Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants
Abstract
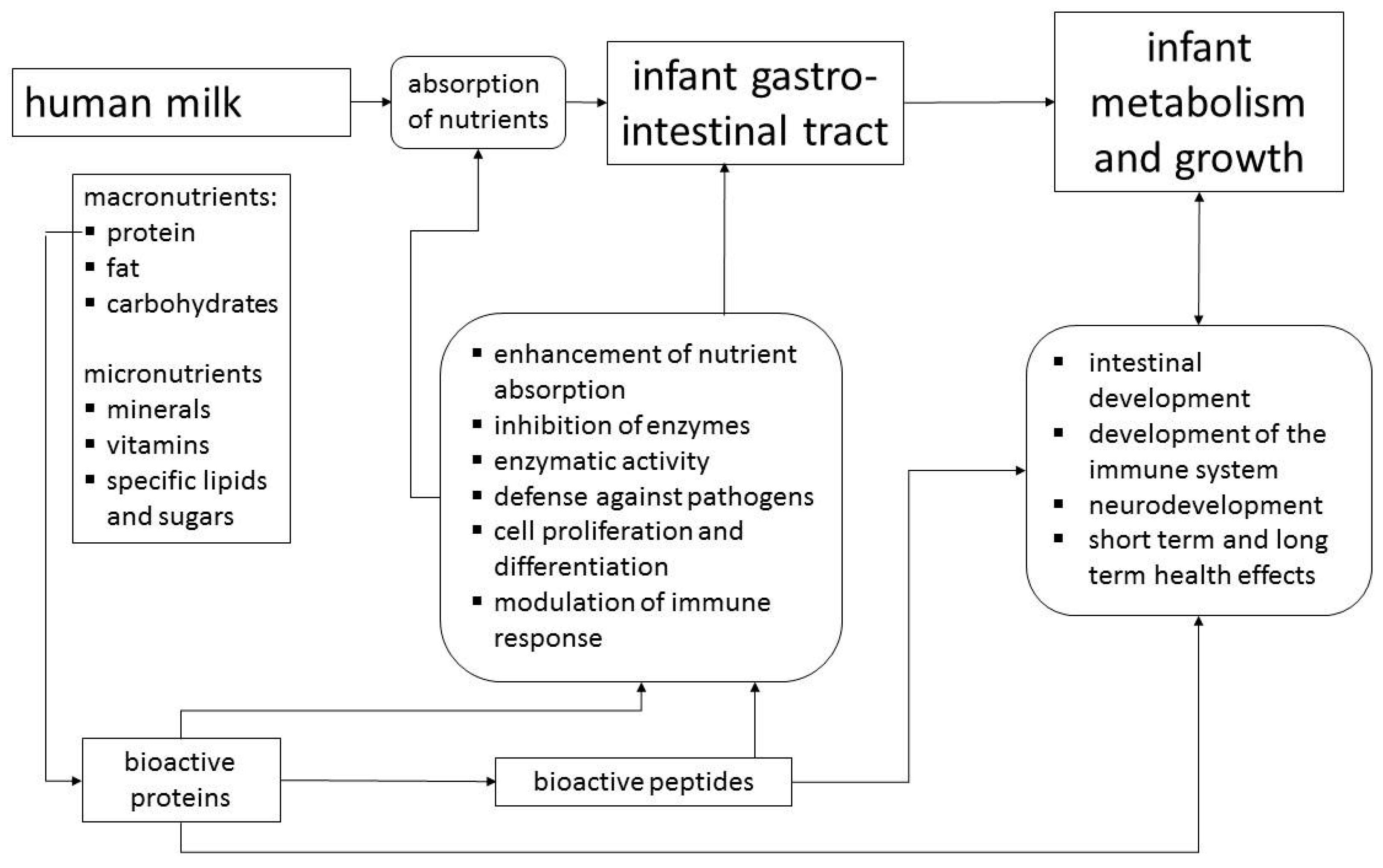
:1. Introduction
2. Human Milk Proteins with Bioactivity
2.1. Casein
2.2. α-Lactalbumin
2.3. Secretory IgA and Lysozyme
2.4. Haptocorrin and Bile Salt Stimulated Lipase
2.5. Lactoferrin
2.6. Osteopontin
2.7. Clinical Trial with OPN
2.8. Milk Fat Globule Membrane
2.9. Clinical Trials with MFGM Components
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prell, C.; Koletzko, B. Breastfeeding and complementary feeding. Dtsch. Arztebl. Int. 2016, 113, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Bioactive proteins in human milk: Mechanisms of action. J. Pediatr. 2010, 156, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch. Gen. Psychiatry 2008, 65, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.W.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell. B 2013, 45, 1730–1747. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Duan, R.D.; Brevaut-Malaty, V.; Gire, C.; Millet, V.; Simeoni, U.; Bernard, M.; Armand, M. Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: An overview. Cell. Mol. Biol. 2013, 59, 108–131. [Google Scholar] [PubMed]
- Hernell, O.; Timby, N.; Domellöf, M.; Lönnerdal, B. Clinical benefits of milk fat globule membranes for infants and children. J. Pediatr. 2016, 173, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Lönnerdal, B. Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Pediatr. Res. 2015, 77, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Deglaire, A.; De Oliveira, S.C.; Jardin, J.; Briard-Bion, V.; Emily, M.; Menard, O.; Bourlieu, C.; Dupont, D. Impact of human milk pasteurization on the kinetics of peptide release during in vitro dynamic term newborn digestion. Electrophoresis 2016, 37, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Smink, C.J.; Robinson, R.C.; Tian, T.; Guerrero, A.; Parker, E.A.; Smilowitz, J.T.; Hettinga, K.A.; Underwood, M.A.; Lebrilla, C.B.; et al. Endogenous human milk peptide release is greater after preterm birth than term birth. J. Nutr. 2015, 145, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Sandström, B.; Lönnerdal, B. The effect of casein phosphopeptides on zinc and calcium absorption from high phytate infant diets assessed in rat pups and Caco-2 cells. Pediatr. Res. 1996, 40, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins—Mechanisms of action. J. Nutr. Biochem. 2014, 25, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine alpha-lactalbumin molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Strömqvist, M.; Falk, P.; Bergström, S.; Hansson, L.; Lönnerdal, B.; Normark, S.; Hernell, O. Human milk kappa-casein and inhibition of Helicobacter pylori adhesion to human gastric mucosa. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Glazier, C. Calcium binding by alpha-lactalbumin in human milk and bovine milk. J. Nutr. 1985, 115, 1209–1216. [Google Scholar] [PubMed]
- Ren, J.; Stuart, D.I.; Acharya, K.R. Alpha-lactalbumin possesses a distinct zinc binding site. J. Biol. Chem. 1993, 268, 19292–19298. [Google Scholar] [PubMed]
- Bruck, W.M.; Kelleher, S.L.; Gibson, G.R.; Nielsen, K.E.; Chatterton, D.E.; Lönnerdal, B. rRNA probes used to quantify the effects of glycomacropeptide and alpha-lactalbumin supplementation on the predominant groups of intestinal bacteria of infant rhesus monkeys challenged with enteropathogenic Escherichia coli. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S. The immune system of human milk: Antimicrobial, antiinflammatory and immunomodulating properties. Pediatr. Infect. Dis. J. 1993, 12, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Antimicrobial activity of lysozyme with special relevance to milk. Afr. J. Biotechnol. 2008, 7, 4856–4867. [Google Scholar]
