Iron Supplementation during Three Consecutive Days of Endurance Training Augmented Hepcidin Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Measurements
2.3.1. Determination of Running Velocity
2.3.2. Blood Sampling and Analyses
2.3.3. Scores of Fatigue and Muscle Soreness
2.3.4. Nutritional Assessment and Standard Meal
2.3.5. Statistical Analyses
3. Results
3.1. General Information during Training Sessions
3.2. Blood Parameters
3.2.1. Iron Parameter
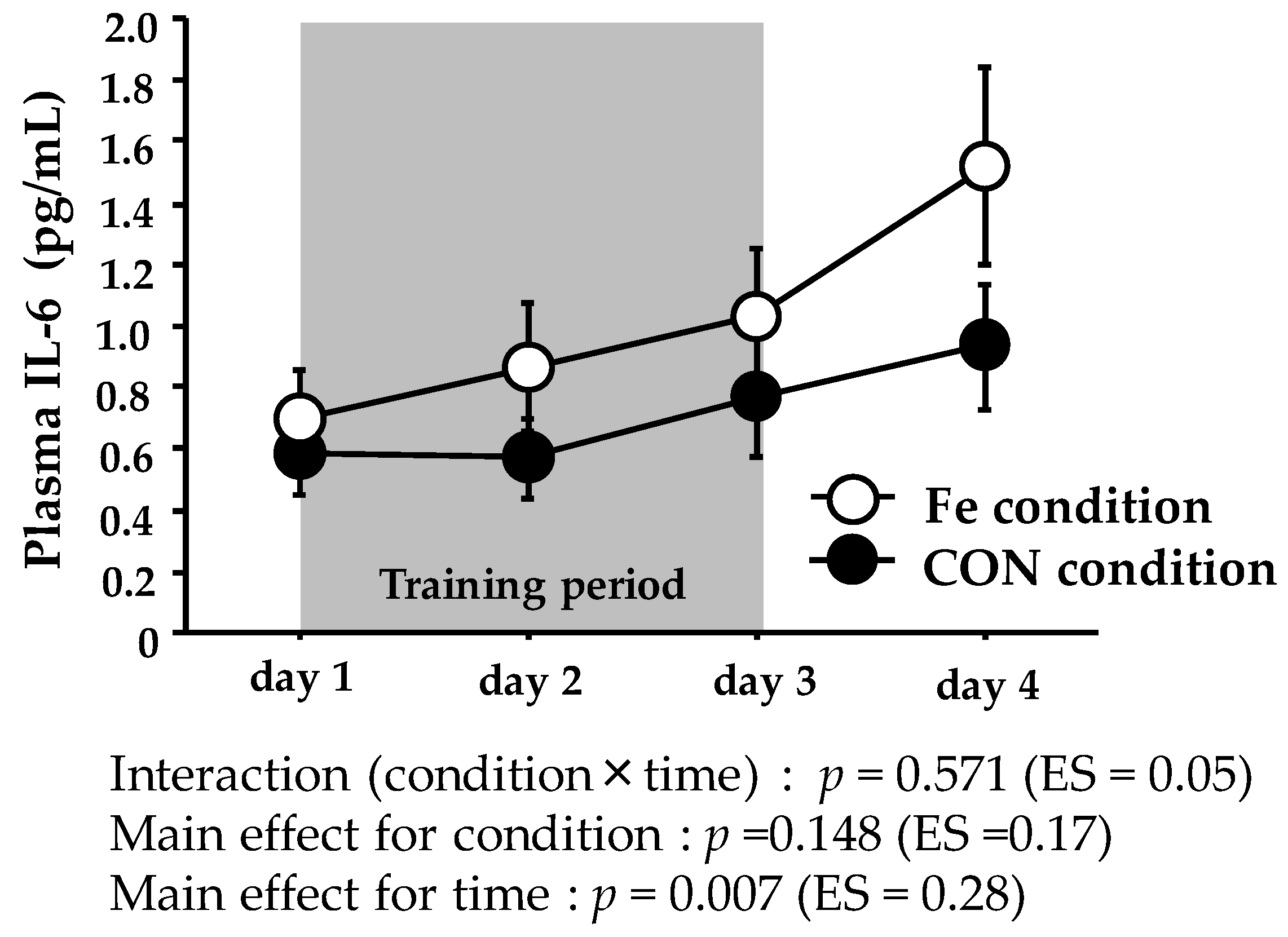
3.2.2. Muscle Damage and Inflammatory Parameters
3.2.3. Serum Hepcidin Level
3.3. Score of Fatigue and Muscle Soreness
3.4. Dietary Intake during the Training Periods
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL-6 | interleukin-6 |
| Hb | hemoglobin |
| TIBC | total iron-binding capacity |
| CK | creatine kinase |
| TSAT | transferrin saturation |
| CV | coefficients of variation |
| ES | effect size |
References
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594S–597S. [Google Scholar] [PubMed]
- Dubnov, G.; Constantini, N.W. Prevalence of iron depletion and anemia in top-level basketball players. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 30–37. [Google Scholar] [CrossRef]
- Eliakim, A.; Nemet, D.; Constantini, N. Screening blood tests in members of the Israeli National Olympic team. J. Sports Med. Phys. Fit. 2002, 42, 250–255. [Google Scholar]
- Stewart, J.G.; Ahlquist, D.A.; McGill, D.B.; Ilstrup, D.M.; Schwartz, S.; Owen, R.A. Gastrointestinal blood loss and anemia in runners. Ann. Int. Med. 1984, 100, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Pate, R.; Burgess, W. Foot impact force and intravascular hemolysis during distance running. Int. J. Sport 1988, 9, 56–60. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Fridlund, K.E.; Askew, E.W. Nutrition issues of military women. J. Am. Coll. Nutr. 1993, 12, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Magnusson, B.; Persson, H.; Hallberg, L. Iron losses in sweat. Am. J. Clin. Nutr. 1986, 43, 438–443. [Google Scholar] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Training surface and intensity: Inflammation, hemolysis, and hepcidin expression. Med. Sci. Sports Exerc. 2009, 41, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron imports IV. Hepcidin and regulation of body iron metabolism. Am. J. Physiol. 2006, 290, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Troesch, B.; Biebinger, R.; Egli, I.; Zeder, C.; Hurrell, R.F. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am. J. Clin. Nutr. 2009, 90, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-N.J.; Tomosugi, N.; Kawabata, H.; Ishikawa, T.; Nishikawa, T.; Yoshizaki, K. Down-regulation of hepcidin resulting from long-term treatment with an anti–IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood 2010, 116, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Pedlar, C.R.; Whyte, G.P.; Burden, R.; Moore, B.; Horgan, G.; Pollock, N. A case study of an iron-deficient female Olympic 1500-m runner. Int. J. Sports. Physiol. Perform. 2013, 8, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.J.; Pollock, N.; Whyte, G.P.; Richards, T.; Moore, B.; Busbridge, M.; Srai, S.K.; Pedlar, C.R. Impact of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Med. Sci. Sports Exerc. 2014, 47, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Giordano, C.; Brownlie, T.; Haas, J.D. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J. Appl. Physiol. 2000, 88, 1103–1111. [Google Scholar] [PubMed]
