Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. ATP and ADP Assay
2.3. cDNAs, Plasmids, Recombinant Viruses
2.4. Other Procedures
2.5. Statistical Analysis
3. Results
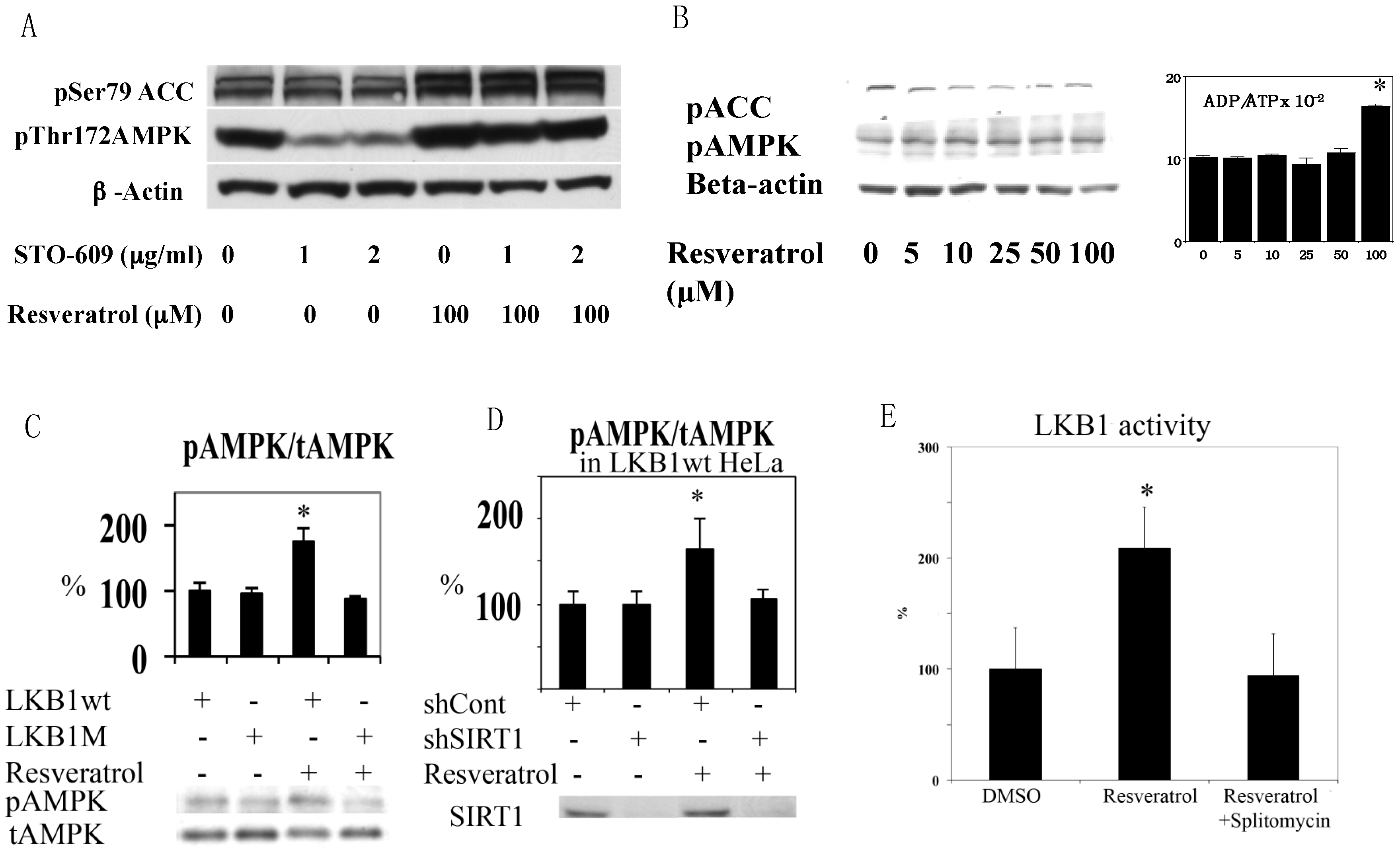
3.1. Resveratrol-Induced AMPK Activation Requires Functional LKB1 and SIRT1 Activity
3.2. Resveratrol Increases LKB1 Activity, Increases S428, T336 Phosphorylation, and Promotes LKB1 Translocation
3.3. Resveratrol May Accelerate LKB1 Degradation by the Proteasome
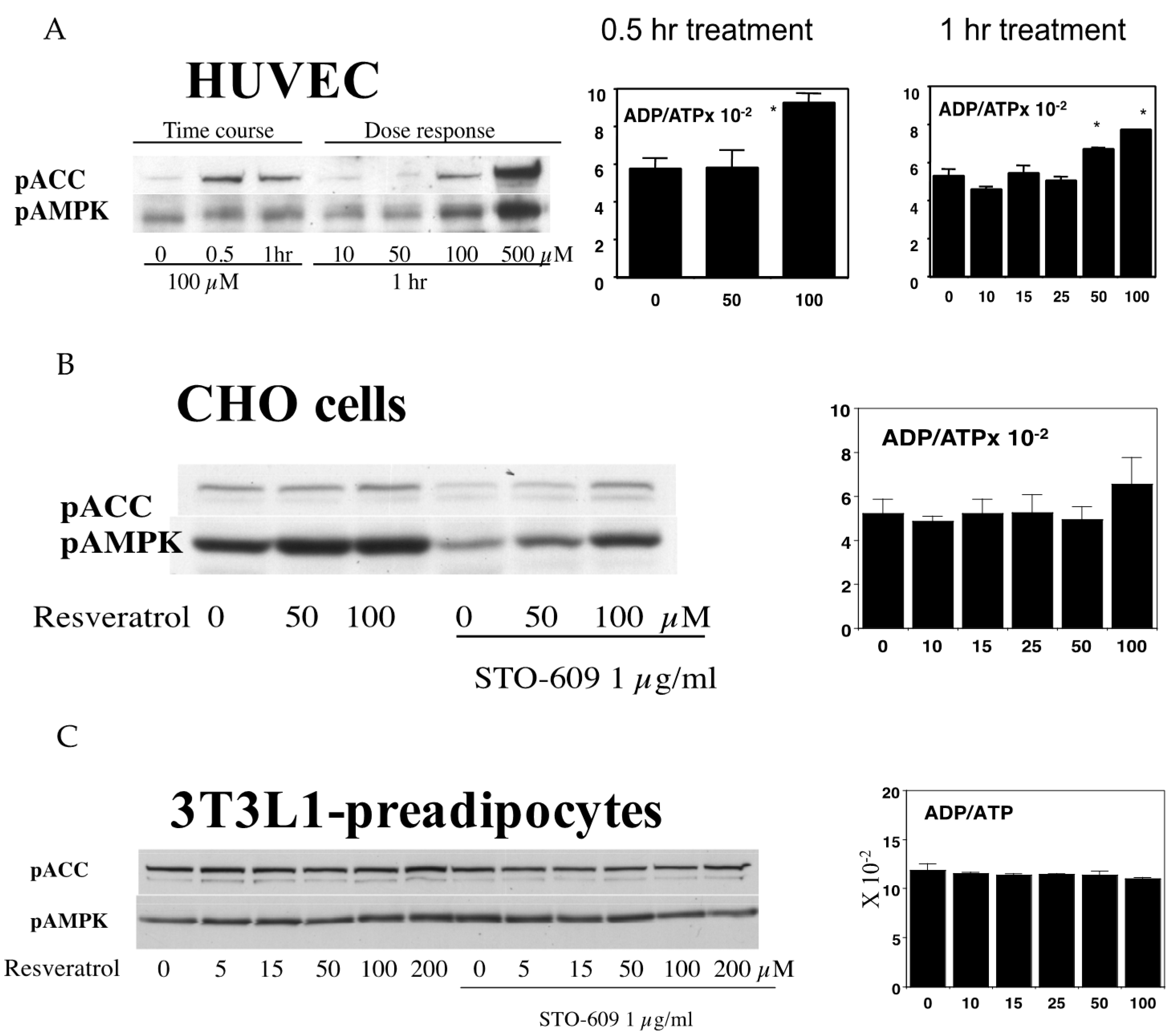
3.4. Resveratrol-Mediated AMPK Activation Was Not Ubiquitously Observed in Different Cell Types
3.5. A Potential Method to Increase Resveratrol Activation of AMPK Based on the Above Findings
4. Discussion
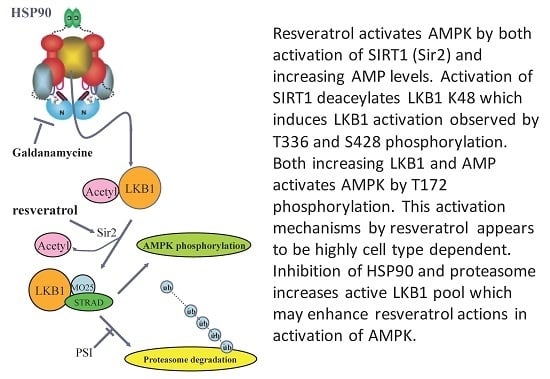
4.1. Resveratrol Activates AMPK by Co-Operative Action of Two Mechanisms
4.2. LKB1 Deacetylation by SIRT1 Increases Activity but Decreases Stability
4.3. Feeding and Starvation Cycles May Regulate the LKB1 Acetylation Cycle
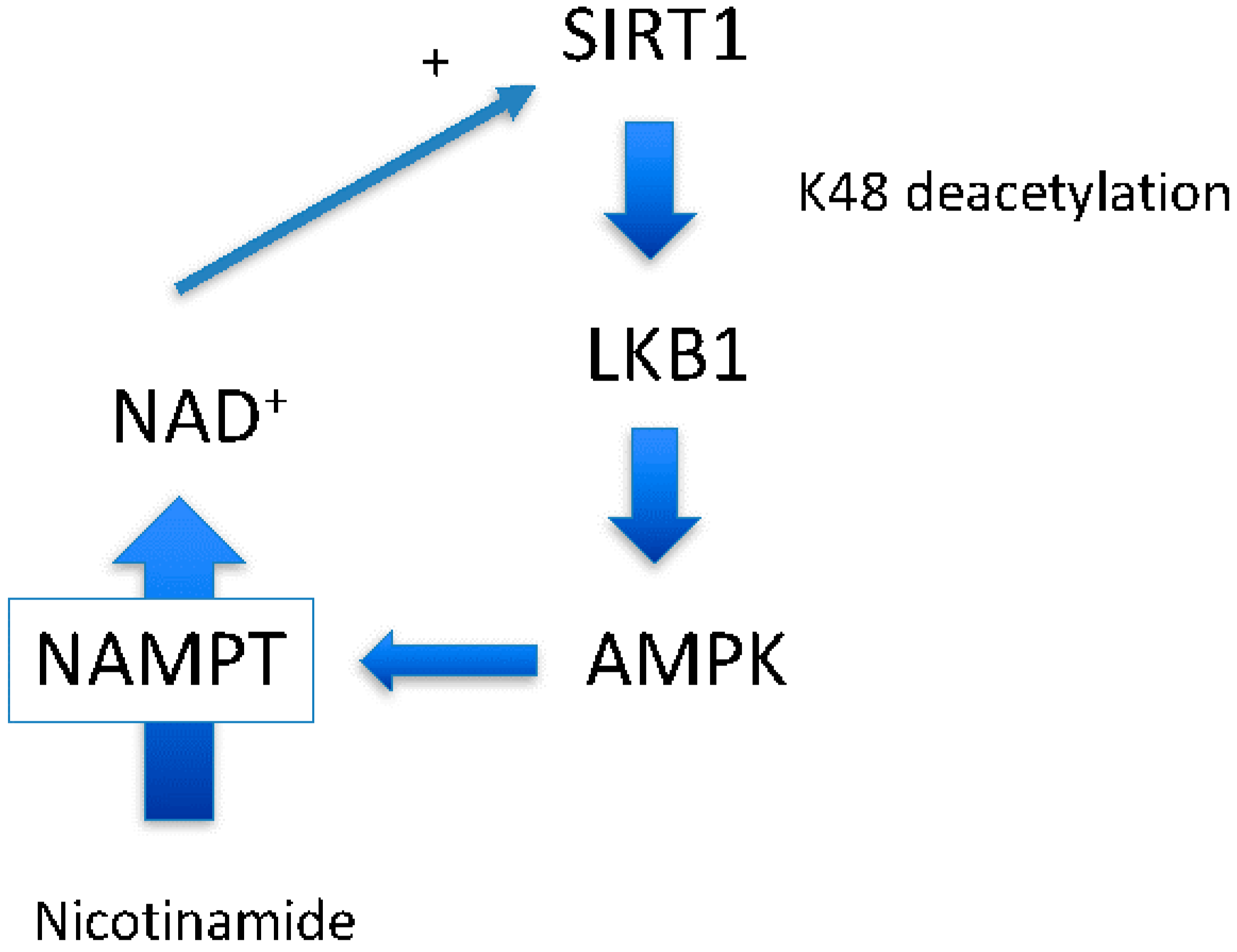
4.4. AMPK Activation May Not Always Increase NAD+
4.5. Potential Method to Improve Resveratrol Efficacy
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPK | AMP (adenosine monophosphate)-activated protein kinase |
| EGCG | epigallocatechin gallate |
| LKB1 | liver kinase B1 |
| NAMPT | nicotinamide phosphoribosyltransferase |
| NMN | nicotinamide mononucleotide |
| NMNAT | nicotinamide mononucleotide adenylyltransferase |
| PPi | pyrophosphate; PRPP: phophoribosyl pyrophosphate |
| PSI | proteasome inhibitor |
References
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Carling, D.; Ruderman, N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: Inhibition by the AMP-activated protein kinase activation. Diabetes 2002, 51, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.A.; Guarente, L. Unlocking the secrets of longevity genes. Sci. Am. 2006, 294, 48–51, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Cacicedo, J.M.; Ido, Y. Activation