- Akinbi, H.; Meinzen-Derr, J.; Auer, C.; Ma, Y.; Pullum, D.; Kusano, R.; Reszka, K.J.; Zimmerly, K. Alterations in the host defense properties of human milk following prolonged storage or pasteurization. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, S.; Kunz, C. Protein and nonprotein nitrogen components in human milk, bovine milk, and infant formula: Quantitative and qualitative aspects in infant nutrition. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Teschemacher, H.; Koch, G.; Brantl, V. Milk protein-derived opioid receptor ligands. Biopolymers 1997, 43, 99–117. [Google Scholar] [CrossRef]
- Kost, N.V.; Sokolov, O.Y.; Kurasova, O.B.; Dmitriev, A.D.; Tarakanova, J.N.; Gabaeva, M.V.; Zolotarev, Y.A.; Dadayan, A.K.; Grachev, S.A.; Korneeva, E.V.; et al. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides 2009, 30, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.; Bernard, H.; Fairweather-Tait, S.; FitzGerald, R.J.; Hartmann, R.; Lane, C.N.; McDonagh, D.; Teucher, B.; Wal, J.M. Detection of caseinophosphopeptides in the distal ileostomy fluid of human subjects. Br. J. Nutr. 2003, 89, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Chabance, B.; Marteau, P.; Rambaud, J.C.; Migliore-Samour, D.; Boynard, M.; Perrotin, P.; Guillet, R.; Jolles, P.; Fiat, A.M. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 1998, 80, 155–165. [Google Scholar] [CrossRef]
- Jackson, J.G.; Janszen, D.B.; Lönnerdal, B.; Lien, E.L.; Pramuk, K.P.; Kuhlman, C.F. A multinational study of alpha-lactalbumin concentrations in human milk. J. Nutr. Biochem. 2004, 15, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Lien, E.L. Nutritional and physiologic significance of alpha-lactalbumin in infants. Nutr. Rev. 2003, 61, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Sandström, O.; Lönnerdal, B.; Graverholt, G.; Hernell, O. Effects of alpha-lactalbumin-enriched formula containing different concentrations of glycomacropeptide on infant nutrition. Am. J. Clin. Nutr. 2008, 87, 921–928. [Google Scholar] [PubMed]
- Rai, D.; Adelman, A.S.; Zhuang, W.; Rai, G.P.; Boettcher, J.; Lönnerdal, B. Longitudinal changes in lactoferrin concentrations in human milk: A global systematic review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Liepke, C.; Adermann, K.; Raida, M.; Magert, H.J.; Forssmann, W.G.; Zucht, H.D. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 2002, 269, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Pezo, A.; Cruz, K.; Chea-Woo, E.; Cleary, T.G. Clinical studies of lactoferrin in children. Biochem. Cell. Biol. 2012, 90, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Lönnerdal, B. Persistence of human-milk proteins in the breast-fed infant. Acta Paediatr. Scand. 1987, 76, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Greibe, E.; Lildballe, D.L.; Streym, S.; Vestergaard, P.; Rejnmark, L.; Mosekilde, L.; Nexo, E. Cobalamin and haptocorrin in human milk and cobalamin-related variables in mother and child: A 9-mo longitudinal study. Am. J. Clin. Nutr. 2013, 98, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Lönnerdal, B. Potential host-defense role of a human milk vitamin B-12-binding protein, haptocorrin, in the gastrointestinal tract of breastfed infants, as assessed with porcine haptocorrin in vitro. Am. J. Clin. Nutr. 2003, 77, 1234–1240. [Google Scholar] [PubMed]
