Nutrition and Allergic Diseases
Abstract
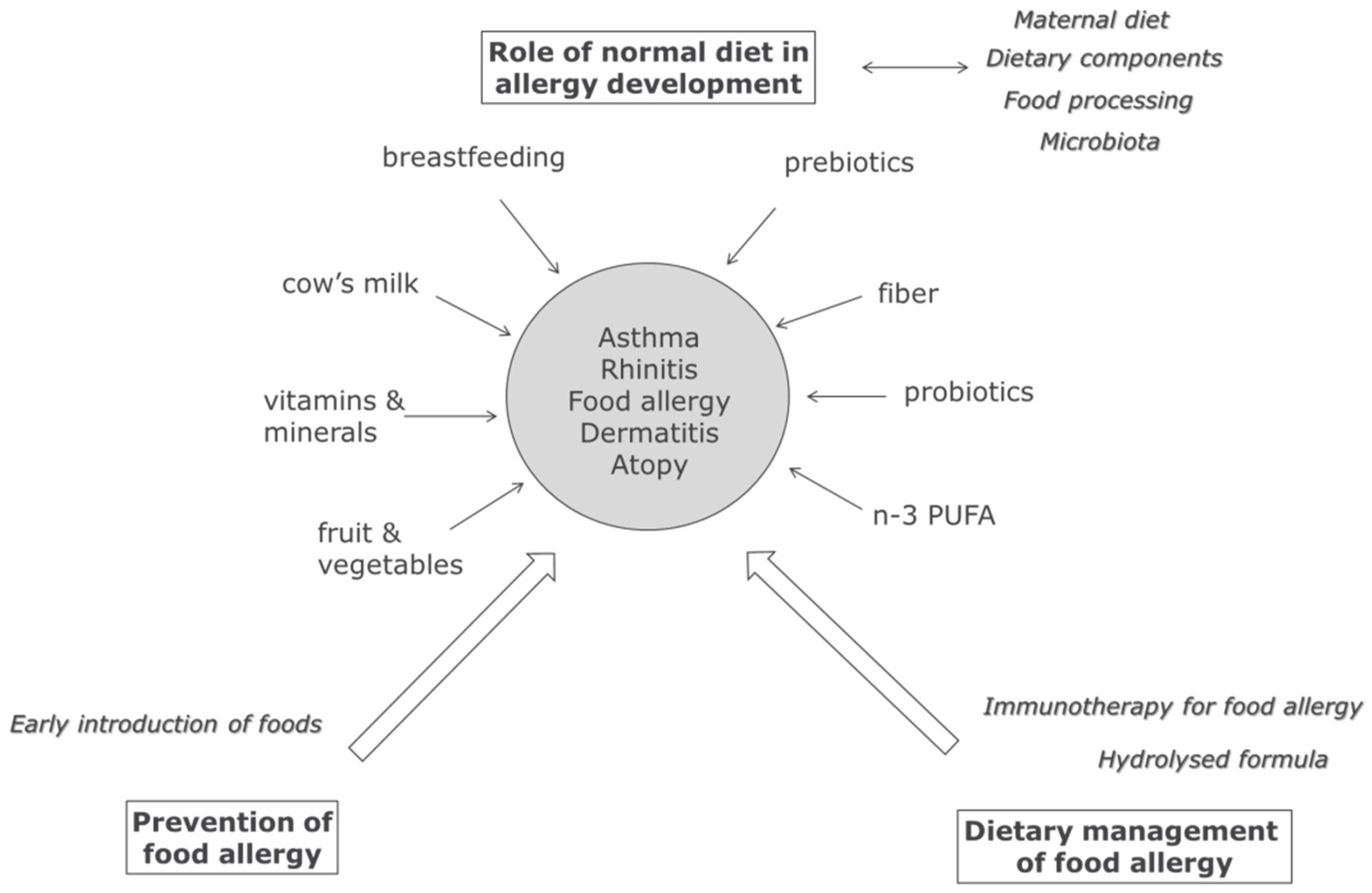
:1. Introduction
2. Maternal Diet, Breastfeeding, and Infant Nutrition & Allergy
3. Modulation of Microbiota and Allergy in Early Life
4. Normal Dietary Components and Allergy
5. IgE Antibody Characteristics and the Allergic Phenotype
6. Food Allergy: Early Introduction and Immunotherapy
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mallol, J.; Crane, J.; von Mutius, E.; Odhiambo, J.; Keil, U.; Stewart, A.; ISAAC Phase Three Study Group. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergol. Immunopathol. (Madr.) 2012, 41, 73–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Sly, R.M. Changing prevalence of allergic rhinitis and asthma. Am. Coll. Allergy Asthma Immunol. 1999, 82, 233–252. [Google Scholar] [CrossRef]
- Asher, M.; Montefort, S.; Bjorksten, B.; Lai, C.K.W.; Strachan, D.P.; Weiland, S.K.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.K.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Mullins, R. Food allergy: Is prevalence increasing? Intern. Med. J. 2017, 47, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Bartra, J.; García-Moral, A.; Enrique, E. Geographical differences in food allergy. Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz 2016, 59, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Mcstay, C.L.; Prescott, S.L.; Bower, C.; Palmer, D.J. Maternal Folic Acid Supplementation during Pregnancy and Childhood Allergic Disease Outcomes: A Question of Timing? Nutrients 2017, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Rueter, K.; Prescott, S.L.; Palmer, D.J. Nutritional approaches for the primary prevention of allergic disease: An update. J. Paediatr. Child Health 2015, 51, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Mckenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Maternal diet and its influence on the development of allergic disease. Clin. Exp. Allergy 2014, 45, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Makrides, M.; Collins, C. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst. Rev. 2015, 22, CD010085. [Google Scholar]
- Miles, E.; Calder, P.C. Can early omega-3 fatty acid exposure reduce risk of 3 childhood allergic disease? Nutrients 2017. submitted. [Google Scholar]
- Matheson, M.C.; Allen, K.J.; Tang, M.L.K. Understanding the evidence for and against the role of breastfeeding in allergy prevention. Clin. Exp. Allergy 2012, 42, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Odijk, J.V.V.; Kull, I.; Borres, M.P.; Brandtzaeg, P.; Edberg, U.; Kuitunen, M.; Olsen, S.F.; Skerfving, S.; Sundell, J.; Wille, S. Breastfeeding and allergic disease: A multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003, 58, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.; Tan, D.; Lau, M.; Dai, X.; Tham, R.; Lowe, A.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Boyle, R.J.; Warner, J.O. Factors affecting breast milk composition and potential consequences for development of the allergic phenotype. Clin. Exp. Allergy 2014, 45, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ramírez, N.; Karmaus, W.; Yousefi, M.; Zhang, H.; Liu, J.; Gangur, V. Maternal immune markers in serum during gestation and in breast milk and the risk of asthma-like symptoms at ages 6 and 12 months: A longitudinal study. Allergy Asthma Clin. Immunol. 2012, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.L.; Havstad, S.; Bobbitt, K.; Woodcroft, K.; Zoratti, E.M.; Nageotte, C.; Misiak, R.; Enberg, R.; Nicholas, C.; Ezell, J.M.; et al. Transforming growth factor beta (TGFbeta1) in breast milk and indicators of infant atopy in a birth cohort. Pediatr. Allergy Immunol. 2014, 25, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Ouwehand, A.; Arvilommi, H.; Kero, P.; Isolauri, E. Transforming growth factor-beta in breast milk: A potential regulator of atopic disease at an early age. J. Allergy Clin. Immunol. 1999, 104, 1251–1257. [Google Scholar] [CrossRef]
