Health Effects of Carotenoids during Pregnancy and Lactation
Abstract
:1. Introduction
2. Pregnancy and Oxidative Stress
3. Carotenoids Intake and Their Plasma Levels in Pregnant Women
4. Carotenoids and the Prevention of Pathology of Pregnancy
5. Maternal-Fetal Transfer of Carotenoids
6. Carotenoid Status in the Newborn
7. Carotenoids in Breast Milk
8. Infant Feeding Method and Infant Carotenoid Status
9. Carotenoids and Infant Health and Development
9.1. Visual Development
9.2. Brain and Cognitive Development
9.3. Preterm Infants
9.4. Long-Term Studies in Infants and Children
10. Safety of Carotenoids and the Intake Recommendations for Pregnant Women and Infants
11. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Supplements. In Carotenoids. Volume 5: Nutrition and Health; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser: Basel, Switzerland, 2009; pp. 67–82. [Google Scholar]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed]
- Cocate, P.G.; Natali, A.J.; Alfenas, R.C.; de Oliveira, A.; dos Santos, E.C.; Hermsdorff, H.H. Carotenoid consumption is related to lower lipid oxidation and DNA damage in middle-aged men. Br. J. Nutr. 2015, 114, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468S–1473S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Strobel, M.; Tinz, J.; Biesalski, H.K. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur. J. Nutr. 2007, 46, I1–I20. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Andreeva, V.A.; Ducros, V.; Jeandel, C.; Julia, C.; Hercberg, S.; Galan, P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br. J. Nutr. 2014, 111, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Cadier, E.; Beulens, J.W.; van der A, D.L.; Spijkerman, A.M.; van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Cetin, I.; Agostoni, C.; Desoye, G.; Devlieger, R.; Emmett, P.M.; Ensenauer, R.; Hauner, H.; Herrera, E.; Hoesli, I.; et al. Pregnancy and infants’ outcome: Nutritional and metabolic implications. Crit. Rev. Food Sci. Nutr. 2016, 56, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Thorne-Lyman, A.L.; Fawzi, W.W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, S36–S54. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Rifas-Shiman, S.L.; Ly, N.P.; Tantisira, K.G.; Rich-Edwards, J.W.; Camargo, C.A., Jr.; Weiss, S.T.; Gillman, M.W.; Gold, D.R. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am. J. Clin. Nutr. 2006, 84, 903–911. [Google Scholar] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Orjuela, M.A.; Titievsky, L.; Liu, X.; Ramirez-Ortiz, M.; Ponce-Castaneda, V.; Lecona, E.; Molina, E.; Beaverson, K.; Abramson, D.H.; Mueller, N.E. Fruit and vegetable intake during pregnancy and risk for development of sporadic retinoblastoma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, C.L.; Sheppard, K.W. Synergistic effects of human milk nutrients in the support of infant recognition memory: An observational study. Nutrients 2015, 7, 9079–9095. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Lieblein-Boff, J.C.; Johnson, E.J.; Kennedy, A.D.; Lai, C.S.; Kuchan, M.J. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PLoS ONE 2015, 10, e0136904. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Lori, I.; Favelli, F.; Frosini, S.; Messner, H.; Wanker, P.; De Marini, S.; Oretti, C.; Boldrini, A.; Massimiliano, C.; et al. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: A randomized controlled study. J. Matern. Fetal Neonatal Med. 2012, 25, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Martel, F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol. Toxicol. 