- Adkins, Y.; Lönnerdal, B. Mechanisms of vitamin B(12) absorption in breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Biochemistry and physiological function of human milk proteins. Am. J. Clin. Nutr. 1985, 42, 1299–1317. [Google Scholar] [PubMed]
- Hamosh, M. Protective function of proteins and lipids in human milk. Biol. Neonate 1998, 74, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Newburg, D.S. Human milk glycoproteins protect infants against human pathogens. Breastfeed Med. 2013, 8, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.; Hernell, O. Lipid digestion and absorption in early life: An update. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Casper, C.; Carnielli, V.P.; Hascoet, J.M.; Lapillonne, A.; Maggio, L.; Timdahl, K.; Olsson, B.; Vagero, M.; Hernell, O. rhBSSL improves growth and LCPUFA absorption in preterm infants fed formula or pasteurized breast milk. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ruvoen-Clouet, N.; Mas, E.; Marionneau, S.; Guillon, P.; Lombardo, D.; Le Pendu, J. Bile-salt-stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem. J. 2006, 393, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M. Enzymes in human milk. In Handbook of Milk Composition; Jensen, R., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 247–388. [Google Scholar]
- Schack, L.; Lange, A.; Kelsen, J.; Agnholt, J.; Christensen, B.; Petersen, T.E.; Sorensen, E.S. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J. Dairy Sci. 2009, 92, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Monaco, M.H.; Drnevich, J.; Kvistgaard, A.S.; Hernell, O.; Lönnerdal, B. Bovine osteopontin modifies the intestinal transcriptome of formula-fed infant rhesus monkeys to be more similar to those that were breastfed. J. Nutr. 2014, 144, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Kvistgaard, A.S.; Peerson, J.M.; Donovan, S.M.; Peng, Y.M. Growth, nutrition, and cytokine response of breast-fed infants and infants fed formula with fdded bovine osteopontin. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Casper, C.; Hascoet, J.M.; Ertl, T.; Gadzinowski, J.S.; Carnielli, V.; Rigo, J.; Lapillonne, A.; Couce, M.L.; Vagero, M.; Palmgren, I.; et al. Recombinant bile balt-stimulated lipase in preterm infant feeding: A randomized phase 3 study. PLoS ONE 2016, 11, e0156071. [Google Scholar] [CrossRef] [PubMed]
- Naarding, M.A.; Dirac, A.M.; Ludwig, I.S.; Speijer, D.; Lindquist, S.; Vestman, E.L.; Stax, M.J.; Geijtenbeek, T.B.; Pollakis, G.; Hernell, O.; et al. Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells. Antimicrob. Agents Chemother. 2006, 50, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537s–1543s. [Google Scholar] [PubMed]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Friedman, R. Evolutionary diversification of the vertebrate transferrin multi-gene family. Immunogenetics 2014, 66, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montoya, I.A.; Cendon, T.S.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. BBA-Gen. Subjects 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Spik, G.; Coddeville, B.; Mazurier, J.; Bourne, Y.; Cambillaut, C.; Montreuil, J. Primary and three-dimensional structure of lactotransferrin (lactoferrin) glycans. Adv. Exp. Med. Biol. 1994, 357, 21–32. [Google Scholar] [PubMed]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lönnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteomics 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.; Wildeboer-Veloo, A.C.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Effects of milk and milk components on calcium, magnesium, and trace element absorption during infancy. Physiol. Rev. 1997, 77, 643–669. [Google Scholar] [PubMed]
