Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women
Abstract
:1. Introduction
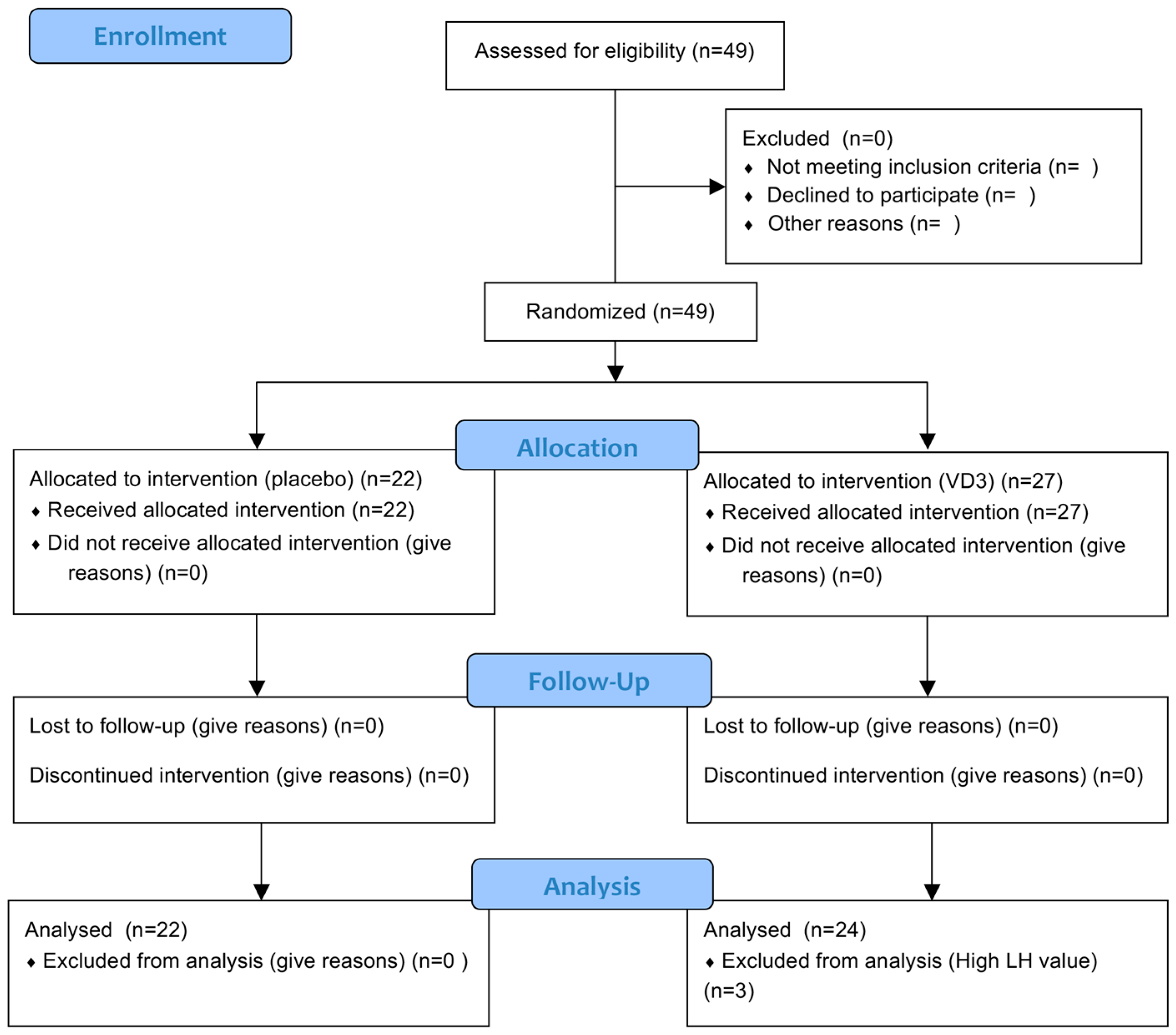
2. Materials and Methods
2.1. Study Participants
2.2. Vitamin D Administration
2.3. Blood Sampling and Assays
2.4. Statistical Analysis
3. Results
3.1. Participant Baseline Characteristics
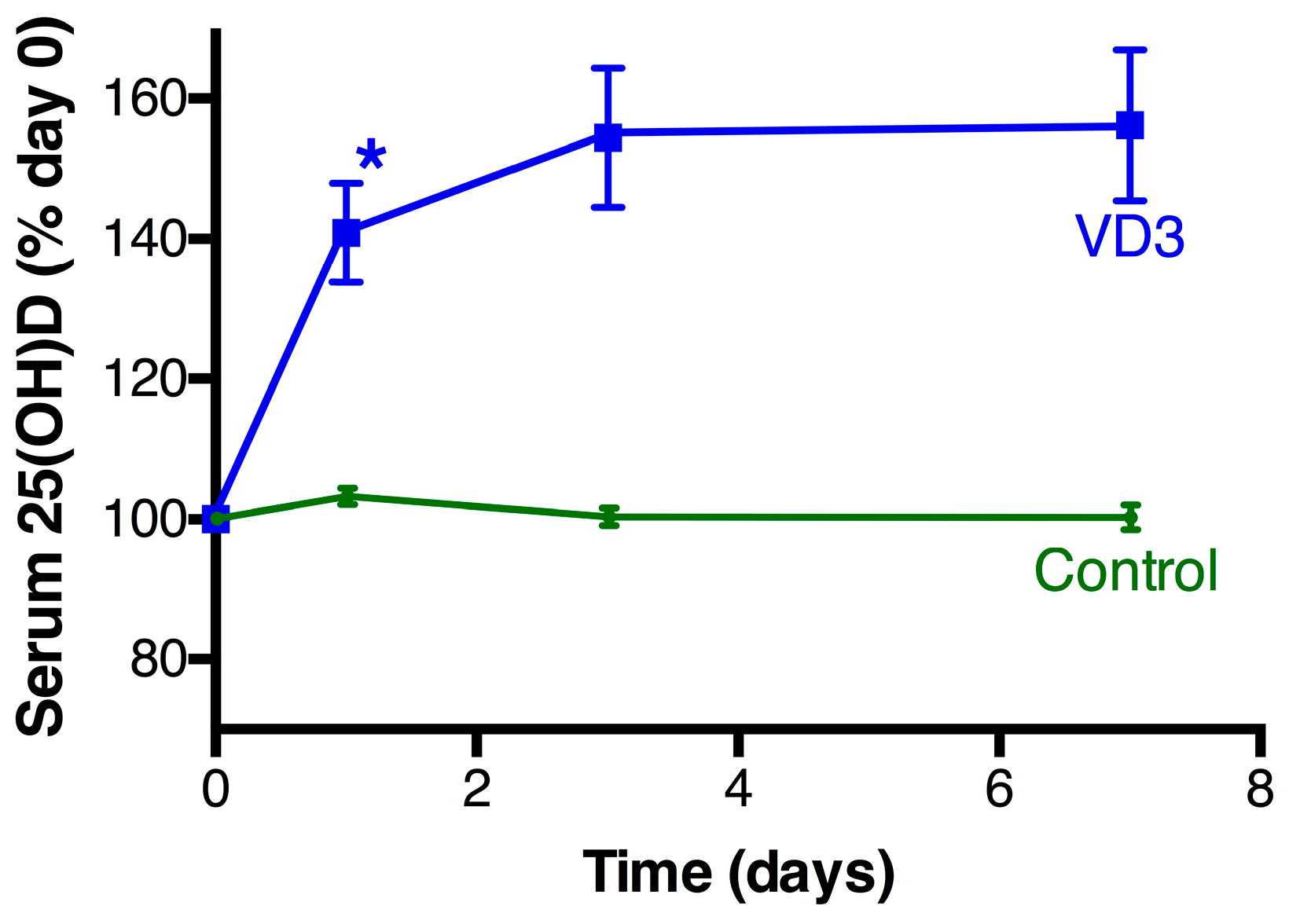
3.2. Vitamin D Changes Following Treatment
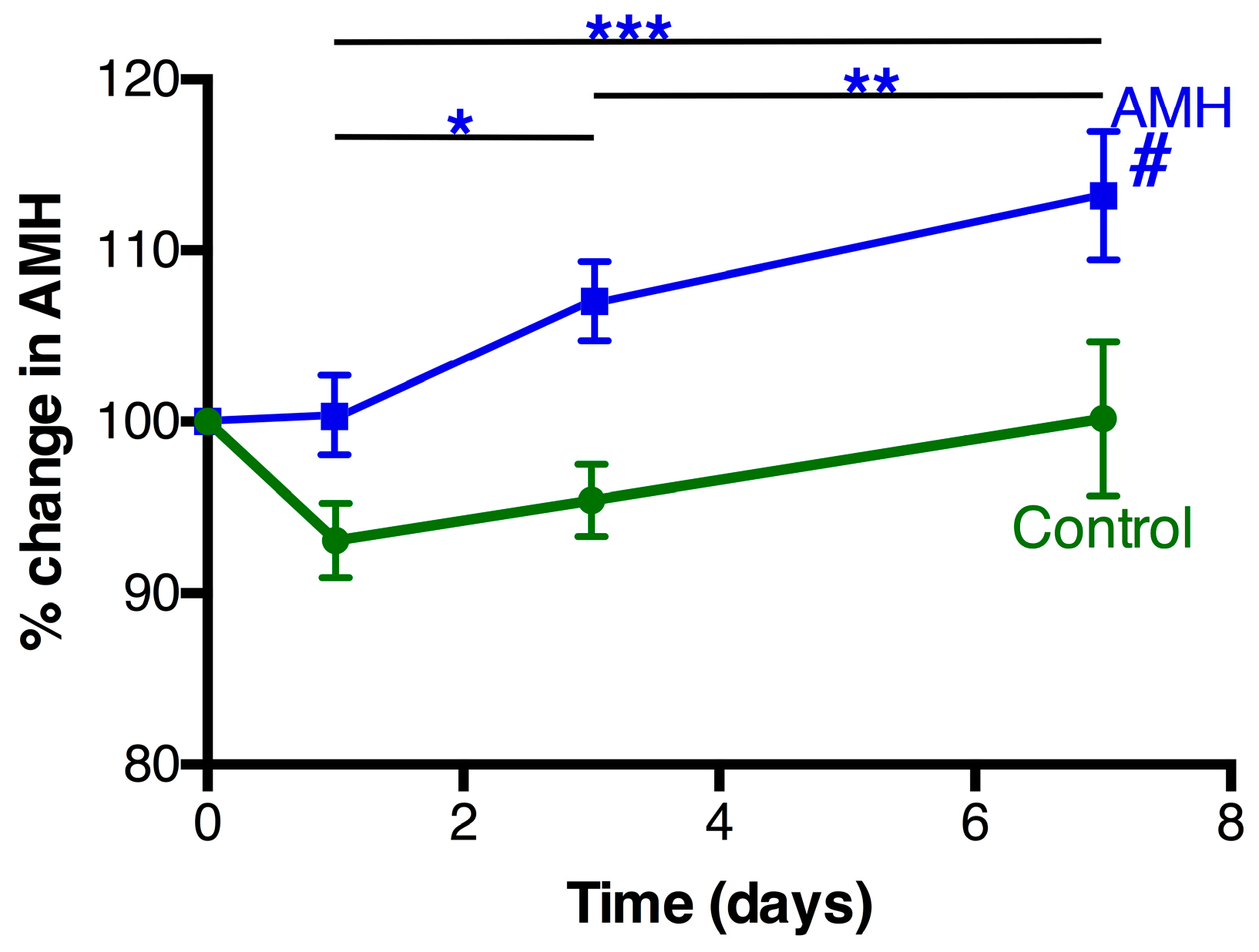
3.3. AMH Changes Following Treatment
3.4. Additional Factors
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luk, J.; Torrealday, S.; Neal Perry, G.; Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 2012, 27, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468. [Google Scholar] [CrossRef] [PubMed]
- McLennan, I.S.; Pankhurst, M.W. Anti-Mullerian hormone is a gonadal cytokine with two circulating forms and cryptic actions. J. Endocrinol. 2015, 226, R45–R57. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Donahoe, P.K.; Hasegawa, T.; Silverman, B.; Crist, G.B.; Best, S.; Hasegawa, Y.; Noto, R.A.; Schoenfeld, D.; MacLaughlin, D.T. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J. Clin. Endocrinol. Metab. 1996, 81, 571–576. [Google Scholar] [PubMed]
