Cyanidin Stimulates Insulin Secretion and Pancreatic β-Cell Gene Expression through Activation of l-type Voltage-Dependent Ca2+ Channels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
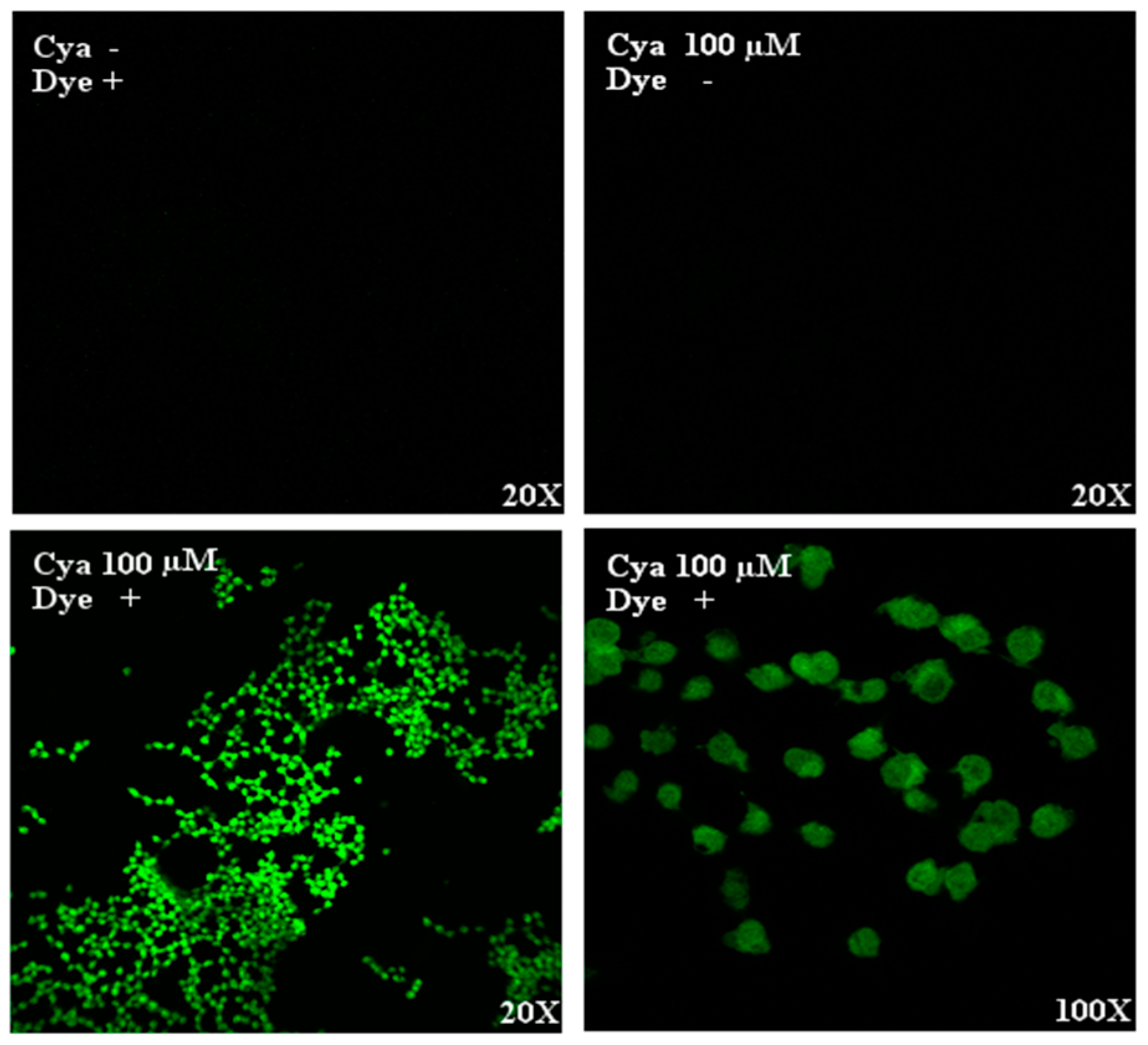
2.3. Cyanidin Localization
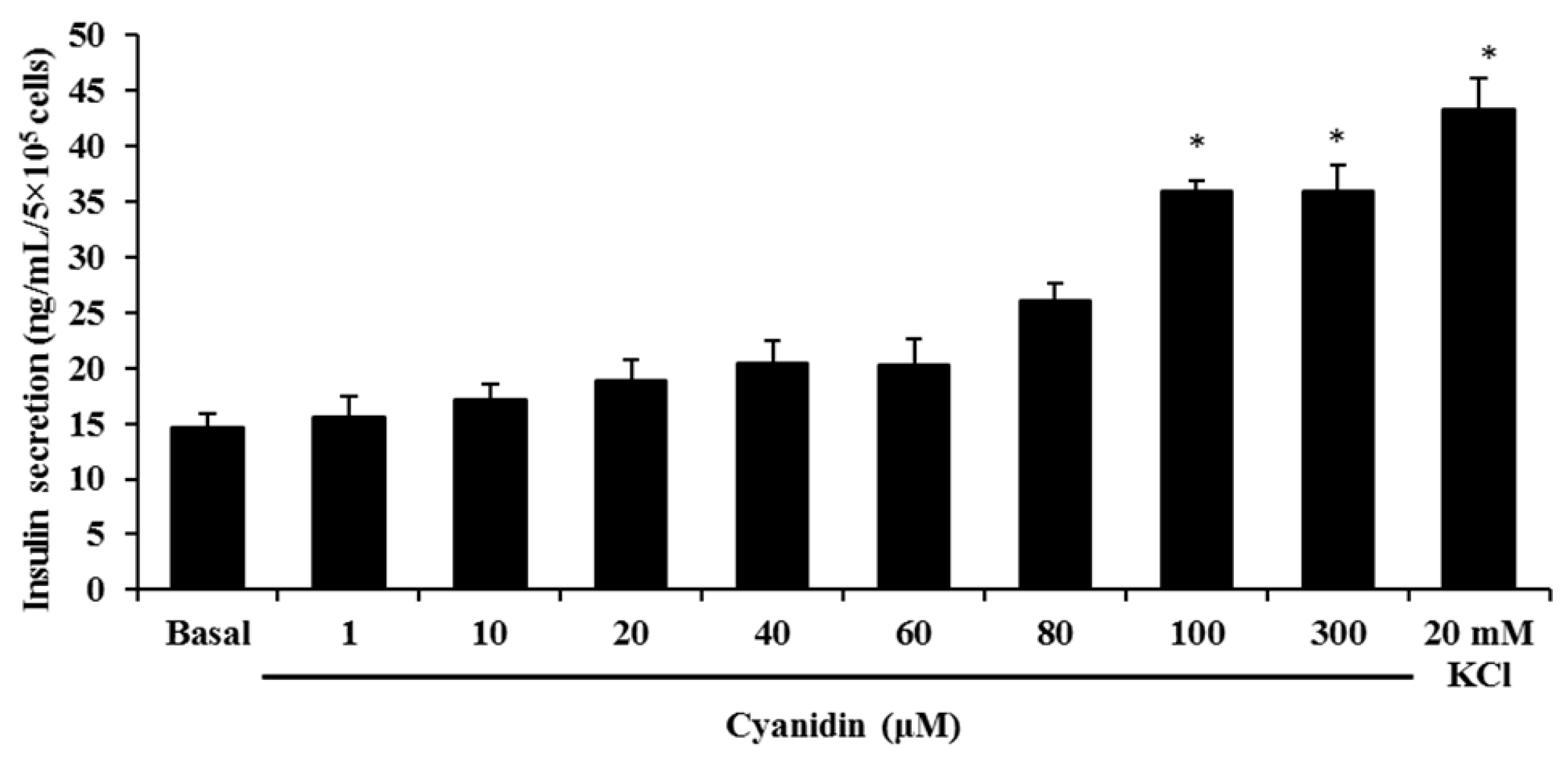
2.4. Static Incubation for Insulin Determination
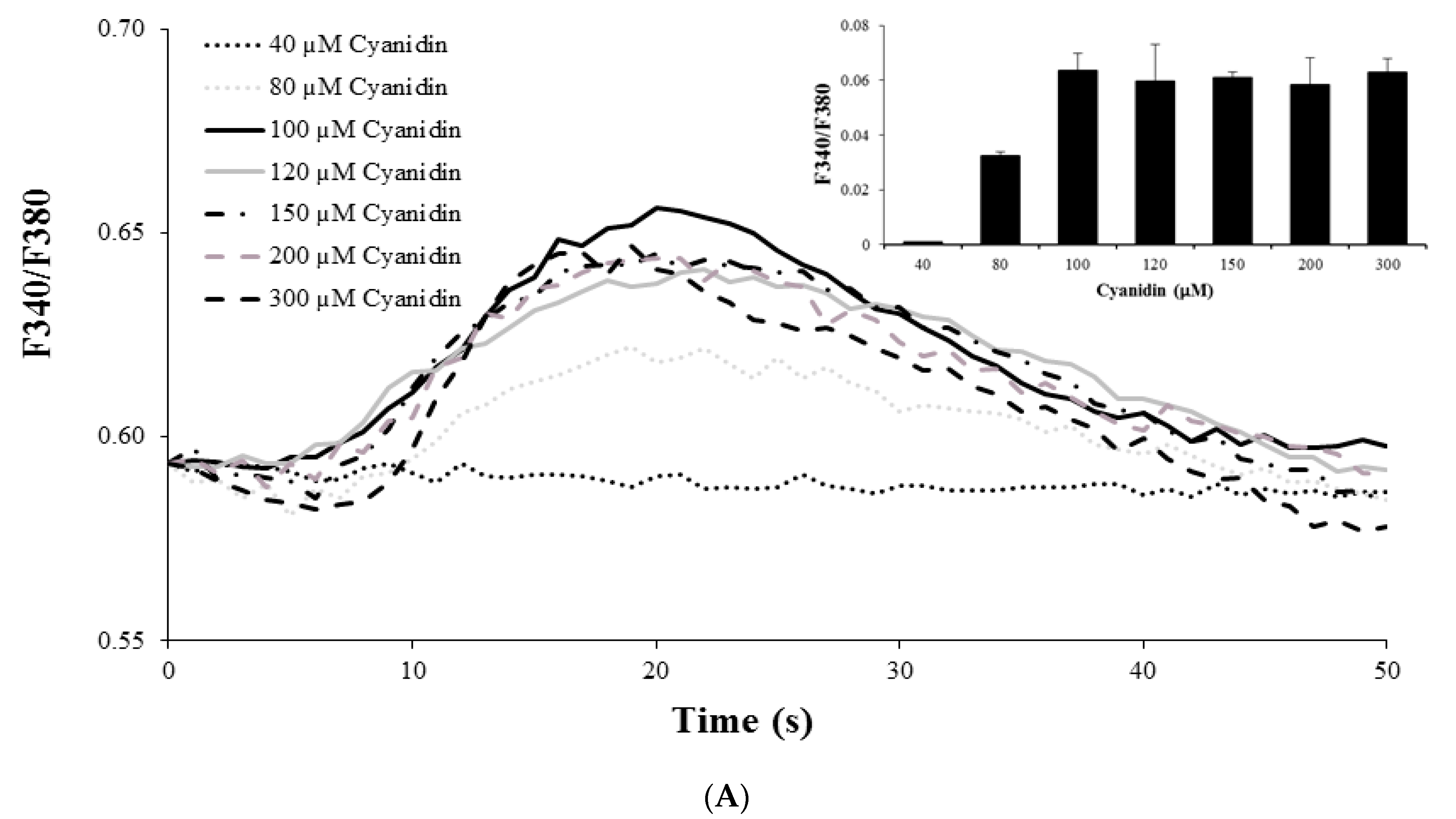
2.5. Real-Time Ca2+ Imaging Analysis
2.6. Electrophysiology
2.7. Real-Time PCR
2.8. Data Analysis
3. Results
3.1. Intracellular Detection of Cyanidin
3.2. Cyanidin Stimulates Insulin Secretion
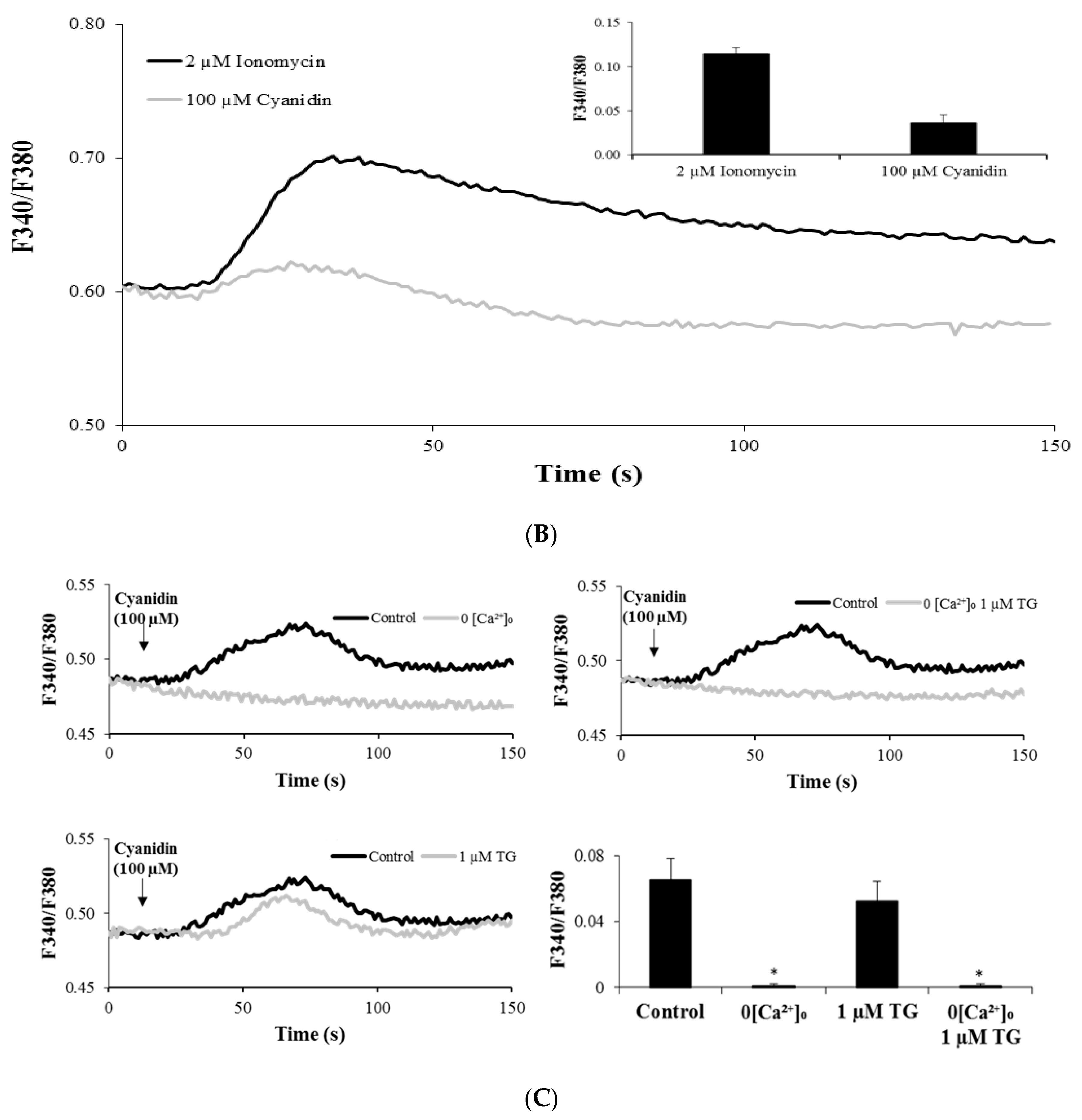
3.3. Cyanidin Increases Intracellular Ca2+ Concentration
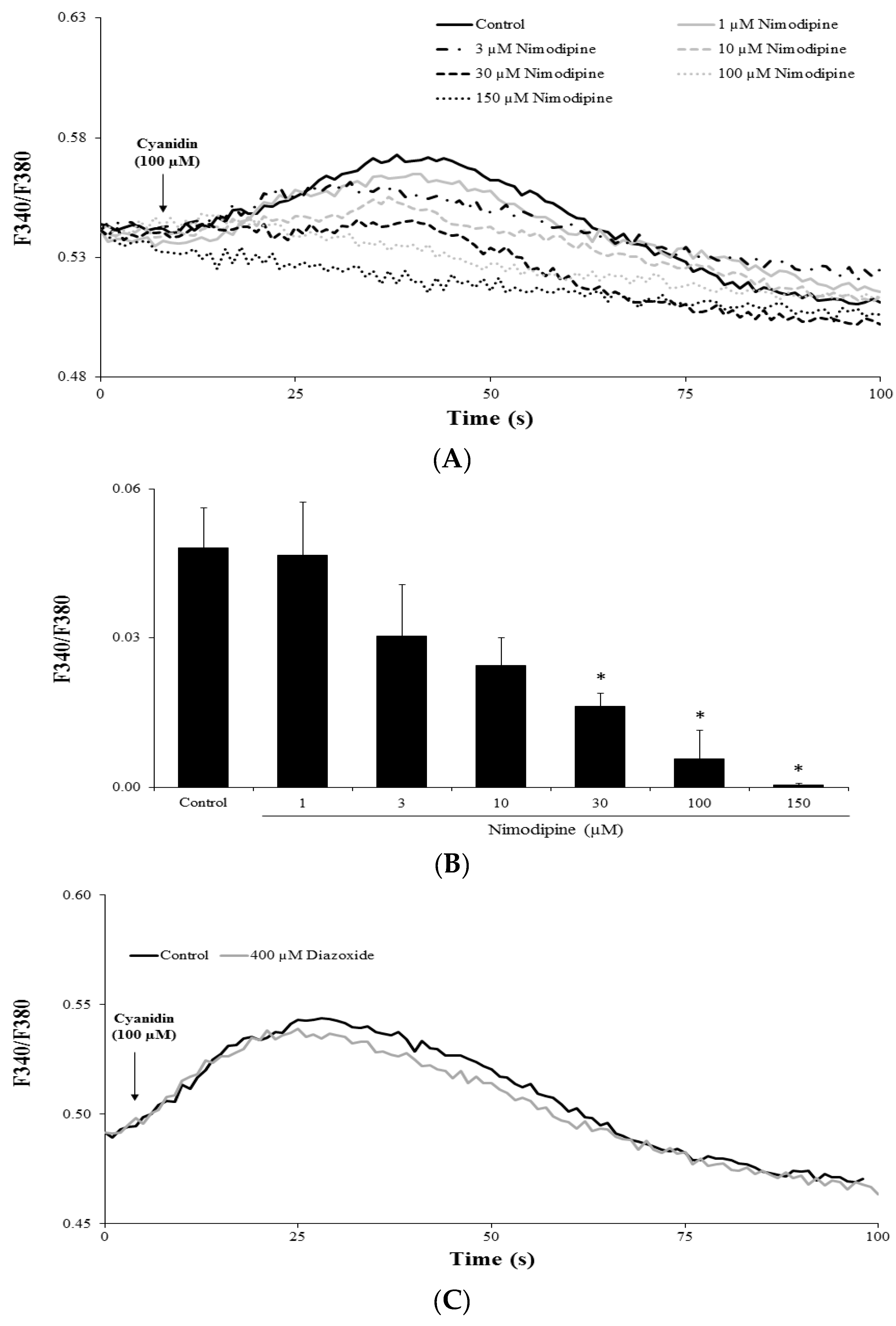
3.4. Cyanidin Increases Intracellular Ca2+ via l-Type Voltage-Dependent Ca2+ Channels
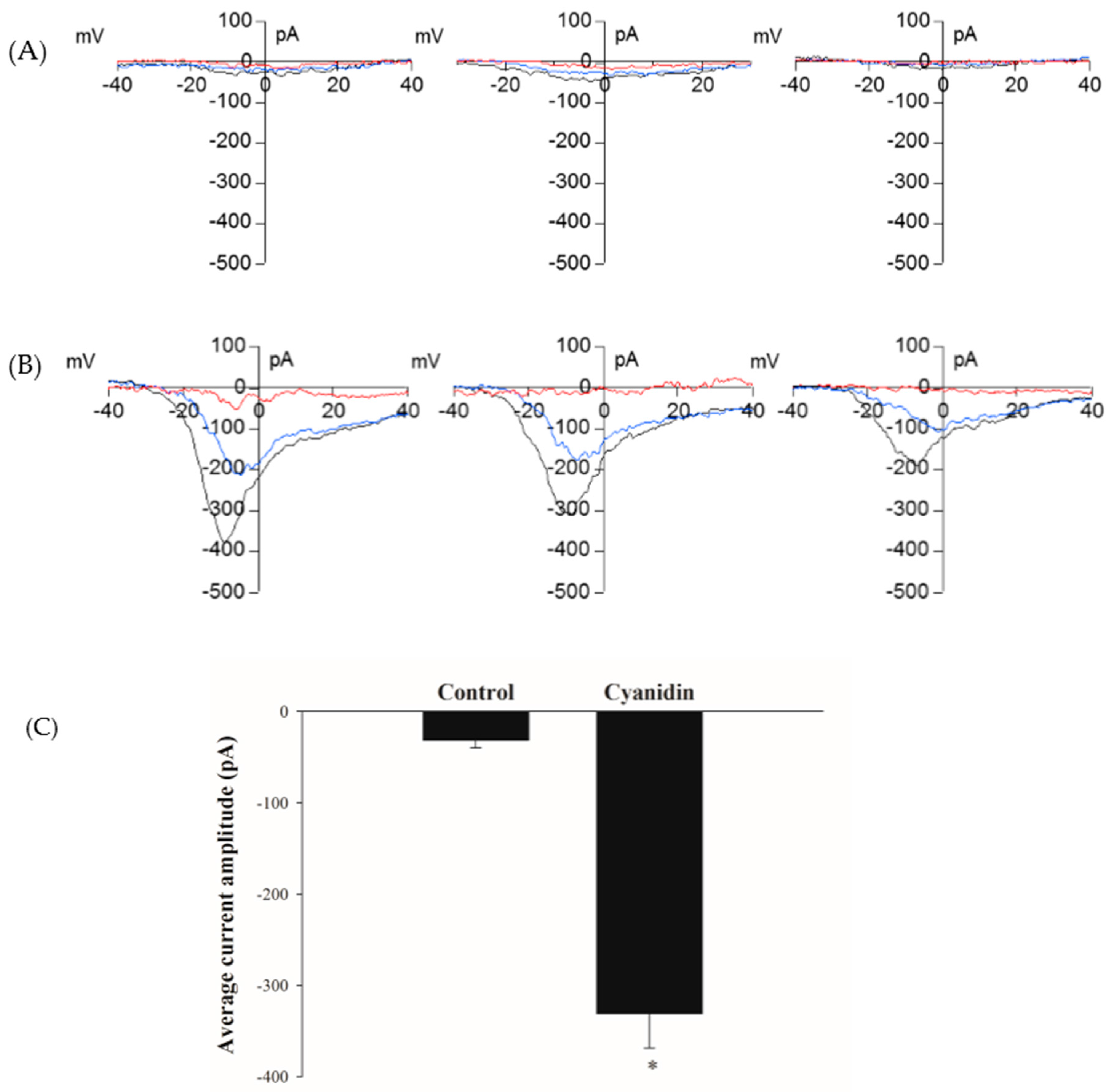
3.5. Cyanidin Directly Activates l-Type Voltage-Dependent Ca2+ Channels
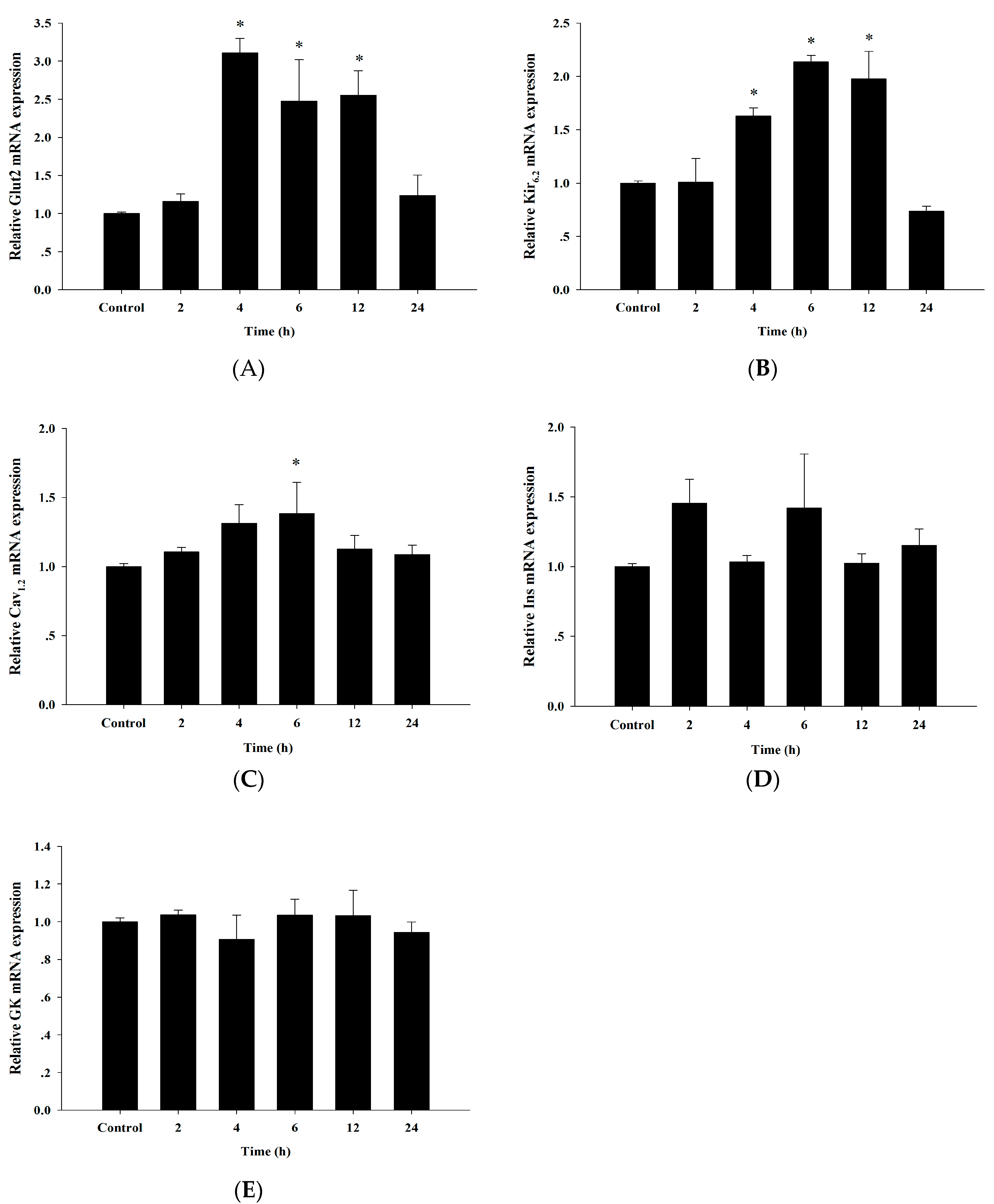
3.6. Up-Regulation of Insulin Secretion Genes by Cyanidin
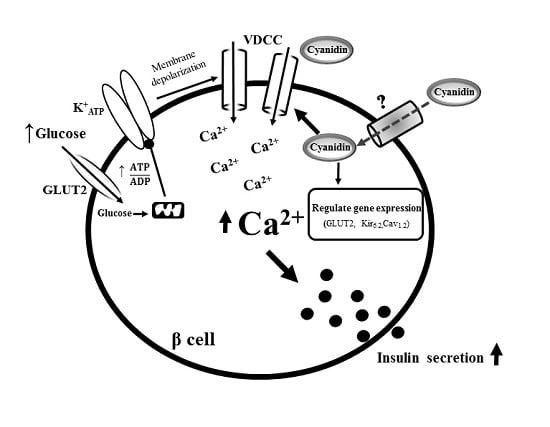
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GLUT1 | Glucose transporter 1 |
| GLUT2 | Glucose transporter 2 |
| SUR1 | Sulfonylurea receptor |
| KATP | ATP-sensitive K+ channels |
| VDCCs | Voltage-dependent Ca2+ channels |
| MG | Methylglyoxal |