- Fransson, G.B.; Lönnerdal, B. Iron in human milk. J. Pediatr. 1980, 96, 380–384. [Google Scholar] [CrossRef]
- Hirai, Y.; Kawakata, N.; Satoh, K.; Ikeda, Y.; Hisayasu, S.; Orimo, H.; Yoshino, Y. Concentrations of lactoferrin and iron in human milk at different stages of lactation. J. Nutr. Sci. Vitaminol. (Tokyo) 1990, 36, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.J.; Rogers, H.J.; Leigh, L. Iron-binding proteins in milk and resistance to Escherichia-Coli infection in infants. Br. Med. J. 1972, 1, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin—Sensitivity of a variety of microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [PubMed]
- Arnold, R.R.; Russell, J.E.; Champion, W.J.; Gauthier, J.J. Bactericidal activity of human lactoferrin—Influence of physical conditions and metabolic state of the target microorganism. Infect. Immun. 1981, 32, 655–660. [Google Scholar] [PubMed]
- Brandenburg, K.; Jurgens, G.; Muller, M.; Fukuoka, S.; Koch, M.H.J. Biophysical characterization of lipopolysaccharide and lipid a inactivation by lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.T.; Giehl, T.J.; Laforce, F.M. Damage of the outer-membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar] [PubMed]
- Ellison, R.T.; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investog. 1991, 88, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.L.; Du, X.G.; Lönnerdal, B. Comparison of bioactivities of talactoferrin and lactoferrins from human and bovine milk. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell. Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Fransson, G.B.; Keen, C.L.; Lönnerdal, B. Supplementation of milk with iron bound to lactoferrin using weanling mice: L. Effects on hematology and tissue iron. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.L.; Jiang, R.L.; Lönnerdal, B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem. Cell. Biol. 2012, 90, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.L.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell. Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Furmanski, P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature 1995, 373, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.L.; Lönnerdal, B. Transcriptomic profiling of intestinal epithelial cells in response to human, bovine and commercial bovine lactoferrins. Biometals 2014, 27, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Shimamura, M.; Matsuda, E.; Fujita, K.; Nomoto, H.; Satoh, J.; Kojima, S.; Alexander, D.B.; Moore, M.A.; Tsuda, H. Orally administered bovine lactoferrin induces caspase-1 and interleukin-18 in the mouse intestinal mucosa: A possible explanation for inhibition of carcinogenesis and metastasis. Cytokine 2004, 25, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. Faseb. J. 2008, 22, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.P. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn. J. Cancer Res. 2000, 91, 1022–1027. [Google Scholar] [CrossRef]
- De la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J. The immunological components of human milk and their effect of immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [PubMed]