- Nielsen, P.; Nachtigall, D. Iron supplementation in athletes. Current recommendations. Sports Med. 1998, 26, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zotter, H.; Robinson, N.; Zorzoli, M.; Schattenberg, L.; Saugy, M.; Mangin, P. Abnormally high serum ferritin levels among professional road cyclists. Br. J. Sports Med. 2004, 38, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Deugnier, Y.; Loréal, O.; Carré, F.; Duvallet, A.; Zoulim, F.; Vinel, J.P.; Paris, J.C.; Blaison, D.; Moirand, R.; Turlin, B.; et al. Increased body iron stores in elite road cyclists. Med. Sci. Sports Exerc. 2002, 34, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Effects of exercise on hepcidin response and iron metabolism during recovery. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 583–597. [Google Scholar] [CrossRef]
- Newlin, M.K.; Williams, S.; McNamara, T.; Tjalsma, H.; Swinkels, D.W.; Haymes, E.M. The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Roecker, L.; Meier-Buttermilch, R.; Brechtel, L.; Nemeth, E.; Ganz, T. Iron-regulatory protein hepcidin is increased in female athletes after a marathon. Eur. J. Appl. Physiol. 2005, 95, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Ronsen, O.; Lea, T.; Bahr, R.; Pedersen, B.K. Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J. Appl. Physiol. 2002, 92, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron sequestration and anemia of inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; McKay, A.K.A.; Pyne, D.B.; Guelfi, K.J.; McCormick, R.H.; Laarakkers, C.M.; Swinkels, D.W.; Garvican-Lewis, L.A.; Ross, M.L.R.; Sharma, A.P.; et al. Factors influencing the post-exercise hepcidin-25 response in elite athletes. Eur. J. Appl. Physiol. 2017, 117, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.J.; Swinkels, D.W.; Tjalsma, H.; Wiegerinck, E.T.; Trinder, D.; Peeling, P. A seven day running training period increases basal urinary hepcidin levels as compared to cycling. J. Int. Soc. Sports Nutr. 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClung, J.P.; Martini, S.; Murphy, N.E.; Montain, S.J.; Margolis, L.M.; Thrane, I.; Spitz, M.G.; Blatny, J.-M.; Young, A.J.; Gundersen, Y.; et al. Effects of a 7-day military training exercise on inflammatory biomarkers, serum hepcidin, and iron status. Nutr. J. 2013, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, A.; Maeda, N.; Sumi, D.; Goto, K. Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners. Nutrients 2017, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Auersperger, I.; Knap, B.; Jerin, A.; Blagus, R.; Lainscak, M.; Skitek, M.; Skof, B. The effects of 8 weeks of endurance running on hepcidin concentrations, inflammatory parameters, and iron status in female runners. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 55–63. [Google Scholar] [CrossRef]
- Rietjens, G.J.; Kuipers, H.; Hartgens, F.; Keizer, H.A. Red blood cell profile of elite olympic distance triathletes. A three-year follow-up. Int. J. Sports Med. 2002, 23, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sumi, D.; Kojima, C.; Goto, K. Impact of Endurance Exercise in Hypoxia on Muscle Damage, Inflammatory and Performance Responses. J. Strength Cond. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- McCormack, H.M.; Horne, D.J.; Sheather, S. Clinical applications of visual analogue scales: A critical review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Dzedzej, A.; Ignatiuk, W.; Jaworska, J.; Grzywacz, T.; Lipińska, P.; Antosiewicz, J.; Korek, A.; Ziemann, E. The effect of the competitive season in professional basketball on inflammation and iron metabolism. Biol. Sport 2016, 33, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Kasprowicz, K.; Kasperska, A.; Zembro, A. Do High Blood Hepcidin Concentrations Contribute to Low Ferritin Levels in Young Tennis Players at the End of Tournament Season? J. Sports Sci. Med. 2013, 12, 249–258. [Google Scholar] [PubMed]