- MacLaughlin, D.T.; Donahoe, P.K. Sex determination and differentiation. N. Engl. J. Med. 2004, 350, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Weenen, C.; Laven, J.S.; von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.; Themmen, A.P. Anti-Mullerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.A.; Themmen, A.P. Anti-Mullerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005, 234, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Malloy, P.J.; Peng, L.; Wang, J.; Feldman, D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology 2009, 150, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Doswell, A.; Krebs, K.; Cipolla, M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J. Clin. Endocrinol. Metab. 2014, 99, E1137–E1145. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, P.; Vijver, v.d.A.; Schutyser, V.; Milatovic, S.; Anckaert, E.; Schiettecatte, J.; Blockeel, C.; Camus, M.; Tournaye, H.; Polyzos, N.P. The effect of serum vitamin D levels on ovarian reserve markers: A prospective cross-sectional study. Hum. Reprod. 2017, 32, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.M.; Kim, Y.S.; Won, H.J.; Yoon, T.K.; Lee, W.S. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J. Clin. Endocrinol. Metab. 2014, 99, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Neville, G.; Martyn, F.; Kilbane, M.; O'Riordan, M.; Wingfield, M.; McKenna, M.; McAuliffe, F.M. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int. J. Gynaecol. Obstet. 2016, 135, 172–176. [Google Scholar] [CrossRef] [PubMed]