- Yoshida, A.; Wei, Z.; Shinmura, Y.; Fukunaga, N. Separation of lactoferrin-a and -b from bovine colostrum. J. Dairy Sci. 2000, 83, 2211–2215. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Jiang, R.L.; Du, X.O. Bovine Lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Maga, E.A.; Murray, J.D. Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: Past, present, and future. Transgenic Res. 2015, 24, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Yemets, A.I.; Tanasienko, I.V.; Krasylenko, Y.A.; Blume, Y.B. Plant-based biopharming of recombinant human lactoferrin. Cell. Biol. Int. 2014, 38, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Lactoferrin, a bird‘s eye view. Biochem. Cell. Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Biological effects of novel bovine milk fractions. Nestle Nutr. Workshop Ser. Pediatr. Program 2011, 67, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- King, J.C., Jr.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sanchez, J.; Alfaro, S.; Lönnerdal, B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chai, L.; Li, H.; Zhang, Y.; Xie, H.M.; Shang, J.; Tian, W.; Yang, P.; Jiang, A.C. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Lan, Z.; Hua, L.; Ying, Z.; Humina, X.; Jia, S.; Weizheng, T.; Ping, Y.; Lingying, C.; Meng, M. Iron metabolism in infants: Influence of bovine lactoferrin from iron-fortified formula. Nutrition 2015, 31, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Abrams, S.A. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2015, 2, CD007137. [Google Scholar] [CrossRef]
- Christensen, B.; Sorensen, E.S. Structure, function and nutritional potential of milk osteopontin. Int. Dairy J. 2016, 57, 1. [Google Scholar] [CrossRef]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegard, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Biological roles of milk osteopontin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, A.; Castronovo, V.; Ogbureke, K.U.; Fisher, L.W.; Fedarko, N.S. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat. Rev. Cancer 2008, 8, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Gimba, E.R.; Tilli, T.M. Human osteopontin splicing isoforms: Known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013, 331, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Mittal, A.; Weiner, H.L. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J. Immunol. 2008, 181, 7480–7488. [Google Scholar] [CrossRef] [PubMed]
- Rittling, S.R.; Singh, R. Osteopontin in immune-mediated diseases. J. Dent. Res. 2015, 94, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.H.; Sanchez-Ross, M.; Kaluski, E.; Klapholz, M. Osteopontin in cardiovascular disease: A potential therapeutic target. Cardiol. Rev. 2010, 18, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, R.; Bernasconi, L.; Losberger, C.; Graber, P.; Kadi, L.; Avellana-Adalid, V.; Picard-Riera, N.; Baron Van Evercooren, A.; Cirillo, R.; Kosco-Vilbois, M.; et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell. Neurosci. 2004, 25, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, T.; Ohga, S.; Takada, H.; Nomura, A.; Hikino, S.; Imura, M.; Ohshima, K.; Hara, T. Microarray analysis of human milk cells: Persistent high expression of osteopontin during the lactation period. Clin. Exp. Immunol. 2004, 138, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.E.; Christou, H.; Park, K.H.; Mantzoros, C.S. Cord blood levels of osteopontin as a phenotype marker of gestational age and neonatal morbidities. Obesity (Silver Spring) 2014, 22, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.R.J.T.; Heegaard, C.W.; Sorensen, E.S.; Petersen, T.E. In vitro digestion of novel milk protein ingredients for use in infant formulas: Research on biological functions. Trends Food Sci. Technol. 2004, 15, 373–383. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Ellen, R.P.; Sorensen, E.S.; Goldberg, H.A.; Zohar, R.; Sodek, J. Osteopontin attenuation of dextran sulfate sodium-induced colitis in mice. Lab. Investig. 