- Karl, J.P.; Lieberman, H.R.; Cable, S.J.; Williams, K.W.; Young, A.J.; McClung, J.P. Randomized, double-blind, placebo-controlled trial of an iron-fortified food product in female soldiers during military training: Relations between iron status, serum hepcidin, and inflammation. Am. J. Clin. Nutr. 2010, 92, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.; Lopez, M.; Gabayan, V.; Ganz, T.; Rivera, S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006, 108, 3073–3075. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Xie, L. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 2014, 146, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Tjalsma, H.; Trinder, D.; Peeling, P. Effect of exercise modality and intensity on post-exercise interleukin-6 and hepcidin levels. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 178–186. [Google Scholar] [CrossRef]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron Status and the Acute Post-Exercise Hepcidin Response in Athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Exercise and interleukin-6. Curr. Opin. Hematol. 2001, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur. J. Appl. Physiol. 2009, 106, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-S.; Enns, C.A. Iron homeostasis: Recently identified proteins provide insight into novel control mechanisms. J. Biol. Chem. 2009, 284, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Steensberg, A.; Pilegaard, H.; Osada, T.; Saltin, B.; Pedersen, B.K.; Neufer, P.D. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: Influence of muscle glycogen content. FASEB J. 2001, 15, 2748–2750. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. Eur. J. Appl. Physiol. 2015, 115, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Timing of post-exercise carbohydrate ingestion: Influence on IL-6 and hepcidin responses. Eur. J. Appl. Physiol. 2015, 115, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Townsend, M.-A.; Trinder, D.; Peeling, P. The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur. J. Appl. Physiol. 2012, 112, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Robson-Ansley, P.; Walshe, I.; Ward, D. The effect of carbohydrate ingestion on plasma interleukin-6, hepcidin and iron concentrations following prolonged exercise. Cytokine 2011, 53, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Sim, M.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Seven days of high carbohydrate ingestion does not attenuate post-exercise IL-6 and hepcidin levels. Eur. J. Appl. Physiol. 2016, 116, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Keen, C.; Commisso, J.; Killip, D.; Ou, C.N.; Rognerud, C.L.; Dennis, K.; Dunn, J.K. The effect of a marathon run on plasma and urine mineral and metal concentrations. J. Am. Coll. Nutr. 1998, 17, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Asobayire, F.S.; Adou, P.; Davidsson, L.; Cook, J.D.; Hurrell, R.F. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: A study in Côte d’Ivoire. Am. J. Clin. Nutr. 2001, 74, 776–782. [Google Scholar] [PubMed]
