Psoralea corylifolia L. Seed Extract Attenuates Diabetic Nephropathy by Inhibiting Renal Fibrosis and Apoptosis in Streptozotocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of PCS Extract
2.3. Animals
2.4. Periodic Acid-Schiff (PAS) Staining
2.5. Biochemical Parameters in Blood and Urine
2.6. Murine Mesangial Cell Culture
2.7. Western Blot Analysis
2.8. Quantitative Real-Time RT-PCR (qRT-PCR) Analysis
2.9. Statistical Analyses
3. Results
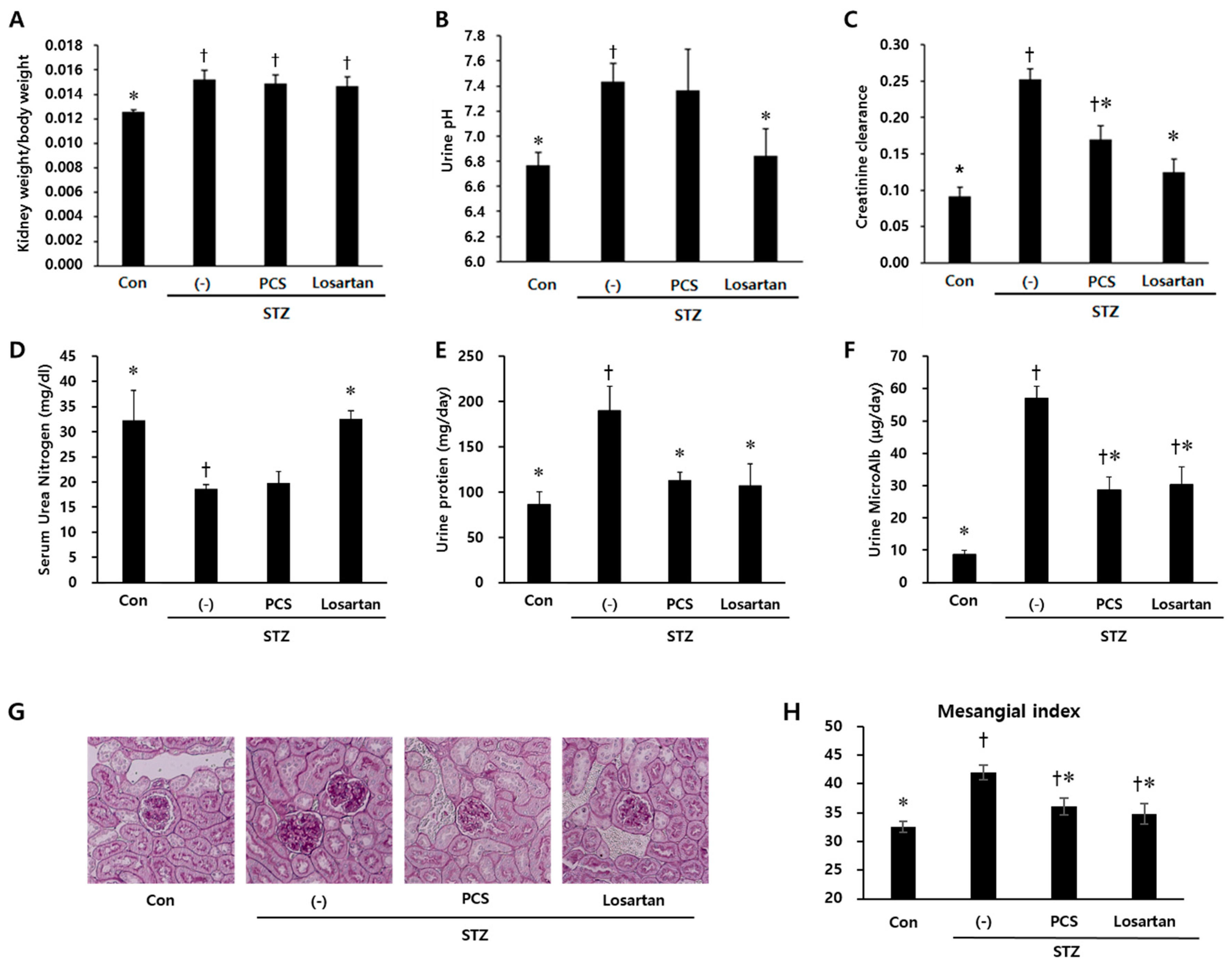
3.1. PCS extract Treatment Improved Renal Function in STZ-Induced Diabetic Mice
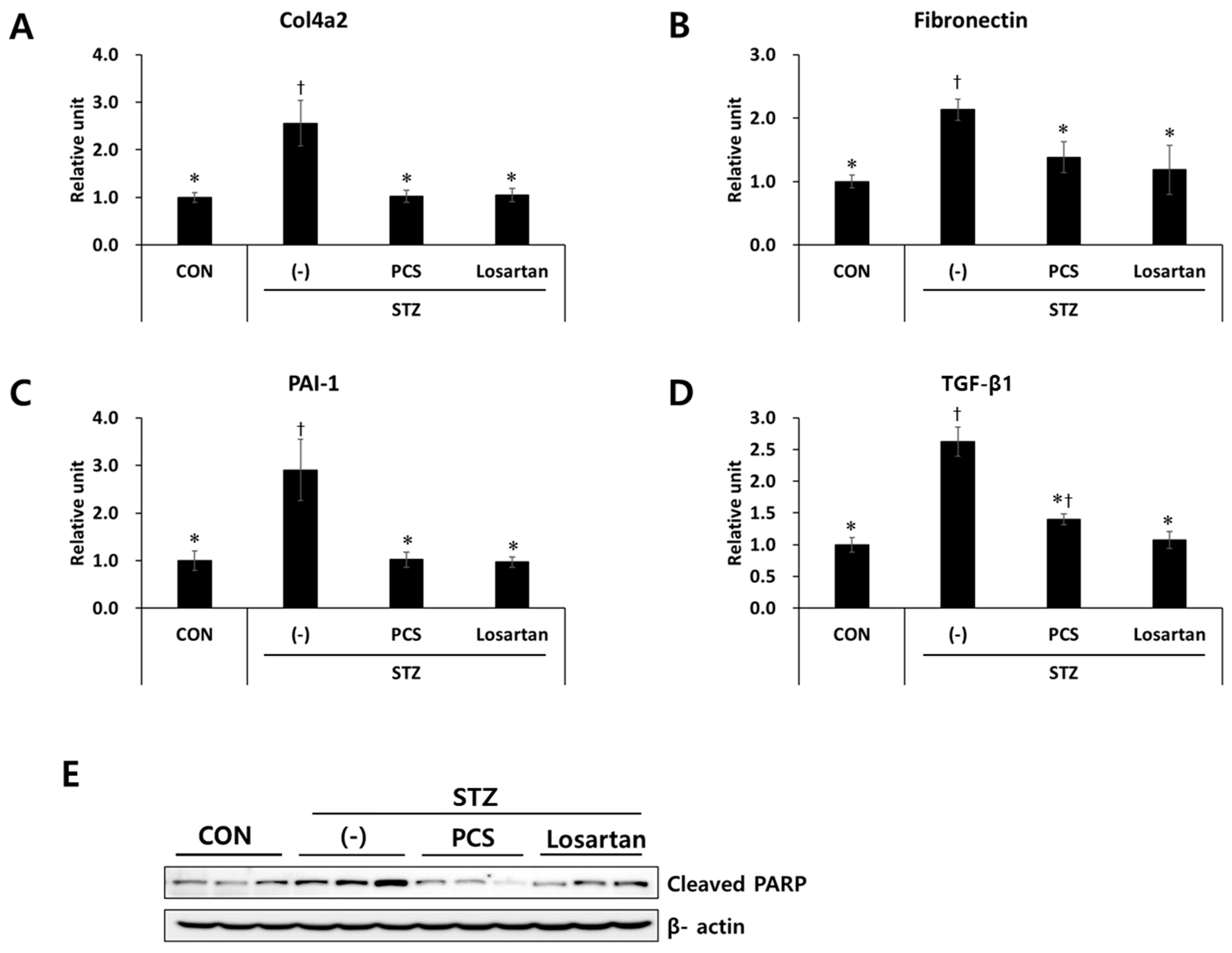
3.2. PCS Extract Inhibited the Expression of Genes Related to Renal Fibrosis and Apoptosis in STZ-Induced Diabetic Mice
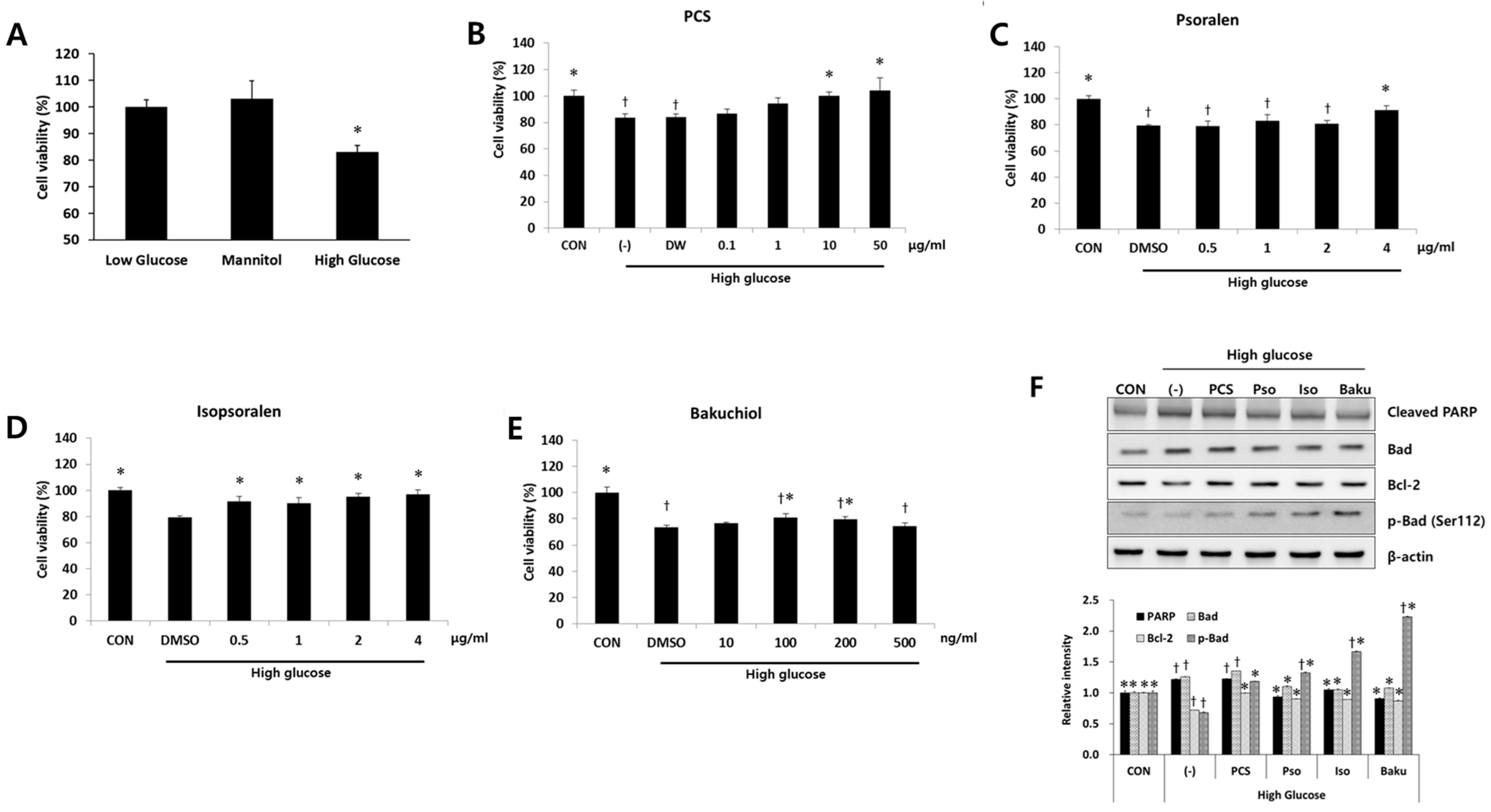
3.3. PCS Extract or Its Components Protected Cells from High Glucose-Induced Apoptosis in Mesangial Cells
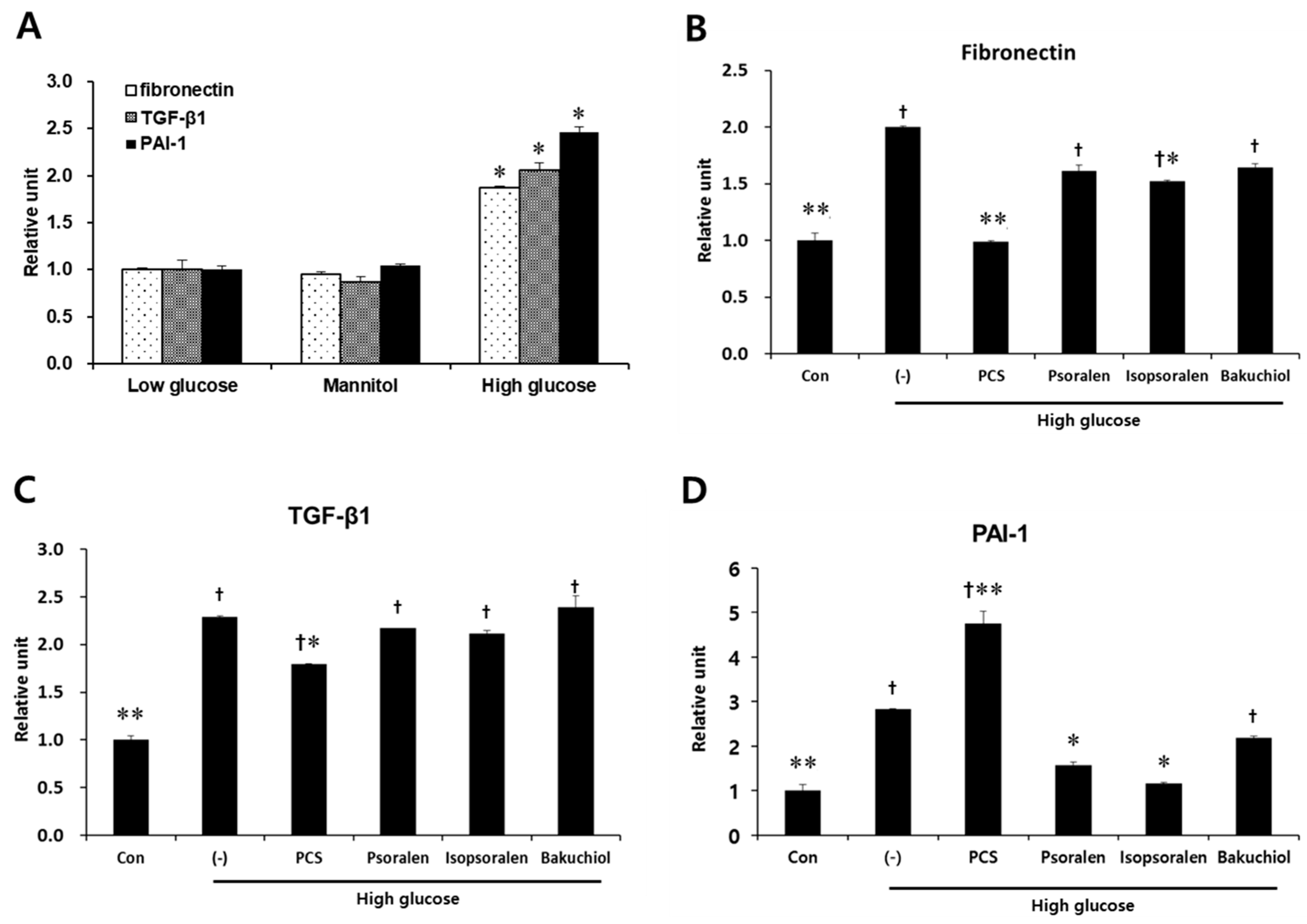
3.4. PCS Extract or Its Components Reduced Expression of Fibrosis-Related Genes in Mesangial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Meigs, J.B.; Wilson, P.W.; Levy, D. Glycemic status and development of kidney disease: The framingham heart study. Diabetes Care 2005, 28, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J. Management of diabetic nephropathy: Recent progress and future perspective. Diabetes Metab. Syndr. 2015, 9, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Suarez, M.L.; Thomas, D.B.; Barisoni, L.; Fornoni, A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J. Diabetes 2013, 4, 245–255. [Google Scholar] [PubMed]