2009, 89, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Rittling, S.R.; Wejse, P.L.; Yagiz, K.; Warot, G.A.; Hui, T. Suppression of tumour growth by orally administered osteopontin is accompanied by alterations in tumour blood vessels. Br. J. Cancer 2014, 110, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Kvistgaard, A.S.; Matulka, R.A.; Dolan, L.C.; Ramanujam, K.S. Pre-clinical in vitro and in vivo safety evaluation of bovine whey derived osteopontin, Lacprodan(R) OPN-10. Food Chem. Toxicol. 2014, 73, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kainonen, E.; Rautava, S.; Isolauri, E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br. J. Nutr. 2013, 109, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Heid, H.W.; Keenan, T.W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell. Biol. 2005, 84, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 2011, 10, 3530–3541. [Google Scholar] [CrossRef] [PubMed]
- Mather, I.H. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci. 2000, 83, 203–247. [Google Scholar] [CrossRef]
- Peterson, J.A.; Hamosh, M.; Scallan, C.D.; Ceriani, R.L.; Henderson, T.R.; Mehta, N.R.; Armand, M.; Hamosh, P. Milk fat globule glycoproteins in human milk and in gastric aspirates of mother's milk-fed preterm infants. Pediatr. Res. 1998, 44, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Patton, S.; Hamosh, M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate. 1998, 74, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, Z.; Chen, C.; Kling, D.E.; Newburg, D.S. Human milk mucin 1 and mucin 4 inhibit Salmonella enterica serovar Typhimurium invasion of human intestinal epithelial cells in vitro. J. Nutr. 2012, 142, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Saeland, E.; de Jong, M.A.; Nabatov, A.A.; Kalay, H.; Geijtenbeek, T.B.; van Kooyk, Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol. Immunol. 2009, 46, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.; Kessen, S.F.; Van Der Voorn, J.P.; Appelmelk, B.J.; Jeurink, P.V.; Knippels, L.M.; Garssen, J.; Van Kooyk, Y. Human milk blocks DC-SIGN-pathogen interaction via MUC1. Front. Immunol. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-derived reactive species: Physiological and Pathological Effects. Oxid. Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R. Milk xanthine oxidase: Properties and physiological roles. Int. Dairy J. 2006, 16, 546–554. [Google Scholar] [CrossRef]
- Stevens, C.R.; Millar, T.M.; Clinch, J.G.; Kanczler, J.M.; Bodamyali, T.; Blake, D.R. Antibacterial properties of xanthine oxidase in human milk. Lancet 2000, 356, 829–830. [Google Scholar] [CrossRef]
- Yolken, R.H.; Peterson, J.A.; Vonderfecht, S.L.; Fouts, E.T.; Midthun, K.; Newburg, D.S. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Investig. 1992, 90, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.F.; Zuo, X.L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.D. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Investig. 2007, 117, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.; Ensslin, M.A.; Shur, B.D. SED1/MFG-E8: A bi-motif protein that orchestrates diverse cellular interactions. J. Cell. Biochem. 2009, 106, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.A.; Reith, W.; Trowsdale, J. Regulation of immunity by butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.L.; Weldon, A.K.; Dobbie, L.; Smith, A.J.; Mather, I.H. Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc. Natl. Acad. Sci. USA 2004, 101, 10084–10089. