Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Intervention
Nutritional Composition of Each Intervention Arm
- Control conditions:This consisted of 350 mL of water served at room temperature.
- High GI (HGI) breakfast:This consisted of 350 mL of water served at room temperature, 200 mL of grape juice (with 569 KJ/136 Kcal), 40 g of white bread (2 slices of 218 KJ/52 Kcal each) and 29 g of strawberry jam (with 313 KJ/75 Kcal) with an overall GI of 64.0.
- Low GI (LGI) breakfast:This consisted of 350 mL of water served at room temperature, a 150 g apple (with 339 KJ/81 Kcal), 125 g low-fat natural yogurt (with 234 KJ/56 Kcal), 3 shelled walnuts (with 163 KJ/39 Kcal per unit), and 17.5 g of 72% dark chocolate (with 427 KJ/102 Kcal) with an overall GI of 29.4.
2.3. Study Protocol
2.4. Outcomes
2.5. Variables and Measurement Instruments
2.5.1. Vascular Function Evaluation Variables
2.5.2. Laboratory Variables
2.5.3. Other Variables
2.6. Ethics
2.7. Statistics
3. Results
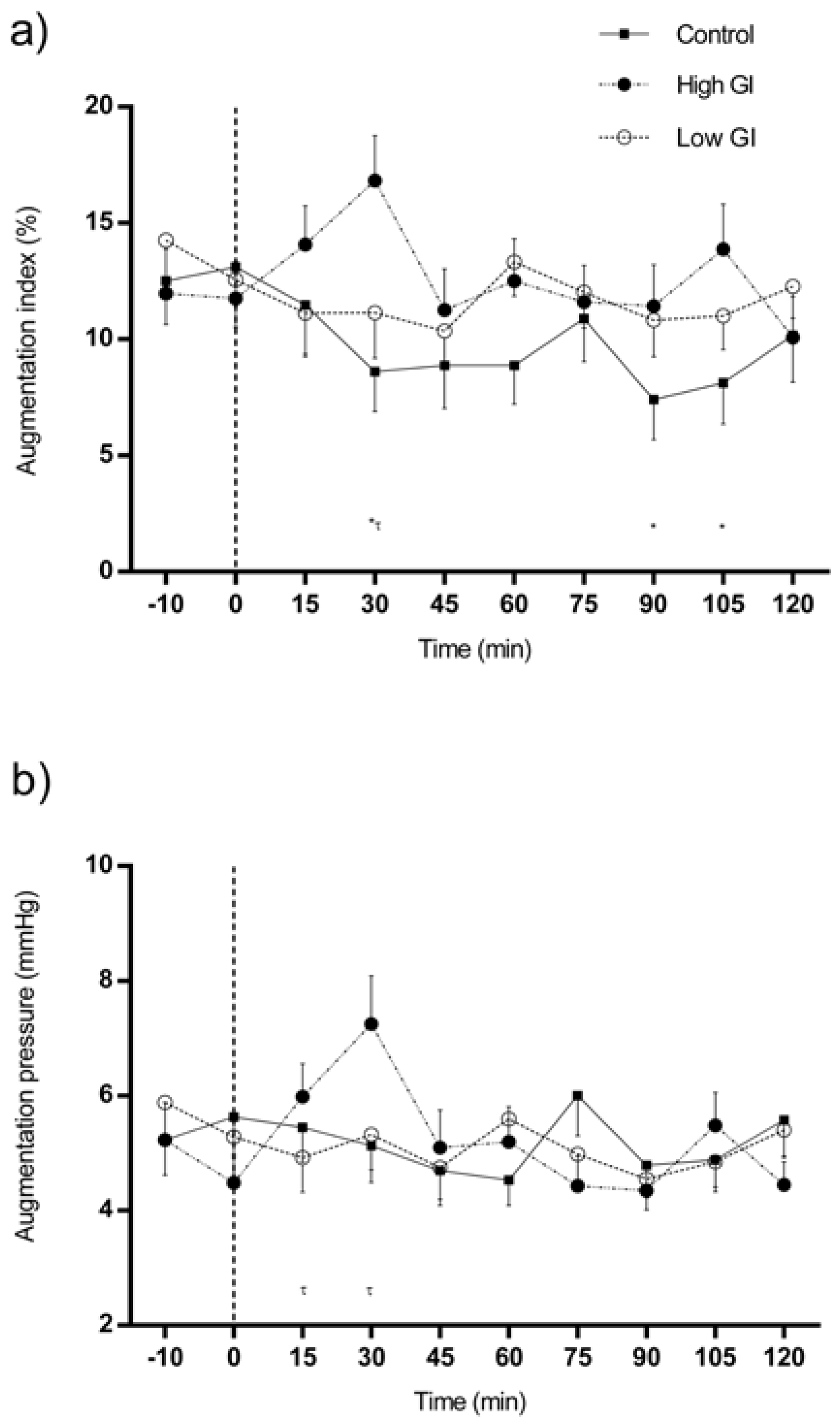
3.1. Changes in Central Hemodynamics in Response to Each Type of Breakfast
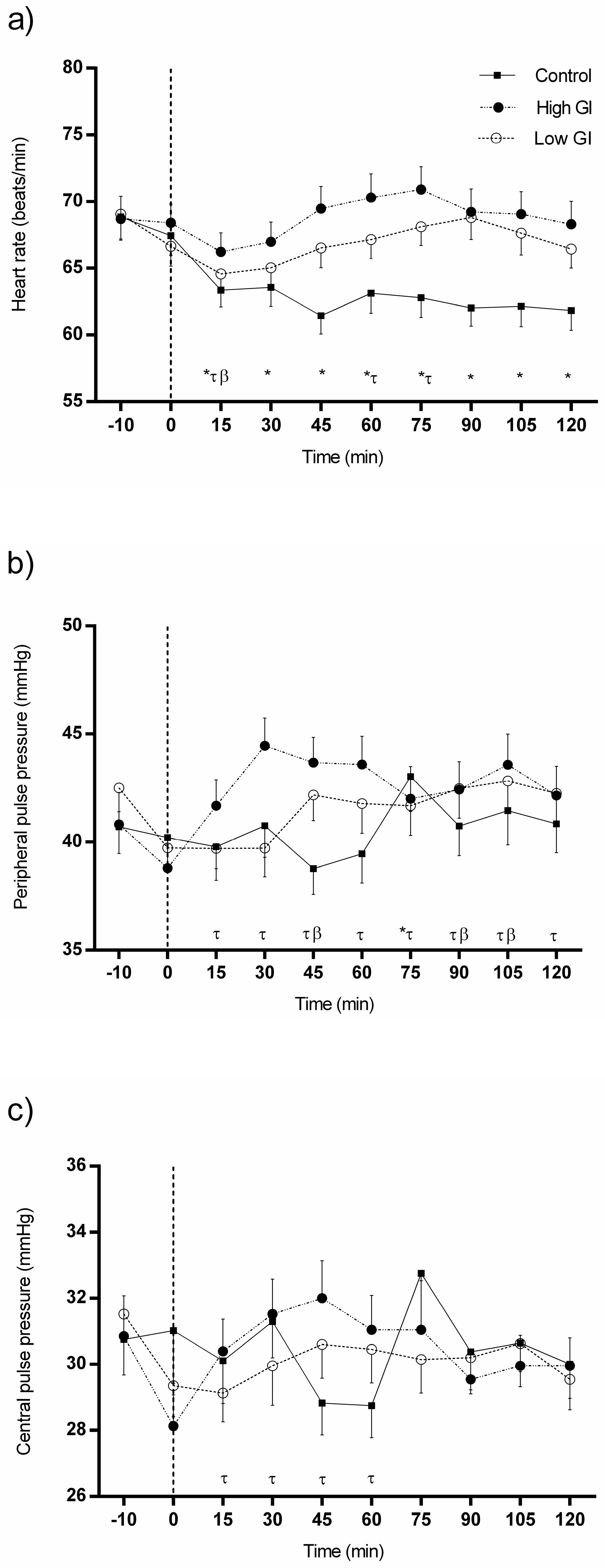
3.2. Changes in Heart Rate and Pulse Pressures in Response to Each Type of Breakfast
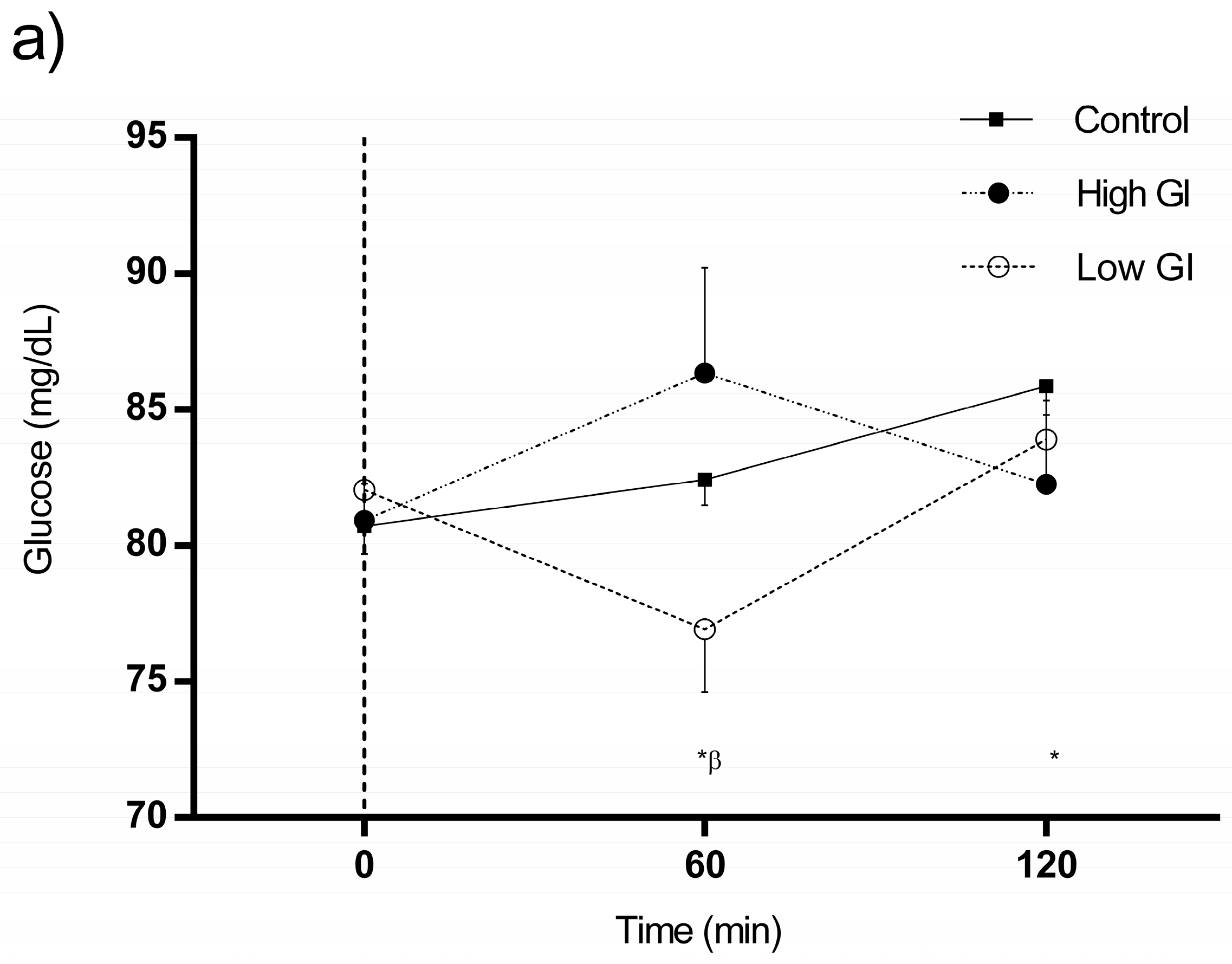
3.3. Changes in Glucose and Insulin in Response to Each Type of Breakfast
3.4. Comparisons between Postprandial Responses to Each Type of Breakfast
3.5. Effects of Type of Breakfast on Postprandial Responses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; O’Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Mattace-Raso, F.U.; van der Cammen, T.J.; Hofman, A.; van Popele, N.M.; Bos, M.L.; Schalekamp, M.A.; Asmar, R.; Reneman, R.S.; Hoeks, A.P.; Breteler, M.M.; et al. Arterial stiffness and risk of coronary heart disease and stroke: The rotterdam study. Circulation 2006, 113, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Lacy, P.S.; Thom, S.M.; Cruickshank, K.; Stanton, A.; Collier, D.; Hughes, A.D.; Thurston, H.; O’Rourke, M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the conduit artery function evaluation (cafe) study. Circulation 2006, 113, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, E.; Mancia, G.; O’Rourke, M.F.; Roman, M.J.; Safar, M.E.; Smulyan, H.; Wang, J.G.; Wilkinson, I.B.; Williams, B.; Vlachopoulos, C. Central blood pressure measurements and antihypertensive therapy: A consensus document. Hypertension 2007, 50, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bodlaj, G.; Berg, J.; Biesenbach, G. Diurnal variation of arterial stiffness and subendocardial perfusion noninvasively assessed using applanation tonometry in healthy young men. Wiener Klin. Wochenschr. 