| Fura-2AM | Fura-2 acetoxymethyl ester |
| KRB | Krebs-Ringer bicarbonate buffer |
| RIA | Radioimmunoassay |
| Vh | holding potential |
| GK | Glucokinase |
| Cav1.2 | l-type Ca2+ channel |
| Ins | Insulin |
| I/V | Current–voltage relationship |
| ER | Endoplasmic reticulum |
| Rutin | Quercetin-3-rutinoside |
References
- Kahn, S. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gembal, M.; Gilon, P.; Henquin, J.C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J. Clin. Investig. 1992, 89, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Nolan, C.J. Islet β cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.T.; Simpson, S.H.; Majumdar, S.R.; Johnson, J.A. Secondary failure rates associated with metformin and sulfonylurea therapy for type 2 diabetes mellitus. Pharmacotherapy 2005, 25, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, H.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yibchok-Anun, S.; Adisakwattana, S.; Yao, C.Y.; Sangvanich, P.; Roengsumran, S.; Hsu, W.H. Slow acting protein extract from fruit pulp of Momordica charantia with insulin secretagogue and insulinomimetic activities. Biol. Pharm. Bull. 2006, 29, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.Y.; Huang, Y.P.; Hsieh, W.B. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell. Adhes. Migr. 2013, 7, 237–245. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.K. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Jeong, D.Y.; Jeong, S.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suantawee, T.; Cheng, H.; Adisakwattana, S. Protective effect of cyanidin against glucose-and methylglyoxal-induced protein glycation and oxidative DNA damage. Int. J. Biol. Macromol. 2016, 93, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Elhabiri, M.; Figueiredo, P.; Fougerousse, A.; Brouillard, R. A convenient method for conversion of flavonols into anthocyanins. Tetrahedron Lett. 1995, 36, 4611–4614. [Google Scholar] [CrossRef]
- Ernst, I.; Wagner, A.; Lipinski, S.; Skrbek, S.; Ruefer, C.E.; Desel, C.; Rimbach, G. Cellular uptake, stability, visualization by ‘Naturstoff reagent A’, and multidrug resistance protein 1 gene-regulatory activity of cyanidin in human keratinocytes. Pharmacol. Res. 2010, 61, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.; Virsolvy, A.; Quignard, J.F.; Ravier, M.A.; Bertrand, G.; Dalle, S.; Cros, D.; Magous, R.; Richard, S.; Oiry, C. Quercetin induces insulin secretion by direct activation of l-type calcium channels in pancreatic beta cells. Br. J. Pharmacol. 2013, 169, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Kappel, V.D.; Frederico, M.J.; Postal, B.G.; Mendes, C.P.; Cazarolli, L.H.; Silva, F.R.M.B. The role of calcium in intracellular pathways of rutin in rat pancreatic islets: Potential insulin secretagogue effect. Eur. J. Pharmacol. 2013, 702, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Shoji, S.; Ichinose, S.; Yamagata, K.; Tagami, M.; Hiraoka, M. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell Calcium 2002, 32, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Lacinova, L.; Klugbauer, N. Voltage-dependent calcium channels: From structure to function. Rev. Physiol. Biochem. Pharmacol. 1999, 139, 33–87. [Google Scholar] [PubMed]
- Kittl, M.; Beyreis, M.; Tumurkhuu, M.; Fuerst, J.; Helm, K.; Pitschmann, A.; Gaisberger, M.; Glasl, S.; Ritter, M.; Jakab, M. Quercetin stimulates insulin secretion and reduces the viability of rat INS-1 beta-Cells. Cell. Physiol. Biochem. 2016, 39, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Hsu, W.; Yibchok-anun, S. Mechanisms of p-methoxycinnamic acid-induced increase in insulin secretion. Horm. Metab. Res. 2011, 43, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Ammälä, C.; Moorhouse, A.; Ashcroft, F. The sulphonylurea receptor confers diazoxide sensitivity on the inwardly rectifying K+ channel Kir6.1 expressed in human embryonic kidney cells. J. Physiol. 