- McLennan, I.S.; Pankhurst, M.W. Is the understanding of AMH being confounded by study designs that do not adequately reflect that it is an atypical hormone? Hum. Reprod. 2017, 32, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Kissell, K.A.; Danaher, M.R.; Schisterman, E.F.; Wactawski-Wende, J.; Ahrens, K.A.; Schliep, K.; Perkins, N.J.; Sjaarda, L.; Weck, J.; Mumford, S.L. Biological variability in serum anti-Mullerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum. Reprod. 2014, 29, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, M.W.; Chong, Y.H.; McLennan, I.S. Enzyme-linked immunosorbent assay measurements of antiMullerian hormone (AMH) in human blood are a composite of the uncleaved and bioactive cleaved forms of AMH. Fertil. Steril. 2014, 101, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, Z.; Wright, D.J.; Rainbow, S.J. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin. Chem. 2005, 51, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, T.W.; Wright, P.; Nelson, S.M.; Anderson, R.A.; Wallace, W.H. A validated model of serum anti-Mullerian hormone from conception to menopause. PLoS ONE 2011, 6, e22024. [Google Scholar] [CrossRef] [PubMed]
- Rockell, J.E.; Skeaff, C.M.; Williams, S.M.; Green, T.J. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos. Int. 2006, 17, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
| Group | Control | Vit D3 |
|---|---|---|
| N | 22 | 24 |
| Age (years) | 21.7 ± 1.4 (19.4–25.2) | 21.7 ± 1.1 (19.6–24.5) |
| Oral contraceptive * | 10 | 11 |
| Asthma medication # | 3 | 2 |
| BMI (kg/m2) | 22.1 ± 2.9 (17.3–29.2) | 23.1 ± 2.4 (17.3–26.9) |
| Initial AMH (pM) | 39.9 ± 30.0 (9.7–139.9) | 32.0 ± 28.0 (7.7–131.6) |
| Initial 25(OH)D (nmol/L) | 54.1± 25.9 16.1–115 | 51.6 ± 22.1 (9.7–109) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, N.A.; Houghton, L.A.; Pankhurst, M.W.; Harper, M.J.; McLennan, I.S. Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women. Nutrients 2017, 9, 719. https://doi.org/10.3390/nu9070719
Dennis NA, Houghton LA, Pankhurst MW, Harper MJ, McLennan IS. Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women. Nutrients. 2017; 9(7):719. https://doi.org/10.3390/nu9070719
Chicago/Turabian StyleDennis, Nicola A., Lisa A. Houghton, Michael W. Pankhurst, Michelle J. Harper, and Ian S. McLennan. 2017. "Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women" Nutrients 9, no. 7: 719. https://doi.org/10.3390/nu9070719





