Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study
Abstract
:1. Introduction
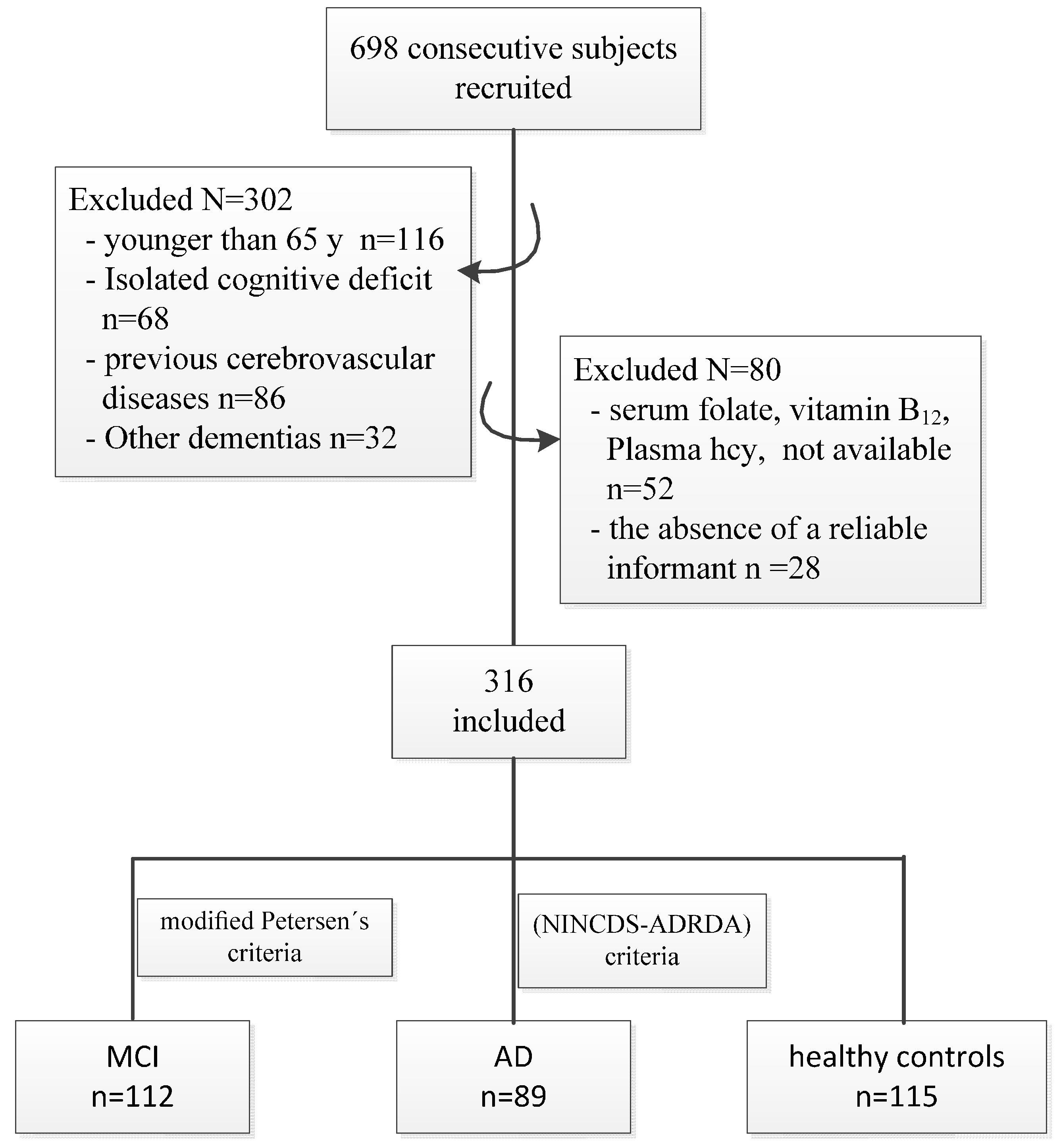
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Diagnosis of MCI and AD
2.4. Blood Sampling and Laboratory Tests
2.5. Statistical Analysis
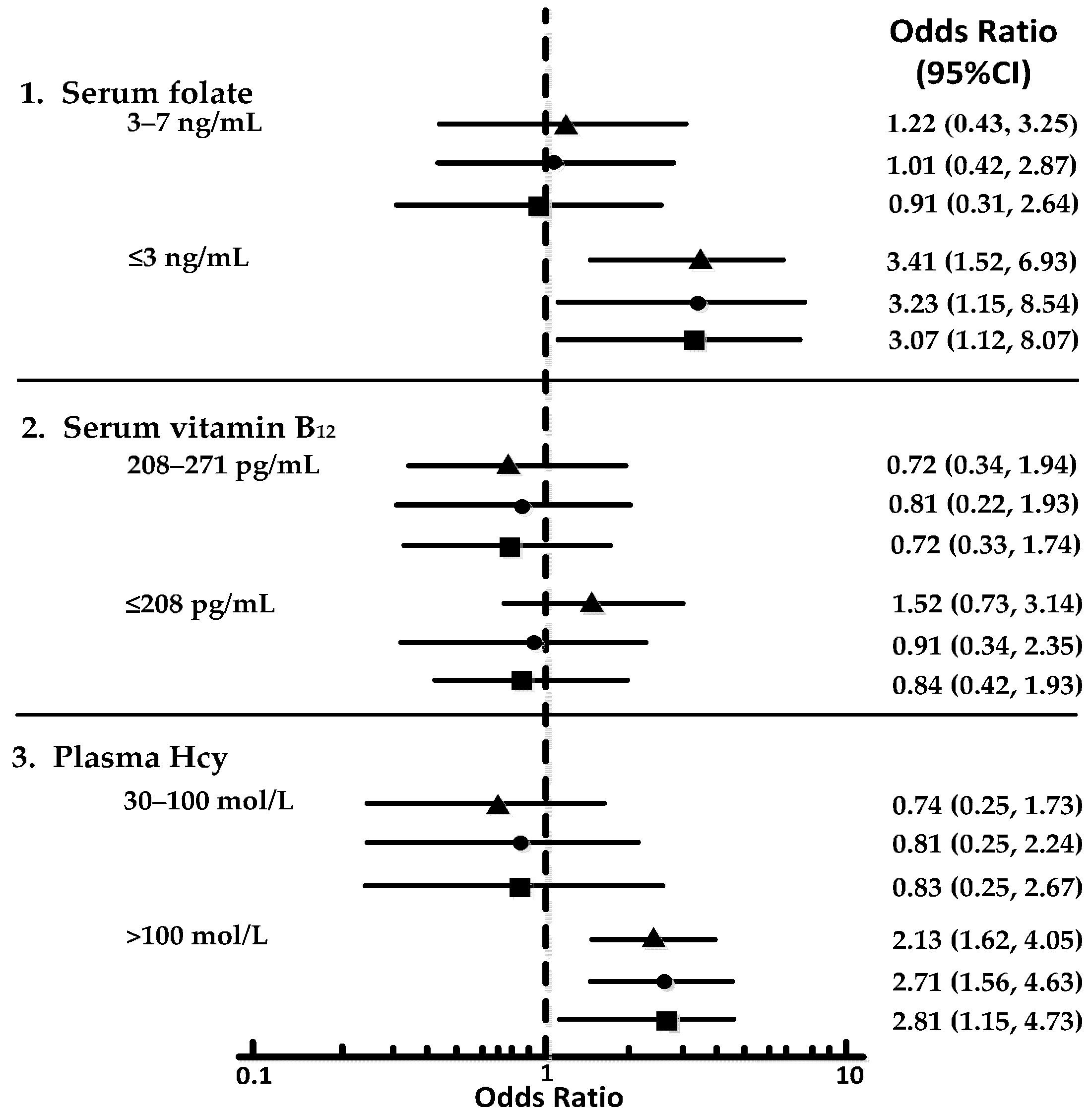
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer Disease |
| ANOVA | Analysis of Variance |
| BMI | Body Mass Index |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders, 4th edition |
| Hcy | Homocysteine |
| IQ | Intelligence Quotient |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini Mental State Examination |
| NINCDS-ADRDA | National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer Disease and Related Disorders Association |
| ORs | Odds Ratios |
| SD | Standard Deviations |
| WAIS-RC | Chinese version of the Wechsler Adult Intelligence Scale-Revised |
References
- Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014, 10, e47–e92. [Google Scholar]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Guralnik, J.M.; Ferrucci, L.; Fried, L.P.; Allen, R.H.; Stabler, S.P. Vitamin B12 deficiency and depression in physically disabled older women: Epidemiologic evidence from the Women’s Health and Aging Study. Am. J. Psychiatry 2000, 157, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Hutto, B.R. Folate and cobalamin in psychiatric illness. Compr. Psychiatry 1997, 38, 305–314. [Google Scholar] [CrossRef]
- Ho, P.I.; Collins, S.C.; Dhitavat, S.; Ortiz, D.; Ashline, D.; Rogers, E.; Shea, T.B. Homocysteine potentiates beta-amyloid neurotoxicity: Role of oxidative stress. J. Neurochem. 2001, 78, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Forloni, G.; Tettamanti, M.; Lucca, U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am. J. Clin. Nutr. 2004, 80, 114–122. [Google Scholar] [PubMed]
- Ravaglia, G.; Forti, P.; Maioli, F.; Muscari, A.; Sacchetti, L.; Arnone, G.; Nativio, V.; Talerico, T.; Mariani, E. Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am. J. Clin. Nutr. 2003, 77, 668–673. [Google Scholar] [PubMed]