- Papanikolaou, G.; Tzilianos, M.; Christakis, J.I.; Bogdanos, D.; Tsimirika, K.; MacFarlane, J.; Goldberg, Y.P.; Sakellaropoulos, N.; Ganz, T.; Nemeth, E. Hepcidin in iron overload disorders. Blood 2005, 105, 4103–4105. [Google Scholar] [CrossRef] [PubMed]
- Deli, C.K.; Fatouros, I.G.; Paschalis, V.; Tsiokanos, A.; Georgakouli, K.; Zalavras, A.; Avloniti, A.; Koutedakis, Y.; Jamurtas, A.Z. Iron supplementation effects on redox status following septic skeletal muscle trauma in adults and children. Oxid. Med. Cell. Longev. 2017, 2017, 4120421. [Google Scholar] [CrossRef] [PubMed]


| Condition | Day 1 | Day 2 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Running distance | Fe | 34.5 | ± | 0.9 | 34.5 | ± | 0.9 | 34.5 | ± | 0.9 |
| (km) | CON | 33.3 | ± | 0.7 | 33.3 | ± | 0.7 | 33.3 | ± | 0.7 |
| Heart rate (bpm) | ||||||||||
| Ex1 | Fe | 158 | ± | 4 | 157 | ± | 4 | 154 | ± | 3 |
| CON | 156 | ± | 5 | 156 | ± | 4 | 154 | ± | 2 | |
| Ex2 | Fe | 158 | ± | 4 | 154 | ± | 4 | 156 | ± | 4 |
| CON | 159 | ± | 5 | 158 | ± | 3 | 153 | ± | 3 | |
| Condition | Day 1 | Day 2 | Day 3 | Day 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferritin | Fe | 47.9 | ± | 9.3 | 52.2 | ± | 9.3 | 56.9 | ± | 9.0 | 61.4 | ± | 10.0 |
| (ng/mL) | CON | 38.0 | ± | 9.5 | 39.8 | ± | 10.8 | 44.9 | ± | 11.2 | 47.6 | ± | 11.1 |
| Iron | Fe | 89.1 | ± | 11.5 | 115.6 | ± | 10.0 | 120.1 | ± | 9.3 | 91.3 | ± | 13.5 |
| (µg/dL) | CON | 73.3 | ± | 15.8 | 143.1 | ± | 23.3 | 120.1 | ± | 13.9 | 129.7 | ± | 7.5 |
| TSAT | Fe | 31.4 | ± | 5.0 | 40.9 | ± | 3.5 | 44.9 | ± | 5.6 | 34.1 | ± | 6.0 |
| (%) | CON | 24.4 | ± | 4.7 | 50.2 | ± | 10.1 | 42.4 | ± | 6.2 | 46.5 | ± | 3.7 |
| Condition | Day 1 | Day 2 | Day 3 | Day 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | Fe | 25 | ± | 7 | 30 | ± | 7 | 41 | ± | 10 | 42 | ± | 6 |
| (mm) | CON | 26 | ± | 4 | 39 | ± | 9 | 43 | ± | 7 | 42 | ± | 7 |
| Muscle Soreness | Fe | 16 | ± | 5 | 36 | ± | 6 | 57 | ± | 9 | 55 | ± | 7 |
| (mm) | CON | 23 | ± | 4 | 43 | ± | 9 | 59 | ± | 5 | 53 | ± | 7 |
| Condition | Day 1 | Day 2 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total energy | Fe | 10,850 | ± | 130 | 11,005 | ± | 110 | 10,761 | ± | 136 |
| (KJ) | CON | 10,560 | ± | 65 | 10,621 | ± | 74 | 10,661 | ± | 71 |
| CHO | Fe | 395.3 | ± | 7.8 | 402.5 | ± | 7.7 | 388.2 | ± | 6.8 |
| (g) | CON | 378.4 | ± | 3.4 | 381.0 | ± | 3.7 | 383.7 | ± | 3.7 |
| Protein | Fe | 87.0 | ± | 0.2 | 87.9 | ± | 0.8 | 88.9 | ± | 1.4 |
| (g) | CON | 86.8 | ± | 0.2 | 88.0 | ± | 0.7 | 87.2 | ± | 0.2 |
| Fat | Fe | 65.2 | ± | 0.04 | 65.9 | ± | 0.47 | 65.3 | ± | 0.04 |
| (g) | CON | 65.2 | ± | 0.04 | 65.2 | ± | 0.04 | 65.3 | ± | 0.04 |
| Iron | Fe | 30.5 | ± | 0.01 | 30.5 | ± | 0.01 | 30.5 | ± | 0.01 |
| (mg) | CON | 6.5 | ± | 0.01 | 6.5 | ± | 0.01 | 6.5 | ± | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, A.; Maeda, N.; Kamei, A.; Goto, K. Iron Supplementation during Three Consecutive Days of Endurance Training Augmented Hepcidin Levels. Nutrients 2017, 9, 820. https://doi.org/10.3390/nu9080820
Ishibashi A, Maeda N, Kamei A, Goto K. Iron Supplementation during Three Consecutive Days of Endurance Training Augmented Hepcidin Levels. Nutrients. 2017; 9(8):820. https://doi.org/10.3390/nu9080820
Chicago/Turabian StyleIshibashi, Aya, Naho Maeda, Akiko Kamei, and Kazushige Goto. 2017. "Iron Supplementation during Three Consecutive Days of Endurance Training Augmented Hepcidin Levels" Nutrients 9, no. 8: 820. https://doi.org/10.3390/nu9080820