- Rigotti, E.; Piacentini, G.L.; Ress, M.; Pigozzi, R.; Boner, A.L.; Peroni, D.G. Transforming growth factor-b1 and interleukin-10 in breast milk and development of atopic diseases in infants. Clin. Exp. Allergy 2006, 36, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Orivuori, L.; Loss, G.; Roduit, C.; Dalphin, J.-C.C.; Depner, M.; Genuneit, J.; Lauener, R.; Pekkanen, J.; Pfefferle, P.; Riedler, J.; et al. Soluble immunoglobulin A in breast milk is inversely associated with atopic dermatitis at early age: The PASTURE cohort study. Clin. Exp. Allergy 2014, 44, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Boix-Amorós, A.; Boyle, R.J.; Carmen Collado, M.; Garssen, J.; Gay, M.C.L.; Geddes, D.T.; Hsu, P.S.; Nanan, R.; Slupsky, C.; et al. Human milk and allergic diseases: Unsolved puzzle. Nutrients 2017. submitted. [Google Scholar]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; De Greef, E.; Devreker, T. Treatment of Cow’s Milk Protein Allergy. Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Ierodiakonou, D.; Khan, T.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Afxentiou, T.; Reeves, T.; Cunha, S.; et al. Hydrolysed formula and risk of allergic or autoimmune disease: Systematic review and meta-analysis. Br. Med. J. 2016, 352, i974. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.; Sinn, J.; Jones, L. Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy. Cochrane Database Syst. Rev. 2017, 3, CD003664. [Google Scholar] [PubMed]
- Vandenplas, Y. Prevention and management of cow milk allergy in non-exclusively breastfed infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Stobberingh, E.E.; Brandt, P.A.V.D.; Thijs, C. The role of the intestinal microbiota in the development of atopic disorders. Allergy 2007, 62, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, F.A.; Penders, J.; Stobberingh, E.E.; Postma, D.S.; Koppelman, G.H.; Kerkhof, M.; Reijmerink, N.E.; Dompeling, E.; van den Brandt, P.A.; Ferreira, I.; et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011, 128, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—Intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Sjogren, Y.M.; Persson, J.O.; Nilsson, C.; Sverremark-Ekstrom, E. Early colonization with a group of lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS ONE 2011, 6, e23031. [Google Scholar] [CrossRef] [PubMed]
- Cuella-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J.; et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Trivedi, M.K.; Jha, A.; Lin, Y.; Dimaano, L.; García-romero, M.T. Synbiotics for Prevention and Treatment of Atopic Dermatitis A Meta-analysis of Randomized Clinical Trials. JAMA Pediatr. 2017, 170, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K.H. Prebiotics in infants for prevention of allergy. Cochrane Database Syst. Rev. 2013, 3, CD006474. [Google Scholar]
- Hulshof, L.; van’t Land, B.; Sprikkelman, A.; Garssen, J. Role of microbial modulation in management of atopic dermatitis in children. Nutrients 2017. submitted. [Google Scholar]
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, G.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 2017, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Gollwitzer, E.S. Host–microorganism interactions in lung diseases. Nat. Rev. Immunol. 