2014, 30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.; Yudkin, P.; Smith, R.F.; Neil, A. Nutrient intakes during pregnancy: The influence of smoking status and age. J. Epidemiol. Community Health 2000, 54, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Scaife, A.R.; McNeill, G.; Campbell, D.M.; Martindale, S.; Devereux, G.; Seaton, A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br. J. Nutr. 2006, 95, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.E.; McDonald, B.W. Seasonal variation of nutrient intake in pregnancy: Effects on infant measures and possible influence on diseases related to season of birth. Eur. J. Clin. Nutr. 2007, 61, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Stettler, N.; Smith, K.M.; Reiss, R. Associations of consumption of fruits and vegetables during pregnancy with infant birth weight or small for gestational age births: A systematic review of the literature. Int. J. Womens Health 2014, 20, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Hamułka, J.; Sulich, A.; Zielińska, M.; Wawrzyniak, A. Assessment of carotenoid intake in a selected group of pregnant women. Probl. Hig. Epidemiol. 2015, 96, 763–768. [Google Scholar]
- Brantsæter, A.L.; Haugen, M.; Rasmussen, S.E.; Alexander, J.; Samuelsen, S.O.; Meltzer, H.M. Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr. 2007, 10, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.K.; Adetona, O.; Aguilar-Villalobos, M.; Cassidy, B.E.; Pfeiffer, C.M.; Schleicher, R.L.; Caldwell, K.L.; Needham, L.L.; Rathbun, S.L.; Vena, J.E.; et al. Changes in the concentrations of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru, 2004–2005. Nutr. J. 2013, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrug, G.S.; Mensink, R.P.; Al, M.D.; van Houwelingen, A.C.; Hornstra, G. Maternal and neonatal plasma antioxidant levels in normal pregnancy, and the relationship with fatty acid unsaturation. Br. J. Nutr. 1998, 80, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chelchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Laskowska-Klita, T.; Leibschang, J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Engel, U.; Kreinberg, R.; Biesalski, H.K. Vitamin A and β-carotene supply of women with gemini or short birth interval. A pilot study. Eur. J. Nutr. 2007, 46, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Bezzerides, E.; Wang, Y.; Connor, S.L. The depletion of maternal stores of lutein and zeaxanthin during pregnancy and lactation. FASEB J. 2008, 22, 313.8. [Google Scholar]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Barden, A.; Beilin, L.J.; Ritchie, J.; Croft, K.D.; Walters, B.N.; Michael, C.A. Plasma and urinary 8-iso-prostane as an indicator of lipid peroxidation in pre-eclampsia and normal pregnancy. Clin. Sci. (Lond.) 1996, 91, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Halvorsen, B.; Ranheim, T.; Henriksen, T. Elevated level of free 8-iso-prostaglandin F2alpha in the decidua basalis of women with preeclampsia. Am. J. Obstet. Gynecol. 1999, 181, 1211–1215. [Google Scholar] [CrossRef]
- Walsh, S.W.; Vaughan, J.E.; Wang, Y.; Roberts, L.J., 2nd. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000, 14, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Palan, P.R.; Mikhail, M.S.; Romney, S.L. Placental and serum levels of carotenoids in preeclampsia. Obstet. Gynecol. 2001, 98, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Woelk, G.B.; King, I.B.; Jenkins, L.; Mahomed, K. Plasma carotenoids, retinol, tocopherols, and lipoproteins in preeclamptic and normotensive pregnant Zimbabwean women. Am. J. Hypertens. 2003, 16, 665–672. [Google Scholar] [CrossRef]
- Moretti, M.; Phillips, M.; Abouzeid, A.; Cataneo, R.N.; Greenberg, J. Increased breath markers of oxidative stress in normal pregnancy and in preeclampsia. Am. J. Obstet. Gynecol. 2004, 190, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Harsem, N.K.; Braekke, K.; Staff, A.C. Augmented oxidative stress as well as antioxidant capacity in maternal circulation in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.B.; Sharma, A.; Bahadur, A.; Vimala, N.; Satyam, A.; Mittal, S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int. J. Gynaecol. Obstet. 2006, 94, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Karacay, O.; Sepici-Dincel, A.; Karcaaltincaba, D.; Sahin, D.; Yalvaç, S.; Akyol, M.; Kandemir, O.; Altan, N. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res. Clin. Pract. 2010, 89, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Catarino, C.; Santos-Silva, A.; Belo, L.; Rocha-Pereira, P.; Rocha, S.; Patrício, B.; Quintanilha, A.; Rebelo, I. Inflammatory disturbances in preeclampsia: Relationship between maternal and umbilical cord blood. J. Pregnancy 2012, 2012, 684384. [Google Scholar] [CrossRef] [PubMed]