of AMPKK-AMPK cascade by silence information regulator 2 (sir2). Diabetes 2005, 54, A383. [Google Scholar]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Shultz, V.; Cacicedo, J.M.; Ido, Y. Resveratrol activates amp-activated protein kinase (AMPK) by increasing LKB1 activity and AMP/ATP ratio. Diabetes 2007, 56, A514. [Google Scholar]
- Hardie, D.G. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology 2006, 131, 973. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000, 130, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Kipp, J.L.; Ramirez, V.D. Effect of estradiol, diethylstilbestrol, and resveratrol on F0F1-ATPase activity from mitochondrial preparations of rat heart, liver, and brain. Endocrine 2001, 15, 165–175. [Google Scholar] [CrossRef]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase—Development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Resveratrol is neuroprotective because it is not a direct activator of SIRT1—A hypothesis. Brain Res. Bull. 2010, 81, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Beher, D.; Wu, J.; Cumine, S.; Kim, K.W.; Lu, S.C.; Atangan, L.; Wang, M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 2009, 74, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarasimhan, M.; Rauh, D.; Schutkowski, M.; Steegborn, C. SIRT1 activation by resveratrol is substrate sequence-selective. Aging 2013, 5, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Guarente, L. It takes two to tango: Nad+ and sirtuins in aging/longevity control. Aging Mech. Dis. 2016, 2, 16017. [Google Scholar]
- Dagher, Z.; Ruderman, N.; Tornheim, K.; Ido, Y. Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 2001, 88, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Schultz, V.; Sussman, I.; Bokvist, K.; Tornheim, K. Bioluminometric assay of ADP and ATP at high ATP/ADP ratios: Assay of ADP after enzymatic removal of ATP. Anal. Biochem. 