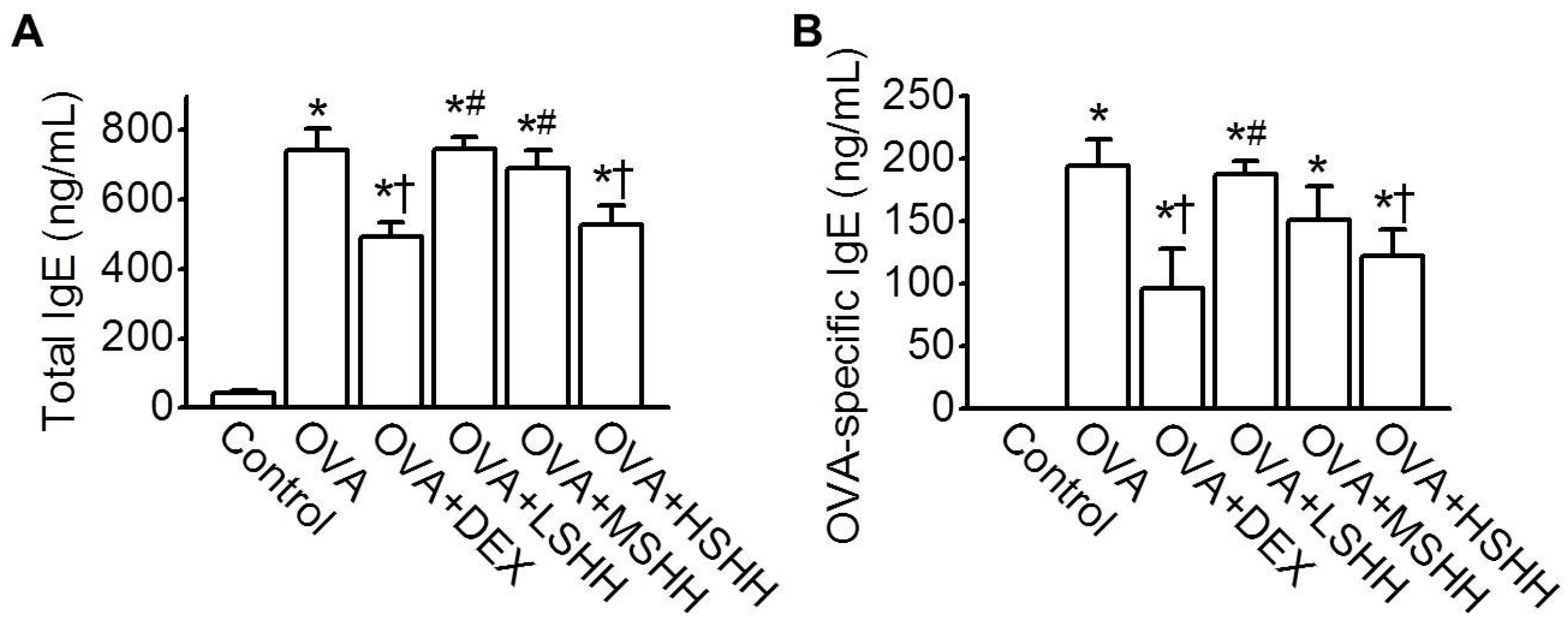
3.2. Inhibition of OVA-Induced Increase in Total and OVA-Specific IgE in Serum by HSHH
3.3. Reduction of OVA-Induced Increase in Th2 Cytokines, Leukotrienes, and Histamine Concentrations in BALF by HSHH
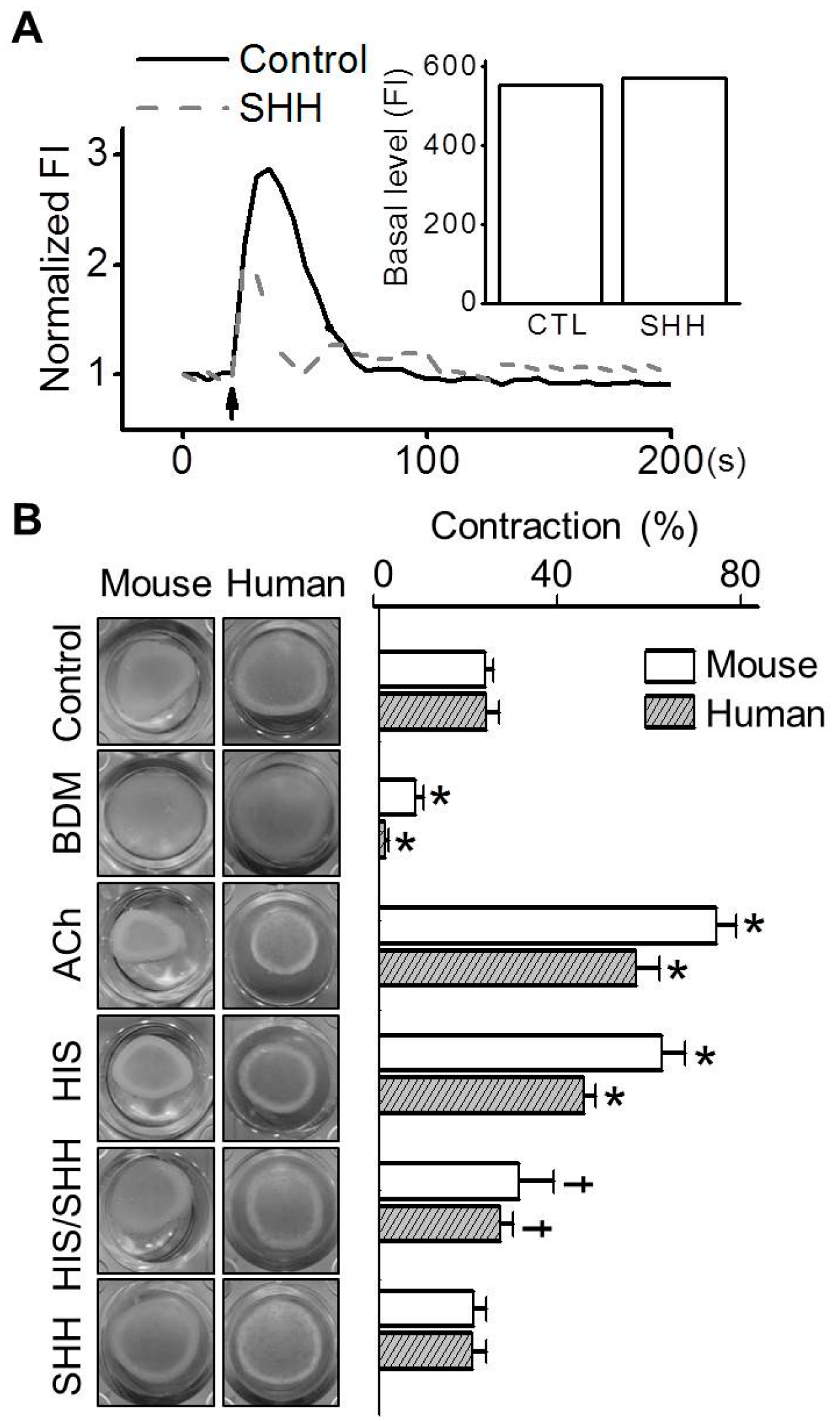
3.4. Inhibition of Histamine-Induced Contraction in ABSMCs by SHH
3.5. No Changes in the Spleen and Thymus by HSHH
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choe, B. Illustrated Encyclopedia of Fauna and Flora of Korea, Mollusca (ii); Ministry of Education: Seoul, Korea, 1992; Volume 33.