2005, 117, 348–352. [Google Scholar] [CrossRef]
- Kollias, G.E.; Stamatelopoulos, K.S.; Papaioannou, T.G.; Zakopoulos, N.A.; Alevizaki, M.; Alexopoulos, G.P.; Kontoyannis, D.A.; Karga, H.; Koroboki, E.; Lekakis, J.P.; et al. Diurnal variation of endothelial function and arterial stiffness in hypertension. J. Hum. Hypertens. 2009, 23, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. Atherogenesis: A postprandial phenomenon. Circulation 1979, 60, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Curry, T.B.; Matzek, L.J.; Joyner, M.J.; Casey, D.P. Acute effects of a mixed meal on arterial stiffness and central hemodynamics in healthy adults. Am. J. Hypertens. 2014, 27, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Akbari, C.M.; Saouaf, R.; Barnhill, D.F.; Newman, P.A.; LoGerfo, F.W.; Veves, A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J. Vasc. Surg. 1998, 28, 687–694. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Baron, A.D.; Brechtel, G. Insulin differentially regulates systemic and skeletal muscle vascular resistance. Am. J. Physiol. 1993, 265, E61–E67. [Google Scholar] [PubMed]
- Dandona, P.; Chaudhuri, A.; Ghanim, H.; Mohanty, P. Insulin as an anti-inflammatory and antiatherogenic modulator. J. Am. Coll. Cardiol. 2009, 53, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.M.; Joy, N.G.; Tate, D.B.; Davis, S.N. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E168–E176. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Arif, H.; Villareal, D.T.; Marzetti, E.; Holloszy, J.O. Endothelial function after high-sugar-food ingestion improves with endurance exercise performed on the previous day. Am. J. Clin. Nutr. 2008, 88, 51–57. [Google Scholar] [PubMed]
- Malin, S.K.; Rynders, C.A.; Weltman, J.Y.; Jackson Roberts, L., 2nd; Barrett, E.J.; Weltman, A. Endothelial function following glucose ingestion in adults with prediabetes: Role of exercise intensity. Obesity 2016, 24, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [PubMed]