- Cooper, M.E. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998, 352, 213–219. [Google Scholar] [CrossRef]
- Isono, M.; Chen, S.; Hong, S.W.; Iglesias-de la Cruz, M.C.; Ziyadeh, F.N. Smad pathway is activated in the diabetic mouse kidney and smad3 mediates tgf-beta-induced fibronectin in mesangial cells. Biochem. Biophys. Res. Commun. 2002, 296, 1356–1365. [Google Scholar] [CrossRef]
- Ziyadeh, F.N.; Sharma, K.; Ericksen, M.; Wolf, G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J. Clin. Investig. 1994, 93, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.P.; Frencher, S.; Reddy, V.; Kessler, A.; Malhotra, A.; Meggs, L.G. High glucose promotes mesangial cell apoptosis by oxidant-dependent mechanism. Am. J. Physiol. Ren. Physiol. 2003, 284, F455–F466. [Google Scholar] [CrossRef] [PubMed]
- Khera, T.; Martin, J.; Riley, S.; Steadman, R.; Phillips, A.O. Glucose enhances mesangial cell apoptosis. Lab. Investig. 2006, 86, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M. Diabetic nephropathy: Preventing progression. BMJ Clin. Evid. 2010, 2010, 0606. [Google Scholar]
- Ajjan, R.A.; Grant, P.J. Cardiovascular disease prevention in patients with type 2 diabetes: The role of oral anti-diabetic agents. Diabetes Vasc. Dis. Res. 2006, 3, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Krum, H. Heart failure in diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet 2015, 385, 2107–2117. [Google Scholar] [CrossRef]