2014, 14, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Wypych, T.P.; Marsland, B.J. Diet Hypotheses in Light of the Microbiota Revolution: New Perspectives. Nutrients 2017, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011, 127, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Mauro, G.; Bernardini, R.; Barberi, S.; Capuano, A.; Correra, A.; De Angelis, G.L.; Iacono, I.D.; de Martino, M.; Ghiglioni, D.; Di Mauro, D.; et al. Prevention of food and airway allergy: Consensus of the Italian Society of Preventive and Social Paediatrics, the Italian Society of Paediatric Allergy and Immunology, and Italian Society of Pediatrics. World Allergy Organ. J. 2016, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Vafa, M.R.; Sharma, S.; Kolahdooz, F. Fruit and vegetable intake and risk of wheezing and asthma: A systematic review and meta-analysis. Nutr. Rev. 2014, 72, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Sunn, J.K.; Osborn, D.A. Polyunsaturated fatty acid supplementation in infancy for the prevention of allergy. Cochrane Database Syst. Rev. 2016, 10, CD010112. [Google Scholar] [PubMed]
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Thijs, C.; Müller, A.; Rist, L.; Kummeling, I.; Snijders, B.E.P.; Huber, M.; van Ree, R.; Simoes-Wust, A.P.; Dagnelie, P.C.; van Den Brandt, P.A.; et al. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy 2010, 66, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Mutius, E.V.; Vercelli, D.; von, M.E.; Von Mutius, E. Farm living: Effects on childhood asthma and allergy. Nat. Rev. Immunol. 2010, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Berthon, B.; Wark, P.; Wood, L. Effects of fruit and vegetable consumption on risk of Asthma, Wheezing and Immune Responses: A systematic review and meta-analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Brick, T.; Ege, M.; Boeren, S.; Böck, A.; von Mutius, E.; Vervoort, J.; Hettinga, K. Effect of processing intensity on immunologically active bovine milk serum proteins. Nutrients 2017. submitted. [Google Scholar]
- Teodorowicz, M.; van Neerven, R.J.; Savelkoul, H.F.J. Food processing: The influence of the Maillard Reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017. submitted. [Google Scholar]
- Jarvinen, K.; Beyer, K.; Vila, L.; Chatchatee, P.; Busse, P.; Sampson, H. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2002, 110, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.; Allen, K. Optimal timing for solids introduction—Why are the guidelines always changing? Clin. Exp. Allergy 2013, 43, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Ierodiakonou, D.; Garcia-Larsen, V.; Logan, A.; Groome, A.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Reeves, T.; et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease. JAMA 2016, 316, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Netting, M.J.; Allen, K.J. Advice about infant feeding for allergy prevention: A confusing picture for Australian consumers? J. Paediatr. Child Health 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Foong, R.-X.; Lack, G. The role of dietary interventions in the prevention of IgE-mediated food allergy in children. Pediatr. Allergy Immunol. 2017, 28, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa, A.; Baker, J.R.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases e sponsored expert panel. Ann. Allergy Asthma Immunol. 2017, 118, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Burks, W.A. Emerging Approaches to Food Desensitization in Children. Curr. Allergy Asthma Rep. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.; Dhami, S.; Arasi, S.; Pajno, G.B.; Fernandez-Rivas, M.; Muraro, A.; Roberts, G.; Akdis, C.; Alvaro-Lozano, M.; Beyer, K.; et al. Allergen Immunotherapy for IgE-mediated food allergy: A systematic review and meta analysis. Allergy 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neerven, R.J.J.v.; Savelkoul, H. Nutrition and Allergic Diseases. Nutrients 2017, 9, 762. https://doi.org/10.3390/nu9070762
Neerven RJJv, Savelkoul H. Nutrition and Allergic Diseases. Nutrients. 2017; 9(7):762. https://doi.org/10.3390/nu9070762
Chicago/Turabian StyleNeerven, R.J.J. van, and Huub Savelkoul. 2017. "Nutrition and Allergic Diseases" Nutrients 9, no. 7: 762. https://doi.org/10.3390/nu9070762