- Mooijaart, S.P.; Gussekloo, J.; Frolich, M.; Jolles, J.; Stott, D.J.; Westendorp, R.G.; de Craen, A.J. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: The Leiden 85-Plus study. Am. J. Clin. Nutr. 2005, 82, 866–871. [Google Scholar] [PubMed]
- Haan, M.N.; Miller, J.W.; Aiello, A.E.; Whitmer, R.A.; Jagust, W.J.; Mungas, D.M.; Allen, L.H.; Green, R. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: Results from the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2007, 85, 511–517. [Google Scholar] [PubMed]
- Ravaglia, G.; Forti, P.; Maioli, F.; Martelli, M.; Servadei, L.; Brunetti, N.; Porcellini, E.; Licastro, F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am. J. Clin. Nutr. 2005, 82, 636–643. [Google Scholar] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Fekkes, D.; van der Cammen, T.J.M.; van Loon, C.P.M.; Verschoor, C.; van Harskamp, F.; de Koning, I.; Schudel, W.J.; Pepplinkhuize, L. Abnormal amino acid metabolism in patients with early stage Alzheimer dementia. J. Neural Transm. 1998, 105, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Dresner Pollak, R.; Pollak, A.; Idelson, M.; Bejarano-Achache, I.; Doron, D.; Blumenfeld, A. The C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene and vascular dementia. J. Am. Geriatr. Soc. 2000, 48, 664–668. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Vettori, C.; Grossi, G.; Bargossi, A.M.; Caldarera, M.; Franceschi, C.; Facchini, A.; Mariani, E.; et al. Elevated plasma homocysteine levels in centenarians are not associated with cognitive impairment. Mech. Ageing Dev. 2000, 121, 251–261. [Google Scholar] [CrossRef]
- Miller, J.W.; Green, R.; Mungas, D.M.; Reed, B.R.; Jagust, W.J. Homocysteine, vitamin B-6, and vascular disease in AD patients. Neurology 2002, 58, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzhimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998; pp. 196–305. [Google Scholar]