- Nishiwaki, S.; Ueda, H.; Makioka, T. Tagging studies on the growth of the sea hare aplysia kurodai on an intertidal rocky shore. Mar. Biol. 1975, 32, 389–395. [Google Scholar] [CrossRef]
- Pereira, R.B.; Andrade, P.B.; Valentão, P. Chemical diversity and biological properties of secondary metabolites from sea hares of aplysia genus. Mar. Drugs 2016, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Kigoshi, H.; Kita, M. Antitumor effects of sea hare-derived compounds in cancer. In Handbook of Anticancer Drugs from Marine Origin; Springer: Cham, Switzerland, 2015; pp. 701–739. [Google Scholar]
- Shin, M.-O. The antioxidative and antimicrobial effects of internal organs of aplysia kurodai fractions. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1433–1438. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Yamashita, Y.; Ohta, T. New cytotoxic and antibacterial compounds isolated from the sea hare, aplysia kurodai. Mar. Drugs 2005, 3, 22–28. [Google Scholar] [CrossRef]
- Ryu, J.H.; Sung, J.; Xie, C.; Shin, M.-K.; Kim, C.-W.; Kim, N.-G.; Choi, Y.J.; Dai Choi, B.; Kang, S.S.; Kang, D. Aplysia kurodai-derived glycosaminoglycans increase the phagocytic ability of macrophages via the activation of amp-activated protein kinase and cytoskeletal reorganization in raw264. 7 cells. J. Funct. Foods 2016, 27, 122–130. [Google Scholar] [CrossRef]
- Ishmael, F.T. The inflammatory response in the pathogenesis of asthma. J. Am. Osteopath. Assoc. 2011, 111, S11–S17. [Google Scholar] [PubMed]
- Maddox, L.; Schwartz, D.A. The pathophysiology of asthma. Annu. Rev. Med. 2002, 53, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H.; Robinson, D.S. The role of eosinophils in airway tissue remodelling in asthma. Curr. Opin. Immunol. 2007, 19, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Burchell, J.T.; Wikstrom, M.E.; Stumbles, P.A.; Sly, P.D.; Turner, D.J. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory t cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L307–L319. [Google Scholar]
- Rosenwasser, L.J.; Boyce, J.A. Mast cells: Beyond ige. J. Allergy Clin. Immunol. 2003, 111, 24–32. [Google Scholar] [CrossRef]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S. The role of the mast cell in asthma: Induction of airway hyperresponsiveness by interaction with smooth muscle? J. Allergy Clin. Immunol. 2004, 114, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.; Hurd, S.; Barnes, P.; Bousquet, J.; Drazen, J.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’byrne, P.; Pedersen, S. Global strategy for asthma management and prevention: Gina executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir. Med. 2006, 100, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Herbert, C.; Foster, P.S. The “classical” ovalbumin challenge model of asthma in mice. Curr. Drug Targets 2008, 9, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.; Sly, P. Animal models of asthma. Clin. Exp. Allergy 2007, 37, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Jeon, S.G.; Lee, C.G.; Oh, M.-H.; Chun, E.-Y.; Gho, Y.S.; Cho, S.-H.; Kim, J.-H.; Min, K.-U.; Kim, Y.-Y.; Kim, Y.-K. Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J. Allergy Clin. Immunol. 2007, 119, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N. Human Anatomy and Physiology, 4th ed.; Benjamin-Cummings Science Publishing: Menlo Park, CA, USA, 1989; Chapter 18; p. 639. [Google Scholar]
- Cho, K.-A.; Suh, J.W.; Sohn, J.H.; Park, J.W.; Lee, H.; Kang, J.L.; Woo, S.-Y.; Cho, Y.J. Il-33 induces th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am. J. Physiology-Lung Cell. Mol. Physiol. 2012, 302, L429–L440. [Google Scholar] [CrossRef] [PubMed]
- Myou, S.; Leff, A.R.; Myo, S.; Boetticher, E.; Tong, J.; Meliton, A.Y.; Liu, J.; Munoz, N.M.; Zhu, X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase–tat. J. Exp. Med. 2003, 198, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.G.; Rennick, D.; Donaldson, D.D.; Venkayya, R.; McArthur, C.; Hansell, E.; Kurup, V.P.; Warnock, M.; Grünig, G. Il-13 and ifn-γ: Interactions in lung inflammation. J. Immunol. 2001, 167, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Yao, Y.; Lu, G.; Wang, Y.; Xia, D.; Zhou, J. Hb-egf–promoted airway smooth muscle cells and their progenitor migration contribute to airway smooth muscle remodeling in asthmatic mouse. J. Immunol. 