- Ma, X.Y.; Liu, J.P.; Song, Z.Y. Glycemic load, glycemic index and risk of cardiovascular diseases: Meta-analyses of prospective studies. Atherosclerosis 2012, 223, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Radulian, G.; Rusu, E.; Dragomir, A.; Posea, M. Metabolic effects of low glycaemic index diets. Nutr. J. 2009, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [PubMed]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Rodrigo-De Pablo, E.; Cabrejas-Sanchez, A.; Arietaleanizbeaskoa, M.S.; Repiso-Gento, I.; Gonzalez-Viejo, N.; Maderuelo-Fernandez, J.A.; Agudo-Conde, C.; et al. Glycemic index, glycemic load, and pulse wave reflection in adults. Nutr. Metab. Cardiovasc. Dis. 2014, 25, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.; Harrington, D.; Starkey, S.; Williams, P.; Hampton, S. Diurnal postprandial responses to low and high glycaemic index mixed meals. Clin. Nutr. 2014, 33, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Ibero-Baraibar, I.; Cuervo, M.; Navas-Carretero, S.; Abete, I.; Zulet, M.A.; Martinez, J.A. Different postprandial acute response in healthy subjects to three strawberry jams varying in carbohydrate and antioxidant content: A randomized, crossover trial. Eur. J. Nutr. 2014, 53, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.; Chiavaroli, L.; Campbell, J.; Ezatagha, A.; Jenkins, A.L.; Esfahani, A.; Kendall, C.W. Post-prandial glucose and insulin responses of hummus alone or combined with a carbohydrate food: A dose-response study. Nutr. J. 2016, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Samaras, K.; Chisholm, D.J.; Campbell, L.V. Effect of postprandial insulinemia and insulin resistance on measurement of arterial stiffness (augmentation index). Int. J. Cardiol. 2007, 114, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Chen, R.; Horowitz, M.; Chapman, I. Blood pressure responses in healthy older people to 50 g carbohydrate drinks with differing glycaemic effects. Br. J. Nutr. 2004, 92, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lavi, T.; Karasik, A.; Koren-Morag, N.; Kanety, H.; Feinberg, M.S.; Shechter, M. The acute effect of various glycemic index dietary carbohydrates on endothelial function in nondiabetic overweight and obese subjects. J. Am. Coll. Cardiol. 