- Han-Byul, J.; Young-Hee, H.; Chandrika, J.P.; Heon, K.; Tawasun, H. Intake and blood concentrations of folate and their association with health-related behaviors in Korean college students. Nutr. Res. Pract. 2013, 7, 216–223. [Google Scholar]
- Mohammad, Y.G.; Ridha, A.G.; Omar, F.K.; Mahmoud, A.A. Hyperhomocysteinemia, Low Folate, and Vitamin B12 Deficiency in Elderly Living at Home and Cwere Residences: A Comparative Study. Labmedicine 2010, 41, 410–414. [Google Scholar]
- Susan, J.T.; Alan, H.; Sheena, B.; Anne, L.; Malcolm, H.; Sean, O.; Bryant, C.N.; Christine, P. International Standard for serum vitamin B(12) and serum folate: International collaborative study to evaluate a batch of lyophilwased serum for B(12) and folate content. Clin. Chem. Lab. Med. 2007, 45, 380–386. [Google Scholar]
- Pitla, S.; Nagalla, B. Gender-related differences in the relationship between plasma homocysteine, anthropometric and conventional biochemical coronary heart disease risk factors in middle-aged Indians. Ann. Nutr. Metab. 2009, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.L.; Won-Ki, K.; Yun-Beom, C.; Shanta, K.; Danielle, M.D.; Posina, V.R.; Derrick, R.A.; Jonathan, S.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-d-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar]
- Beal, M.F.; Swartz, K.J.; Finn, S.F.; Mazurek, M.F.; Kowall, N.W. Neurochemical characterization of excitoxin lesions in the cerebral cortex. J. Neurosci. 1991, 11, 147–158. [Google Scholar] [PubMed]
- Paul, S.A.; Susan, E.; Howard, A.; Ramon, D.A.; Myron, W.; Charles, D.; William, J.; Joshua, W.M.; Ralph, G.; Karen, B.; et al. A pilot study of vitamins to lower plasma homocysteine levels in Alzheimer disease. Am. J. Geriatr. Psychiatry 2003, 1, 246–249. [Google Scholar]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.F.