1996, 494, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, D. Long-term exposure to genistein improves insulin secretory function of pancreatic β-cells. Eur. J. Pharmacol. 2009, 616, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Van Camp, J.; Vissenaekens, H.; Raes, K.; Smagghe, G.; Grootaert, C. Review on the use of cell cultures to study metabolism, transport, and accumulation of flavonoids: From mono-cultures to co-culture systems. Compr. Rev. Food Sci. Food Saf. 2015, 14, 741–754. [Google Scholar] [CrossRef]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Chabane, M.N.; Ahmad, A.A.; Peluso, J.; Muller, C.D.; Ubeaud-Séquier, G. Quercetin and naringenin transport across human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2009, 61, 1473–1483. [Google Scholar] [CrossRef]
- Glaeser, H.; Bujok, K.; Schmidt, I.; Fromm, M.F.; Mandery, K. Organic anion transporting polypeptides and organic cation transporter 1 contribute to the cellular uptake of the flavonoid quercetin. Naunyn-Schmiedebergs Arch. Pharmacol. 2014, 387, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; Freitas, D.V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell. Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bonny, C.; Roduit, R.; Gremlich, S.; Nicod, P.; Thorensb, B.; Waebera, G. The loss of GLUT2 expression in the pancreatic β-cells of diabetic db/db mice is associated with an impaired DNA-binding activity of islet-specific trans-acting factors. Mol. Cell. Endocrinol. 1997, 135, 59–65. [Google Scholar] [PubMed]
- Orci, L.; Ravazzola, M.; Baetens, D.; Inman, L.; Amherdt, M.; Peterson, R.G.; Newgard, C.B.; Johnson, J.H.; Unger, R.H. Evidence that down-regulation of beta-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia. Proc. Natl. Acad. Sci. USA 1990, 87, 9953–9957. [Google Scholar] [PubMed]
- Chen, F.; Zheng, D.; Xu, Y.; Luo, Y.; Li, H.; Yu, K.; Song, Y.; Zhong, W.; Ji, Y. Down-regulation of Kir6.2 affects calcium influx and insulin secretion in HIT-T15 cells. J. Pediatr. Endocrinol. Metab. 2010, 23, 709–717. [Google Scholar] [PubMed]
- Cernea, S.; Dobreanu, M. Diabetes and beta cell function: From mechanisms to evaluation and clinical implications. Biochem. Medica 2013, 23, 266–280. [Google Scholar]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Chang, Y.Y.; Kuo, H.F.; Hsu, Y.W.; Pan, T.M. Ankaflavin: A natural novel PPARγ agonist upregulates Nrf2 to attenuate methylglyoxal-induced diabetes in vivo. Free Radic. Biol. Med. 2012, 53, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Oksbjerg, N.; Young, J.; Jeppesen, P. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes. Metab. 