- Heo, J. Dongui Bogam; Original Work Published in 1613; Namsandang: Seoul, Korea, 1980. [Google Scholar]
- Guo, J.; Weng, X.; Wu, H.; Li, Q.; Bi, K. Antioxidants from a Chinese Medicinal Herb—Psoralea corylifolia L. Food Chem. 2005, 91, 287–292. [Google Scholar]
- Cho, H.; Jun, J.Y.; Song, E.K.; Kang, K.H.; Baek, H.Y.; Ko, Y.S.; Kim, Y.C. Bakuchiol: A hepatoprotective compound of psoralea corylifolia on tacrine-induced cytotoxicity in hep g2 cells. Planta Med. 2001, 67, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Zhao, Y.Z.; Kim, Y.C.; Sohn, D.H. Protective effect of (s)-bakuchiol from psoralea corylifolia on rat liver injury in vitro and in vivo. Planta Med. 2005, 71, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, C.; Zhou, C.; Xu, D.; Qu, H.B. Screening antitumor compounds psoralen and isopsoralen from Psoralea corylifolia L. Seeds. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.R.; Chang, C.L.; Hsieh, P.Y.; Lin, L.W.; Ching, H. Psoralen and isopsoralen, two coumarins of psoraleae fructus, can alleviate scopolamine-induced amnesia in rats. Planta Med. 2007, 73, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Lee, E.K.; Lee, C.S.; Chun, K.H.; Lee, M.Y.; Jun, H.S. Psoralea corylifolia L. Seed extract ameliorates streptozotocin-induced diabetes in mice by inhibition of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Oh, Y.S.; Kim, D.; Lee, M.Y.; Chae, S.; Jun, H.S. Protective role of Psoralea corylifolia L. Seed extract against hepatic mitochondrial dysfunction induced by oxidative stress or aging. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Oh, Y.S.; Jun, H.S. Psoralea corylifolia L. Seed extract attenuates nonalcoholic fatty liver disease in high-fat diet-induced obese mice. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Pesce, C.; Menini, S.; Pricci, F.; Favre, A.; Leto, G.; DiMario, U.; Pugliese, G. Glomerular cell replication and cell loss through apoptosis in experimental diabetes mellitus. Nephron 2002, 90, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Cho, H.; Oh, G.S.; Kim, N.Y.; Song, E.K.; Kim, Y.C.; Yun, Y.G.; Kang, C.L.; Kim, J.D.; Kim, J.M.; et al. Bakuchiol from psoralea corylifolia inhibits the expression of inducible nitric oxide synthase gene via the inactivation of nuclear transcription factor-kappab in raw 264.7 macrophages. Int. Immunopharmacol. 2001, 1, 1849–1855. [Google Scholar] [CrossRef]
- Im, A.R.; Chae, S.W.; Zhang, G.J.; Lee, M.Y. Neuroprotective effects of psoralea corylifolia linn seed extracts on mitochondrial dysfunction induced by 3-nitropropionic acid. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H.; Allen, T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 2007, 12, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.J.; Scheinman, J.I.; Mauer, S.M.; Michael, A.F. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes 1983, 32 (Suppl. 2), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.V.; Neves, F.A.; Bastos, M.G.; Franca, A.K.; Brito, D.J.; Santos, E.M.; Salgado Filho, N. Monitoring renal function: Measured and estimated glomerular filtration rates—A review. Braz. J. Med. Biol. Res. 2010, 43, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Ma, Z.; Li, Y.; Guo, J.; Meng, Q.; Bian, H. A novel formula from mulberry leaf ameliorates diabetic nephropathy in rats via inhibiting the tgf-beta1 pathway. Food Funct. 2015, 6, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. 2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Schnaper, H.W.; Hayashida, T.; Hubchak, S.C.; Poncelet, A.C. Tgf-beta signal transduction and mesangial cell fibrogenesis. Am. J. Physiol. Ren. Physiol. 2003, 284, F243–F252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Jin, Y.; Guo, J.; Ziyadeh, F.N. Neutralization of tgf-beta by anti-tgf-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in stz-induced diabetic mice. Diabetes 1996, 45, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Molecular basis of renal fibrosis. Pediatr. Nephrol. 2000, 15, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Kashihara, N.; Makino, H.; Yamasaki, Y.; Ota, A. Apoptosis in glomerular sclerosis. Kidney Int. 1996, 49, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Rao, Y.K.; Chen, K.; Lee, Y.C.; Chung, Y.S.; Tzeng, Y.M. Andrographolide and 14-deoxy-11,12-didehydroandrographolide from andrographis paniculata attenuate high glucose-induced fibrosis and apoptosis in murine renal mesangeal cell lines. J. Ethnopharmacol. 