- Mert, I.; Oruc, A.S.; Yuksel, S.; Cakar, E.S.; Buyukkagnici, U.; Karaer, A.; Danisman, N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012, 38, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Evans, R.W.; Kahn, S.R. The association between maternal antioxidant levels in midpregnancy and preeclampsia. Am. J. Obstet. Gynecol. 2015, 213, 695.e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.; Basu, A.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Scholz, H.; Henriksen, T.; Garg, S.K.; Hammad, S.M.; Scardo, J.A.; et al. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: A longitudinal study. Diabetes Care 2011, 34, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Williams, M.A.; Sanchez, S.E.; King, I.B.; Ware-Jauregui, S.; Larrabure, G.; Bazul, V.; Leisenring, W.M. Plasma concentrations of carotenoids, retinol, and tocopherols in preeclamptic and normotensive pregnant women. Am. J. Epidemiol. 2001, 153, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Mishra, G.D. The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med. 2014, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Engeset, D.; Alsaker, E.; Ciampi, A.; Lund, E. Dietary patterns and lifestyle factors in the Norwegian EPIC cohort: The Norwegian Women and Cancer (NOWAC) study. Eur. J. Clin. Nutr. 2005, 59, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dam, R.M.; Li, T.; Spiegelman, D.; Franco, O.H.; Hu, F.B. Combined impact of lifestyle factors on mortality: Prospective cohort study in US women. BMJ 2008, 337, a1440. [Google Scholar] [PubMed]
- Sharma, J.B.; Ashok, K.; Kumar, A.; Malhotra, M.; Arora, R.; Prasad, S.; Batra, S. Effect of lycopene on pre-eclampsia and intra-uterine growth retardation in primigravidas. Int. J. Gynaecol. Obstet. 2003, 81, 257–262. [Google Scholar] [CrossRef]
- Banerjee, S.; Jeyaseelan, S.; Guleria, R. Trial of lycopene to prevent pre-eclampsia in healthy primigravidas: Results show some adverse effects. J. Obstet. Gynaecol. Res. 2009, 35, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Antartani, R.; Ashok, K. Effect of lycopene in prevention of preeclampsia in high risk pregnant women. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Rahmana, A.; Bokharia, S.S.I.; Waqara, M.A. Beta-carotene degradation by cigarette smoke in hexane solution in vitro. Nutr. Res. 2001, 21, 821–829. [Google Scholar] [CrossRef]
- Cohen, J.M.; Beddaoui, M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Kahn, S.R. Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0135192. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Hauguel-de Mouzon, S.; Jawerbaum, A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef] [PubMed]
- Grissa, O.; Atègbo, J.M.; Yessoufou, A.; Tabka, Z.; Miled, A.; Jerbi, M.; Dramane, K.L.; Moutairou, K.; Prost, J.; Hichami, A.; et al. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl. Res. 2007, 150, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, F.; Giampietri, M.; Ferri, G.; Lunardi, S.; Madrigali, V.; Battini, L.; Boldrini, A.; Ghirri, P. Lutein administration to pregnant women with gestational diabetes mellitus is associated to a decrease of oxidative stress in newborns. Gynecol. Endocrinol. 2013, 29, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Clerici, G.; Slavescu, C.; Fiengo, S.; Kanninen, T.T.; Romanelli, M.; Biondi, R.; Di Renzo, G.C. Oxidative stress in pathological pregnancies. J. Obstet. Gynaecol. 