2009, 53, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Kahlhofer, J.; Peter, A.; Bosy-Westphal, A. Effects of low versus high glycemic index sugar-sweetened beverages on postprandial vasodilatation and inactivity-induced impairment of glucose metabolism in healthy men. Nutrients 2016, 8, 802. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, R.; Di Castelnuovo, A.; Melegari, C.; De Lucia, F.; Santimone, I.; Sciarretta, A.; Barisciano, P.; Persichillo, M.; De Curtis, A.; Zito, F.; et al. Typical breakfast food consumption and risk factors for cardiovascular disease in a large sample of italian adults. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, P.; Guo, H.; Ling, W. Taking a low glycemic index multi-nutrient supplement as breakfast improves glycemic control in patients with type 2 diabetes mellitus: A randomized controlled trial. Nutrients 2014, 6, 5740–5755. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Lim, S.; Egger, G. The effect of a low glycaemic index breakfast on blood glucose, insulin, lipid profiles, blood pressure, body weight, body composition and satiety in obese and overweight individuals: A pilot study. J. Am. Coll. Nutr. 2008, 27, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Robertson, I.K.; Ball, M.J. Acute effects of food on postprandial blood pressure and measures of arterial stiffness in healthy humans. Am. J. Clin. Nutr. 2009, 90, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.; Yang, E.Y.; Brunner, G.; Kumar, A.; Lakkis, N.; Misra, A.; Virani, S.S.; Hartley, C.J.; Morrisett, J.D.; Ballantyne, C.M.; et al. Postprandial effects on arterial stiffness parameters in healthy young adults. Vasc. Med. 2015, 20, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aguadero, N.; Garcia-Ortiz, L.; Patino-Alonso, M.C.; Mora-Simon, S.; Gomez-Marcos, M.A.; Alonso-Dominguez, R.; Sanchez-Salgado, B.; Recio-Rodriguez, J.I. Postprandial effect of breakfast glycaemic index on vascular function, glycaemic control and cognitive performance (bgi study): Study protocol for a randomised crossover trial. Trials 2016, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.; Gohlisch, C.; Harsch-Gladisch, C.; Tolle, M.; Zidek, W.; van der Giet, M. Oscillometric estimation of central blood pressure: Validation of the mobil-o-graph in comparison with the sphygmocor device. Blood Press Monit. 