1993, 215, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Tokumitsu, H.; Inuzuka, H.; Ishikawa, Y.; Ikeda, M.; Saji, I.; Kobayashi, R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 2002, 277, 15813–15818. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, M.; Hong, S.P.; Carlson, M. Mammalian TAK1 activates SNF1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 2006, 281, 25336–25343. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Xu, M.; Chen, Z.J. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 2007, 26, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Makela, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Goransson, O.; Hardie, D.G.; Alessi, D.R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: Effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E310–E317. [Google Scholar] [CrossRef] [PubMed]
- Posakony, J.; Hirao, M.; Stevens, S.; Simon, J.A.; Bedalov, A. Inhibitors of SIR2: Evaluation of splitomicin analogues. J. Med. Chem. 2004, 47, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Boudeau, J.; Kieloch, A.; Alessi, D.R.; Stella, A.; Guanti, G.; Resta, N. Functional analysis of LKB1/STK11 mutants and two aberrant isoforms found in peutz-jeghers syndrome patients. Hum. Mutat. 2003, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef] [PubMed]
- Boudeau, J.; Deak, M.; Lawlor, M.A.; Morrice, N.A.; Alessi, D.R. Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability. Biochem. J. 2003, 370, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Nony, P.; Gaude, H.; Rossel, M.; Fournier, L.; Rouault, J.P.; Billaud, M. Stability of the peutz-jeghers syndrome kinase LKB1 requires its binding to the molecular chaperones Hsp90/Cdc37. Oncogene 2003, 22, 9165–9175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, H.; Balschi, J.A. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic amp concentration. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H457–H466. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.; Riek, U.; Tuerk, R.; Schlattner, U.; Wallimann, T.; Neumann, D. Dissecting the role of 5’-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 2006, 281, 32207–32216. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.J.; Grondin, P.O.; Hegarty, B.D.; Snowden, M.A.; Carling, D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007, 403, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y. Diabetic complications within the context of aging: Nicotinamide adenine dinucleotide redox, insulin C-peptide, sirtuin 1-liver kinase B1-adenosine monophosphate-activated protein kinase positive feedback and forkhead box O3. J. Diabetes Investig. 2016, 7, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. Sirt1 expression is auto-regulated through expression of LKB1 and AMPK via negative feedback. Diabetes 2011, 60, A461. [Google Scholar]
- Jaleel, M.; Villa, F.; Deak, M.; Toth, R.; Prescott, A.R.; Van Aalten, D.M.; Alessi, D.R. The ubiquitin-associated domain of AMPK-related kinases regulates conformation and LKB1-mediated phosphorylation and activation. Biochem. J. 2006, 394, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Li, C.F.; Jin, G.; Cai, Z.; Han, F.; Chan, C.H.; Yang, W.L.; Li, B.K.; Rezaeian, A.H.; Li, H.Y.; et al. Skp2-dependent ubiquitination and activation of LKB1 is essential for cancer cell survival under energy stress. Mol. Cell 2015, 57, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Burgos, E.S.; Schramm, V.L. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry 2008, 47, 11086–11096. [Google Scholar] [CrossRef] [PubMed]
- Devin, A.; Guerin, B.; Rigoulet, M. Cytosolic NAD+ content strictly depends on ATP concentration in isolated liver cells. FEBS Lett. 1997, 410, 329–332. [Google Scholar] [CrossRef]
- Schweiger, M.; Hennig, K.; Lerner, F.; Niere, M.; Hirsch-Kauffmann, M.; Specht, T.; Weise, C.; Oei, S.L.; Ziegler, M. Characterization of recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 2001, 492, 95–100. [Google Scholar] [CrossRef]