2010, 132, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jun, H.S.; Oh, Y.S. Protective effect of Psoralea corylifolia L. Seed extract against palmitate-induced neuronal apoptosis in pc12 cells. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Inagaki, Y.; Okamoto, T.; Amano, S.; Koga, K.; Takeuchi, M. Advanced glycation end products inhibit de novo protein synthesis and induce tgf-beta overexpression in proximal tubular cells. Kidney Int. 2003, 63, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Imaizumi, T. Advanced glycation end products (ages) and diabetic vascular complications. Curr. Diabetes Rev. 2005, 1, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, E.; Kang, H.; Oh, Y.S.; Jun, H.-S. Psoralea corylifolia L. Seed Extract Attenuates Diabetic Nephropathy by Inhibiting Renal Fibrosis and Apoptosis in Streptozotocin-Induced Diabetic Mice. Nutrients 2017, 9, 828. https://doi.org/10.3390/nu9080828
Seo E, Kang H, Oh YS, Jun H-S. Psoralea corylifolia L. Seed Extract Attenuates Diabetic Nephropathy by Inhibiting Renal Fibrosis and Apoptosis in Streptozotocin-Induced Diabetic Mice. Nutrients. 2017; 9(8):828. https://doi.org/10.3390/nu9080828
Chicago/Turabian StyleSeo, Eunhui, Hwansu Kang, Yoon Sin Oh, and Hee-Sook Jun. 2017. "Psoralea corylifolia L. Seed Extract Attenuates Diabetic Nephropathy by Inhibiting Renal Fibrosis and Apoptosis in Streptozotocin-Induced Diabetic Mice" Nutrients 9, no. 8: 828. https://doi.org/10.3390/nu9080828