2012, 17, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; West, S.G.; Augustin, L.S.; Esfahani, A.; Vidgen, E.; Bashyam, B.; Sauder, K.A.; Campbell, J.; Chiavaroli, L.; Jenkins, A.L.; et al. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Gordin, D.; Saraheimo, M.; Tuomikangas, J.; Soro-Paavonen, A.; Forsblom, C.; Paavonen, K.; Steckel-Hamann, B.; Vandenhende, F.; Nicolaou, L.; Pavo, I.; et al. Influence of postprandial hyperglycemic conditions on arterial stiffness in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Matheson, P.J.; Wilson, M.A.; Garrison, R.N. Regulation of intestinal blood flow. J. Surg. Res. 2000, 93, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Szinnai, C.; Mottet, C.; Gutzwiller, J.P.; Drewe, J.; Beglinger, C.; Sieber, C.C. Role of gender upon basal and postprandial systemic and splanchnic haemodynamics in humans. Scand. J. Gastroenterol. 2001, 36, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Marchesi, S.; Siepi, D.; Lupattelli, G.; Vaudo, G.; Pasqualini, L.; Mannarino, E. Gender differences in postprandial endothelial function. Am. J. Cardiol. 2001, 87, 1323–1325. [Google Scholar] [CrossRef]
- Adkisson, E.J.; Casey, D.P.; Beck, D.T.; Gurovich, A.N.; Martin, J.S.; Braith, R.W. Central, peripheral and resistance arterial reactivity: Fluctuates during the phases of the menstrual cycle. Exp. Biol. Med. 2010, 235, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [PubMed]

| Type of Breakfast | GI, % | Energy, Kcal/KJ | Carbohydrates, g (%) | Protein, g (%) | Fat, g (%) | Saturated Fat a, g | Fiber, g (%) | Soluble Fiber b, g |
|---|---|---|---|---|---|---|---|---|
| Control conditions | - | - | - | - | - | - | - | - |
| High GI | 64.0 | 315/1318 | 72.0 (91.4) | 3.9 (5.0) | 0.9 (2.6) | 0.29 | 1.6 (1.0) | 0.36 |
| Low GI | 29.4 | 356/1489 | 31.5 (35.4) | 9.7 (10.9) | 19.9 (50.3) | 1.54 | 6.0 (3.4) | 1.57 |
| Global (N = 40) Mean or n/SD or % | Males (n = 20) Mean or n/SD or % | Females (n = 20) Mean or n/SD or % | |
|---|---|---|---|
| Age (years) | 28.1 (6.3) | 28.7 (6.8) | 27.4 (5.9) |
| Diet Quality Index, DQI (total score) | 39.5 (2.8) | 38.4 (2.6) | 40.6 (2.5) |
| Physical Activity (METS/min/week) | 1973.1 (1239.5) | 2448.0 (1290.4) | 1498.2 (1006.8) |
| Current smoker (n, %) | 3 (7.5) | 2 (10.0) | 1 (5.0) |
| Alcohol consumption (g/week) | 40.8 (48.1) | 52.5 (55.9) | 29.0 (36.5) |