- Moses, M.A.; Henry, E.C.; Ricke, W.A.; Gasiewicz, T.A. The heat shock protein 90 inhibitor, (−)–epigallocatechin gallate, has anticancer activity in a novel human prostate cancer progression model. Cancer Prev. Res. 2015, 8, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Landis-Piwowar, K.; Chan, T.H.; Dou, Q.P. Green tea polyphenols as proteasome inhibitors: Implication in chemoprevention. Curr. Cancer Drug Targets 2011, 11, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Bieganowski, P.; Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD+ in fungi and humans. Cell 2004, 117, 495–502. [Google Scholar] [CrossRef]
- Hammes, H.P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D.; et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Stracke, H.; Gaus, W.; Achenbach, U.; Federlin, K.; Bretzel, R.G. Benfotiamine in diabetic polyneuropathy (BENDIP): Results of a randomised, double blind, placebo-controlled clinical study. Exp. Clin. Endocrinol. Diabetes 2008, 116, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Foldi, M.; Stickeler, E.; Bau, L.; Kretz, O.; Watermann, D.; Gitsch, G.; Kayser, G.; Zur Hausen, A.; Coy, J.F. Transketolase protein TKTl1 overexpression: A potential biomarker and therapeutic target in breast cancer. Oncol. Rep. 2007, 17, 841–845. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, F.; Weikel, K.A.; Cacicedo, J.M.; Ido, Y. Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application. Nutrients 2017, 9, 751. https://doi.org/10.3390/nu9070751
Lan F, Weikel KA, Cacicedo JM, Ido Y. Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application. Nutrients. 2017; 9(7):751. https://doi.org/10.3390/nu9070751
Chicago/Turabian StyleLan, Fan, Karen A. Weikel, Jose M. Cacicedo, and Yasuo Ido. 2017. "Resveratrol-Induced AMP-Activated Protein Kinase Activation Is Cell-Type Dependent: Lessons from Basic Research for Clinical Application" Nutrients 9, no. 7: 751. https://doi.org/10.3390/nu9070751