2014, 16, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Díaz-García, C.M.; Larqué, C.; Hiriart, M. Modulation of ionic channels and insulin secretion by drugs and hormones in pancreatic beta cells. Mol. Pharmacol. 2016, 90, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Nitert, M.D.; Nagorny, C.L.; Wendt, A.; Eliasson, L.; Mulder, H. CaV1.2 rather than CaV1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. J. Mol. Endocrinol. 2008, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moritz, W.; Leech, C.A.; Ferrer, J.; Habener, J.F. Regulated expression of adenosine triphosphate-sensitive potassium channel subunits in pancreatic β-cells. Endocrinology 2001, 142, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Bedoui, J.; Madeira, S.F.; Chalupsky, K.; Schini-Kerth, V. Delphinidin and cyanidin inhibit PDGFAB-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br. J. Pharmacol. 2006, 149, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hoang, M.H.; Jun, H.J.; Lee, J.H.; Lee, S.J. Cyanidin, a natural flavonoid, is an agonistic ligand for liver X receptor alpha and beta and reduces cellular lipid accumulation in macrophages and hepatocytes. Bioorganic Med. Chem. Lett. 2013, 23, 4185–4190. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kim, J.Y.; Jun, H.J.; Kim, S.J.; Kim, S.J.; Lee, J.H.; Hoang, M.H.; Kim, H.S.; Chang, H.I.; Hwang, K.Y.; et al. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim. Biophys. Acta 2013, 1831, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Vitaglione, P.; Fogliano, V.; Vanella, L.; Felgines, C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann. Ist. Super. Sanita 2006, 43, 382–393. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suantawee, T.; Elazab, S.T.; Hsu, W.H.; Yao, S.; Cheng, H.; Adisakwattana, S. Cyanidin Stimulates Insulin Secretion and Pancreatic β-Cell Gene Expression through Activation of l-type Voltage-Dependent Ca2+ Channels. Nutrients 2017, 9, 814. https://doi.org/10.3390/nu9080814
Suantawee T, Elazab ST, Hsu WH, Yao S, Cheng H, Adisakwattana S. Cyanidin Stimulates Insulin Secretion and Pancreatic β-Cell Gene Expression through Activation of l-type Voltage-Dependent Ca2+ Channels. Nutrients. 2017; 9(8):814. https://doi.org/10.3390/nu9080814
Chicago/Turabian StyleSuantawee, Tanyawan, Sara T. Elazab, Walter H. Hsu, Shaomian Yao, Henrique Cheng, and Sirichai Adisakwattana. 2017. "Cyanidin Stimulates Insulin Secretion and Pancreatic β-Cell Gene Expression through Activation of l-type Voltage-Dependent Ca2+ Channels" Nutrients 9, no. 8: 814. https://doi.org/10.3390/nu9080814