| Systolic blood pressure (mmHg) | 106.4 (9.8) | 112.0 (7.5) | 100.7 (8.5) |
| Diastolic blood pressure (mmHg) | 66.2 (6.9) | 67.1 (6.9) | 65.4 (6.9) |
| Heart rate (bpm) | 70.2 (12.5) | 65.7 (11.3) | 74.8 (12.2) |
| Body mass index (kg/m2) | 23.5 (3.6) | 24.5 (3.1) | 22.5 (3.9) |
| Waist circumference (cm) | 78.8 (10.1) | 84.4 (8.5) | 73.2 (8.4) |
| Hip circumference (cm) | 102.2 (7.5) | 103.9 (7.1) | 100.4 (7.7) |
| Total cholesterol (mg/dL) | 169.2 (29.4) | 168.7 (30.4) | 169.8 (29.0) |
| HDL-cholesterol (mg/dL) | 61.9 (15.3) | 54.5 (12.2) | 69.3 (14.8) |
| LDL-cholesterol (mg/dL) | 92.5 (27.5) | 97.9 (28.6) | 87.1 (25.9) |
| Triglycerides (mg/dL) | 74.3 (31.2) | 81.3 (33.6) | 67.2 (27.6) |
| Creatinine (mg/dL) | 0.76 (0.19) | 0.88 (0.17) | 0.64 (0.12) |
| Augmentation index (AIx), % | 0 min-AIx | 30 min-AIx | 60 min-AIx | 120 min-AIx | ||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | Mean | SD | p | |
| Control conditions | 13.10 | 10.67 | 0.864 | 8.60 | 10.87 | 0.008 | 8.88 | 10.60 | 0.139 | 10.18 | 12.75 | 0.599 |
| HGI breakfast | 11.75 | 9.96 | 16.83 ¥ | 12.20 | 12.50 | 11.60 | 10.08 | 11.07 | ||||
| LGI breakfast | 12.55 | 12.81 | 11.15 | 12.42 | 13.33 | 9.38 | 12.28 | 8.65 | ||||
| Augmentation pressure (AP), mmHg | 0 min-AP | 30 min-AP | 60 min-AP | 120 min-AP | ||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | Mean | SD | p | |
| Control conditions | 5.63 | 4.49 | 0.417 | 5.13 | 4.10 | 0.069 | 4.53 | 2.81 | 0.355 | 5.58 | 4.22 | 0.265 |
| HGI breakfast | 4.48 | 3.11 | 7.25 | 5.32 | 5.20 | 3.92 | 4.45 | 2.58 | ||||
| LGI breakfast | 5.28 | 4.18 | 5.33 | 3.90 | 5.60 | 3.26 | 5.40 | 2.87 | ||||
| Heart rate (HR), bpm | 0 min-HR | 30 min-HR | 60 min-HR | 120 min-HR | ||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | Mean | SD | p | |
| Control conditions | 67.45 | 9.28 | 0.696 | 63.58 | 8.94 | 0.249 | 63.13 | 9.50 | 0.007 | 61.85 | 9.50 | 0.013 |
| HGI breakfast | 68.40 | 8.85 | 66.98 | 9.36 | 70.30 ¥ | 11.19 | 68.30 ¥ | 10.86 | ||||
| LGI breakfast | 66.65 | 9.43 | 65.03 | 9.01 | 67.15 | 9.04 | 66.45 | 9.16 | ||||
| Peripheral pulse pressure (PPP), mmHg | 0 min-PPP | 30 min-PPP | 60 min-PPP | 120 min-PPP | ||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | Mean | SD | p | |
| Control conditions | 40.20 | 8.57 | 0.678 | 40.75 | 9.26 | 0.040 | 39.45 | 8.50 | 0.094 | 40.85 | 8.50 | 0.721 |