2016, 196, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jeon, B.; Kim, H.; Roh, G.; Shin, J.H.; Sung, N.J.; Han, J.; Kang, D. Aged red garlic extract reduces lipopolysaccharide-induced nitric oxide production in raw 264.7 macrophages and acute pulmonary inflammation through haeme oxygenase-1 induction. Acta Physiol. 2012, 205, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Park, H.-J.; Cho, Y.-W.; Kim, E.-J.; Lee, J.D.; Kang, K.R.; Han, J.; Kang, D. Cigarette smoke extract-induced reduction in migration and contraction in normal human bronchial smooth muscle cells. Korean J. Physiol. Pharmacol. 2011, 15, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Farman, M.; Najmi, M.H.; Mian, K.B.; Hasan, A. Pharmacological basis for use of pistacia integerrima leaves in hyperuricemia and gout. J. Ethnopharmacol. 2008, 117, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.P.R.; Pinheiro, N.M.; Mernak, M.I.B.; Righetti, R.F.; Martins, M.A.; Lago, J.H.G.; dos Santos Lopes, F.D.T.Q.; Tibério, I.F.L.C.; Prado, C.M. Evidences of herbal medicine-derived natural products effects in inflammatory lung diseases. Mediat. Inflamm. 2016, 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeeny, N.A.; Nader, M.A.; Attia, G.M.; Ateyya, H. Agmatine protects rat liver from nicotine-induced hepatic damage via antioxidative, antiapoptotic, and antifibrotic pathways. Naunyn-Schmiedebergs Arch. Pharmacol. 2016, 389, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.R.; Simes, J.C.; Moya, M.; Soriano, F.; Palma, J.A.; Campana, V. Inflammatory and oxidative stress markers in experimental crystalopathy: Their modification by photostimulation. Photomed. Laser Surg. 2009, 27, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Sathyamurthy, R.; Manney, S.; Webbster, C.; Krishna, M.T.; Mansur, A.H. Biomarkers of oxidative stress and antioxidants in severe asthma: A prospective case-control study. Ann. Allergy. Asthma Immunol. 2017, 118, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Choi, B.; Choi, Y. Extraction of glycosaminoglycan from sea hare, aplysia kurodai, and its functional properties 1. Optimum extraction of polysaccharide and purification of glycosaminoglycan. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1640–1646. [Google Scholar] [CrossRef]
- Miyamoto, T.; Higuchi, R.; Komori, T.; Fujioka, T.; Mihashi, K. Isolation and structures of aplykurodins a and b, two new isoprenoids from the marine mollusk aplysia kurodai. Tetrahedron Lett. 1986, 27, 1153–1156. [Google Scholar] [CrossRef]
- Miyamoto, T.; Higuchi, R.; Marubayashi, N.; Komori, T. Studies on the constituents of marine opisthobranchia, iv. Two new polyhalogenated monoterpenes from the sea hare aplysia kurodai. Liebigs Ann. Chem. 1988, 1988, 1191–1193. [Google Scholar]
- Ojika, M.; Yoshida, Y.; Okumura, M.; Ieda, S.; Yamada, K. Aplysiadiol, a new brominated diterpene from the marine mollusc aplysia kurodai. J. Nat. Prod. 1990, 53, 1619–1622. [Google Scholar] [CrossRef]
- Kigoshi, H.; Imamura, Y.; Yoshikawa, K.; Yamada, K. Three new cytotoxic alkaloids, aplaminone, neoaplaminone and neoaplaminone sulfate from the marine mollusc aplysia kurodai. Tetrahedron Lett. 1990, 31, 4911–4914. [Google Scholar] [CrossRef]
- Lever, R.; Page, C. Glycosaminoglycans, airways inflammation and bronchial hyperresponsiveness. Pulm. Pharmacol. Ther. 2001, 14, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Simonaro, C.M.; D’Angelo, M.; He, X.; Eliyahu, E.; Shtraizent, N.; Haskins, M.E.; Schuchman, E.H. Mechanism of glycosaminoglycan-mediated bone and joint disease: Implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008, 172, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Adage, T.; del Bene, F.; Fiorentini, F.; Doornbos, R.P.; Zankl, C.; Bartley, M.R.; Kungl, A.J. Pa401, a novel cxcl8-based biologic therapeutic with increased glycosaminoglycan binding, reduces bronchoalveolar lavage neutrophils and systemic inflammatory markers in a murine model of lps-induced lung inflammation. Cytokine 2015, 76, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Blake, R.J.; Garcia-Caraballo, S.; Gibson, P.G. Airway and circulating levels of carotenoids in asthma and healthy controls. J. Am. Coll. Nutr. 2005, 24, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Chang, J.-H.; Moon, D.-O.; Choi, Y.H.; Choi, I.-W.; Park, Y.-M.; Kim, G.-Y. Lycopene suppresses ovalbumin-induced airway inflammation in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008, 374, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhang, X.-G.; Wu, J.; Xu, W.-C.; Li, L.-Q.; Liu, F.; Yu, J.-E. Effect of treatment with geraniol on ovalbumin-induced allergic asthma in mice. Ann. Allergy. Asthma Immunol. 2016, 116, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Khan, H. Alkaloids: Potential therapeutic modality in the management of asthma. J. Ayurvedic Herb. Med. 2015, 1, 3. [Google Scholar]