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.A.; Knezevic, B.R.; Ammann, J.U.; Rhodes, D.A.; Aw, D.; Palmer, D.B.; Mather, I.H.; Trowsdale, J. BTN1A1, the mammary gland butyrophilin, and BTN2A2 are both inhibitors of T cell activation. J. Immunol. 2010, 184, 3514–3525. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Zhang, W.; Liu, L.; Pang, X.; Zhang, S.; Lv, J. Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chem. 2016, 196, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, A.M.; Kelly, P.M.; Stanton, C.; Devery, R.A. Milk fat globule membrane-a source of polar lipids for colon health? A review. Int. J. Dairy Technol. 2012, 65, 315–333. [Google Scholar] [CrossRef]
- Nilsson, A. Role of sphingolipids in infant gut health and immunity. J. Pediatr. 2016, 173, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Motouri, M.; Matsuyama, H.; Yamamura, J.; Tanaka, M.; Aoe, S.; Iwanaga, T.; Kawakami, H. Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.D.; Nilsson, A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 2009, 48, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kiyono, H. Immunological function of sphingosine 1-phosphate in the intestine. Nutrients 2012, 4, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Haruta-Ono, Y.; Setoguchi, S.; Ueno, H.M.; Higurashi, S.; Ueda, N.; Kato, K.; Saito, T.; Matsunaga, K.; Takata, J. Orally administered sphingomyelin in bovine milk is incorporated into skin sphingolipids and is involved in the water-holding capacity of hairless mice. J. Dermatol. Sci. 2012, 68, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; McJarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R. The role of dietary gangliosides on immunity and the prevention of infection. Br. J. Nutr. 2007, 98, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Wang, M.; Monaco, M.H.; Alexander, L.S.; Mudd, A.T.; Chichlowski, M.; Waworuntu, R.V.; Berg, B.M.; Miller, M.J.; Dilger, R.N.; et al. Prebiotics and bioactive milk fractions affect gut development, microbiota, and neurotransmitter expression in piglets. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, G.; Allaire, J.M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk fat globule membrane supplementation in formula modulates the neonatal gut microbiome and normalizes intestinal development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef] [PubMed]
- Claumarchirant, L.; Cilla, A.; Matencio, E.; Sanchez-Siles, L.M.; Castro-Gomez, P.; Fontecha, J.; Alegría, A.; Lagarda, M.J. Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. Int. Dairy J. 2016, 61, 228–238. [Google Scholar] [CrossRef]
- O'Riordan, N.; Kane, M.; Joshi, L.; Hickey, R.M. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology 2014, 24, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E. Early determinants of development: A lipid perspective. Am. J. Clin. Nutr. 2009, 89, 1523S–1529S. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.; Moreira, E.M.; Kiefte-de Jong, J.C.; Darweesh, S.K.; Visser, T.; Voortman, T.; Bautista, P.K.; Chowdhury, R.; Gorman, D.; Bramer, W.M.; et al. Effects of choline on health across the life course: A systematic review. Nutr. Rev. 2015, 73, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; McGregor, R.A.; Wiessing, K.R.; Goyal, V.K.; Chitkara, A.J.; Gupta, S.; Palmano, K.; Kuhn-Sherlock, B.; McConnell, M.A. Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in northern Indian infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clin. Med. Insights Pediatr. 2014, 8, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Comment on “Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants”. Clin. Med. Insights Pediatr. 2015, 9, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 months of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Timby, N.; Lönnerdal, B.; Hernell, O.; Domellöf, M. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr. Res. 2014, 76, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Timby, N.; Hernell, O.; Vaarala, O.; Melin, M.; Lönnerdal, B.; Domellöf, M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef] [PubMed]