| HGI breakfast | 38.80 | 7.01 | 44.45 # | 8.17 | 43.60 | 8.19 | 42.15 | 8.60 | ||||
| LGI breakfast | 39.73 | 5.76 | 39.73 | 8.43 | 41.78 | 8.68 | 42.25 | 8.78 | ||||
| Central pulse pressure (CPP), mmHg | 0 min-CPP | 30 min-CPP | 60 min-CPP | 120 min-CPP | ||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | Mean | SD | p | |
| Control conditions | 31.03 | 10.05 | 0.232 | 31.30 | 6.91 | 0.558 | 28.75 | 6.13 | 0.252 | 30.00 | 6.53 | 0.912 |
| HGI breakfast | 28.13 | 6.03 | 31.53 | 6.70 | 31.05 | 6.60 | 29.48 | 5.35 | ||||
| LGI breakfast | 29.35 | 5.87 | 29.95 | 7.49 | 30.45 | 6.41 | 29.55 | 5.84 | ||||
| Glucose, mg/dL | 0 min-Glucose | 60 min-Glucose | 120 min-Glucose | |||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | ||||
| Control conditions | 80.73 | 6.54 | 0.702 | 82.43 | 5.99 | 0.045 | 85.88 | 6.80 | 0.462 | |||
| HGI breakfast | 80.93 | 8.70 | 86.35# | 24.43 | 82.28 | 19.46 | ||||||
| LGI breakfast | 82.05 | 7.36 | 76.93 | 14.65 | 83.90 | 8.76 | ||||||
| Insulin, mg/dL | 0 min-Insulin | 60 min-Insulin | 120 min-Insulin | |||||||||
| Mean | SD | p | Mean | SD | p | Mean | SD | p | ||||
| Control conditions | 5.55 | 3.68 | 0.858 | 5.00 | 3.44 | <0.001 | 4.54 | 3.31 | <0.001 | |||
| HGI breakfast | 5.96 | 4.54 | 35.98 ¥ # | 26.71 | 18.72 ¥ # | 12.76 | ||||||
| LGI breakfast | 5.51 | 3.77 | 16.60 ¥ | 12.58 | 9.80 ¥ | 6.31 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Aguadero, N.; Patino-Alonso, M.C.; Mora-Simon, S.; Gomez-Marcos, M.A.; Alonso-Dominguez, R.; Sanchez-Salgado, B.; Recio-Rodriguez, J.I.; Garcia-Ortiz, L. Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial. Nutrients 2017, 9, 712. https://doi.org/10.3390/nu9070712
Sanchez-Aguadero N, Patino-Alonso MC, Mora-Simon S, Gomez-Marcos MA, Alonso-Dominguez R, Sanchez-Salgado B, Recio-Rodriguez JI, Garcia-Ortiz L. Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial. Nutrients. 2017; 9(7):712. https://doi.org/10.3390/nu9070712
Chicago/Turabian StyleSanchez-Aguadero, Natalia, Maria C. Patino-Alonso, Sara Mora-Simon, Manuel A. Gomez-Marcos, Rosario Alonso-Dominguez, Benigna Sanchez-Salgado, Jose I. Recio-Rodriguez, and Luis Garcia-Ortiz. 2017. "Postprandial Effects of Breakfast Glycemic Index on Vascular Function among Young Healthy Adults: A Crossover Clinical Trial" Nutrients 9, no. 7: 712. https://doi.org/10.3390/nu9070712