- Premprasert, C.; Tewtrakul, S.; Plubrukarn, A.; Wungsintaweekul, J. Anti-inflammatory activity of diterpenes from croton stellatopilosus on lps-induced raw264.7 cells. J. Nat. Med. 2013, 67, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, V.; Giridhar, P. Antioxidant potential of free diterpenes cafestol and kahweol rich extractives of coffee beans. 2015. [Google Scholar]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Cho, Y.-S.; Je, J.-Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Bae, I.Y.; Lee, S.; Lee, D.-H.; Hur, B.-S.; Lee, H.G. Evaluation of wheat gluten hydrolysates as taste-active compounds with antioxidant activity. J. Food Sci. Technol. 2014, 51, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M.; Karp, C.L. Eosinophils in asthma: Remodeling a tangled tale. Science 2004, 305, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, B.K.; seok Kim, J.; Lee, J.-W.; Park, H.A.; Ryu, H.W.; Lee, S.U.; Hwang, K.W.; Yun, W.-K.; Kim, H.-C. Picroside ii attenuates airway inflammation by downregulating the transcription factor gata3 and th2-related cytokines in a mouse model of hdm-induced allergic asthma. PLoS ONE 2016, 11, e0167098. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role played by th2 type cytokines in ige mediated allergy and asthma. Lung India 2010, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. Ige and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Hollins, F.; Woodman, L.; Yang, W.; Monk, P.; May, R.; Bradding, P.; Brightling, C. Mast cells express il-13rα1: Il-13 promotes human lung mast cell proliferation and fcεri expression. Allergy 2006, 61, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Erle, D.J.; Sheppard, D. The cell biology of asthma. J Cell Biol. 2014, 205, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Hauber, H.-P.; Lavigne, F.; Hung, H.-L.; Levitt, R.C.; Hamid, Q. Effect of th2 type cytokines on hclca1 and mucus expression in cystic fibrosis airways. J. Cyst. Fibros. 2010, 9, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, B.; Knobloch, J.; Schulz, V.M.; Wecker, C.; Schlimm, M.; Scholz, P.; Jansen, F.; Stoelben, E.; Philippou, S.; Hecker, E. Olfactory receptors modulate physiological processes in human airway smooth muscle cells. Front. Physiol. 2016, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, V.; Hapgood, K.; McVicker, C.; Lee, T.; Ward, J. Mechanisms of leukotriene d4-induced constriction in human small bronchioles. Br. J. Pharmacol. 2001, 133, 243–252. [Google Scholar] [CrossRef] [PubMed]
- West, A.R.; Syyong, H.T.; Siddiqui, S.; Pascoe, C.D.; Murphy, T.M.; Maarsingh, H.; Deng, L.; Maksym, G.N.; Bossé, Y. Airway contractility and remodeling: Links to asthma symptoms. Pulm. Pharmacol. Ther. 2013, 26, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pauly, T.; Thiel, H.; Saalm, A. Infection with classical swine fever virus: Effects on phenotype and immune responsiveness of porcine t lymphocytes. J. Gen. Virol. 1998, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xie, Y.; Depierre, J. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol. 2000, 122, 219–226. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.H.; Xie, C.; Kim, E.-J.; Park, S.-H.; Choi, Y.J.; Kang, S.S.; Shin, M.-K.; Kang, D. Reduction of Asthmatic Parameters by Sea Hare Hydrolysates in a Mouse Model of Allergic Asthma. Nutrients 2017, 9, 699. https://doi.org/10.3390/nu9070699
Ryu JH, Xie C, Kim E-J, Park S-H, Choi YJ, Kang SS, Shin M-K, Kang D. Reduction of Asthmatic Parameters by Sea Hare Hydrolysates in a Mouse Model of Allergic Asthma. Nutrients. 2017; 9(7):699. https://doi.org/10.3390/nu9070699
Chicago/Turabian StyleRyu, Ji Hyeon, Chengliang Xie, Eun-Jin Kim, Si-Hyang Park, Yeung Joon Choi, Sang Soo Kang, Min-Kyoung Shin, and Dawon Kang. 2017. "Reduction of Asthmatic Parameters by Sea Hare Hydrolysates in a Mouse Model of Allergic Asthma" Nutrients 9, no. 7: 699. https://doi.org/10.3390/nu9070699