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993, 270, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Hyland, K.; Reynolds, E.H. The clinical potential of adometionine (S-adenosylmethionine) in neurological disorders. Drugs 1994, 48, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Basun, H.; Fratiglioni, L.; Winbland, B. Cobalamin levels are not reduced in Alzheimer’s disease: Results from a population-based study. J. Am. Geriatr. Soc. 1994, 42, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Crystal, H.A.; Ortof, E.; Frishman, W.H.; Gruber, A.; Hershman, D.; Aronson, M. Serum vitamin B-12 levels and incidence of dementia in a healthy elderly population: A report from the Bronx Longitudinal Aging Study. J. Am. Geriatr. Soc. 1994, 42, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Blom, A.H.; Van Boxtel, M.P.; Bosma, H.; de Bruijn, C.; Jolles, J.; Wauters, B.A.; Steinbusch, H.W.; de Vente, J. Homocysteine: A marker for cognitive performance? A longitudinal follow-up study. J. Nutr. Health Aging 2003, 7, 153–159. [Google Scholar] [PubMed]
- David, A.S.; Christine, L.T.; Charles, D.S.; Kathryn, P.R.; William, R.M. Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: Findings from the Nun Study. Am. J. Clin. Nutr. 2000, 71, 993–998. [Google Scholar]
- Wang, H.-X.; Wahlin, A.; Basun, H.; Fastbom, J.; Winblad, B.; Fratiglioni, L. Vitamin B12 and folate in relation to the development of Alzheimer’s disease. Neurology 2001, 56, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.; Lesaffre, E.; Riezler, R.; Ghekiere, V.; Dereymaeker, L.; Pelemans, W.; Dejaeger, E. Is metabolic evidence for vitamin B-12 and folate deficiency more frequent in elderly patients with Alzheimer’s disease? J. Gerontol. 1997, 52, M76–M79. [Google Scholar] [CrossRef]
- Lehmann, M.; Gottfries, C.G.; Regland, B. Identification of cognitive impairment in the elderly: Homocysteine is an early marker. Dement. Geriatr. Cogn. Disord. 1999, 10, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef] [PubMed]


| Profile | Control Group (n = 115) | MCI Group (n = 112) | AD Group (n = 89) |
|---|---|---|---|
| Demographics | |||
| Age (years) | 72.82 ± 8.87 | 73.23 ± 8.67 | 74.62 ± 8.01 |
| Female | 69 (60.00) | 68 (60.71) | 56 (62.92) |
| Total education (years) | 10.92 ± 1.53 | 10.42 ± 2.01 | 10.33 ± 2.37 |
| Clinical characteristics | |||