| Protein | Human Milk Concentration (mg/L) | Molecular Weight (kDa) | Human Milk Concentration (µmol/L) | Bioactivity a | Bioactivity in Cell or Animal Models (1) or in Clinical Trials (2) | Resistance to Digestion |
|---|---|---|---|---|---|---|
| β-casein | 3000–5000 [21] | 24 | 125–208 | peptides | (1) casein phosphopeptides support Ca absorption [11], (1) β-casomorphins act as opioid receptor ligands [22] (2) β-casomorphins appear in infant plasma [23] | casein phosphopeptides detected in ileostomy fluids of adults [24] |
| κ-casein | 1000–3000 [21] | 30 | 33–100 | protein/peptides | (1) inhibition of H. pylori adhesion to gastric mucosa cells [14] (1) glycomacropeptide shows bifidogenic and pathogen inhibiting effects in the gut and anti-inflammatory effects after absorption [12] | κ-caseinglycopeptide detected in the duodenum of adults after milk consumption [25] |
| α-lactalbumin | 1800–3100 [26] | 14 | 129–221 | peptides | (1) activity against gram positive bacteria [13] (1) immunostimulation by GLF peptides [27] (2) increased serum Fe [28] | slower digested than caseins, but no intact α-lactalbumin detected in infant faecal samples [27] |
| lactoferrin | 1200–3000 [29] | 77 | 16–39 | protein/peptides | (1) antimicrobial and immunomodulating effects, influence on iron absorption in various models [30] (1) antimicrobial activity of lactoferricin H and B [31] (1) bifidogenic activity [32] (2) beneficial effects [33] | detected in fecal samples of breast fed infants [34] |
| haptocorrin | <0.7–7 | 68 | <0.01–0.1 (age 4 months) [35] | protein | (1) bacteriostatic effect of porcine haptocorrin [36] (1) contribution to Vit-B12 absorption [37] | in vitro resistance to proteolysis for porcine haptocorrin [36] |
| lysozyme | 50–250 [38] | 15 | 3–17 | protein | (1) cleavage of cell wall of gram positive bacteria [19] | resistant to peptic digestion, but susceptible to tryptic digestion [39] |
| secretory IgA | 500–1000 [38] | 420 | 1–2 | protein | (1) antibody, antimicrobial activity [18] | secretory IgA detected in fecal samples of breast fed infants [34] |
| bile-salt stimulated lipase | 100–200 [40] | 120–140 | <1–2 | protein | (1) lipolytic activity [41] (2) increased long chain fatty acid absorption [42] (1) inhibits the attachment of Norwalk virus-like particles to its cellular ligand [43] | stable at pH > 3 [44] |
| osteopontin | 60–220 [45] | 60 | 1–4 | protein/peptides | (1) altered intestinal gene expression in rhesus monkeys [46] (2) less fever and altered cytokine pattern [47] | human and bovine osteopontin are partially resistant to proteolysis by infant gastric juice at pH 4 [41] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. https://doi.org/10.3390/nu9080817
Demmelmair H, Prell C, Timby N, Lönnerdal B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients. 2017; 9(8):817. https://doi.org/10.3390/nu9080817
Chicago/Turabian StyleDemmelmair, Hans, Christine Prell, Niklas Timby, and Bo Lönnerdal. 2017. "Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants" Nutrients 9, no. 8: 817. https://doi.org/10.3390/nu9080817