| Disease duration (months) | 27.67 ± 22.24 | ||
| MMSE score | 28.53 ± 1.74 | 16.23 ± 8.07 ‡ | 12.83 ± 8.13 ‡ |
| BMI (kg/m2) | 25.87 ± 5.13 | 25.14 ± 3.96 | 24.73 ± 5.54 |
| Current smokers | 24 (20.87) | 24 (21.43) | 20 (22.47) |
| Alcohol (units/week) | 9.12 ± 4.53 | 8.14 ± 3.22 | 8.57 ± 3.21 |
| Total cholesterol (mmol/L) | 5.83 ± 1.35 | 6.02 ± 1.36 | 5.74 ± 1.43 |
| Systolic blood pressure (mmHg) | 143.33 ± 25.20 | 143.31 ± 25.21 | 144.44 ± 26.47 |
| Hypertension | 50 (43.48) | 49 (43.75) | 38 (42.70) |
| Diabetes | 20 (17.39) | 21 (18.75) | 16 (17.98) |
| Biochemical measures | |||
| Plasma Hcy (μmol/L) | 13.21 ± 4.05 | 15.35 ± 8.44 ‡ | 16.37 ± 7.46 ‡ |
| Serum folate (ng/mL) | 7.03 ± 3.68 | 5.74 ± 2.63 † | 5.13 ± 3.57 † |
| Serum vitamin B12 (pg/mL) | 573.17 ± 75.41 | 538.82 ± 84.06 † | 531.21 ± 44.33 † |
| B vitamin supplements, n (%) * | 31 (26.96) | 31 (27.68) | 23 (25.84) |
| Use of fish-oils, omega-3, n (%) | 16 (13.91) | 16 (14.28) | 12 (13.48) |
| Tertiles of Duration of Memory Impairment, Years | MMSE Score | Biochemical Variables | ||
|---|---|---|---|---|
| Hcy, μmol/L | Folate, ng/mL | Vitamin B12, pg/mL | ||
| I <2 | 17.80 ± 7.99 | 17.25 ± 6.82 | 5.67 ± 4.11 | 525.31 ± 37.21 |
| II 2–4 | 14.22 ± 8.44 | 16.47 ± 5.75 | 5.11 ± 5.17 | 528.47 ± 63.41 |
| III >4 | 7.55 ± 6.49 | 16.79 ± 4.15 | 5.05 ± 7.39 | 517.45 ± 43.49 |
| p value | 0.007 | 0.452 | 0.321 | 0.367 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Wu, T.; Zhao, J.; Ji, L.; Song, A.; Zhang, M.; Huang, G. Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study. Nutrients 2017, 9, 725. https://doi.org/10.3390/nu9070725
Ma F, Wu T, Zhao J, Ji L, Song A, Zhang M, Huang G. Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study. Nutrients. 2017; 9(7):725. https://doi.org/10.3390/nu9070725
Chicago/Turabian StyleMa, Fei, Tianfeng Wu, Jiangang Zhao, Lu Ji, Aili Song, Meilin Zhang, and Guowei Huang. 2017. "Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study" Nutrients 9, no. 7: 725. https://doi.org/10.3390/nu9070725