2012, 32, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Perrone, S.; Vezzosi, P.; Marzocchi, B.; Kenanidis, A.; Centini, G.; Rosignoli, L.; Buonocore, G. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin. Biochem. 2007, 40, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Kahn, S.R.; Platt, R.W.; Genest, J.; Rozen, R.; Chen, M.F.; Goulet, L.; Séguin, L.; Dassa, C.; Lydon, J.; et al. Antioxidant vitamins, long-chain fatty acids, and spontaneous preterm birth. Epidemiology 2009, 20, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.L.; Yang, W.; Shaw, G.M.; National Birth Defects Prevention Study. Maternal dietary nutrient intake and risk of preterm delivery. Am. J. Perinatol. 2013, 30, 579–588. [Google Scholar] [PubMed]
- Englund-Ögge, L.; Brantsæter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saker, M.; Soulimane Mokhtari, N.; Merzouk, S.A.; Merzouk, H.; Belarbi, B.; Narce, M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Stuetz, W.; Bernhard, W.; Franz, A.; Raith, M.; Grune, T.; Breusing, N. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur. J. Clin. Nutr. 2014, 68, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Kahn, S.R.; Platt, R.W.; Basso, O.; Evans, R.W.; Kramer, M.S. Small-for-gestational-age birth and maternal plasma antioxidant levels in mid-gestation: A nested case-control study. BJOG 2015, 122, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, B.S.; Chan, G.; Hoffman, R.O.; Sharifzadeh, M.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5568–5578. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.T.; Jedrychowski, W.; Schleicher, R.L.; Tsai, W.Y.; Tu, Y.H.; Camann, D.; Tang, D.; Perera, F.P. Relation between prenatal lipid-soluble micronutrient status, environmental pollutant exposure, and birth outcomes. Am. J. Clin. Nutr. 2007, 86, 1139–1145. [Google Scholar] [PubMed]
- Christian, P.; Klemm, R.; Shamim, A.A.; Ali, H.; Rashid, M.; Shaikh, S.; Wu, L.; Mehra, S.; Labrique, A.; Katz, J.; et al. Effects of vitamin A and β-carotene supplementation on birth size and length of gestation in rural Bangladesh: A cluster-randomized trial. Am. J. Clin. Nutr. 2013, 97, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Nagao, A. Absorption and metabolism of xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Amusquivar, E.; López-Soldado, I.; Ortega, H. Maternal lipid metabolism and placental lipid transfer. Horm. Res. 2006, 65, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Panova, I.G.; Tatikolov, A.S.; Sukhikh, G.T. Correlation between the content of albumin and carotenoids in human vitreous body during prenatal development. Bull. Exp. Biol. Med. 2007, 144, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, O.; Cagliero, P. Lipid metabolism in the human fetus development. In Human Fetal Growth and Development. First and Second Trimesters; Bhattacharya, N., Stubblefield, P.G., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 183–195. [Google Scholar]
- Manago, M.; Tamai, H.; Ogihara, T.; Mino, M. Distribution of circulating beta-carotene in human plasma lipoproteins. J. Nutr. Sci. Vitaminol. 1992, 38, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Costabile, B.K.; Kim, Y.K.; Iqbal, J.; Zuccaro, M.V.; Wassef, L.; Narayanasamy, S.; Curley, R.W., Jr.; Harrison, E.H.; Hussain, M.M.; Quadro, L. β-apo-10’-carotenoids modulate placental microsomal triglyceride transfer protein expression and function to optimize transport of intact β-carotene to the embryo. J. Biol. Chem. 2016, 291, 18525–18535. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.M.; Saunders, C.; Ramalho, A. Placenta: A possible predictor of vitamin A deficiency. Br. J. Nutr. 2010, 103, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Karakilcik, A.Z.; Aksakal, M.; Baydas, G.; Sozen, R.; Ayata, A.; Simsek, M. Plasma beta-carotene concentrations in pregnancies, newborn infants and their mothers. J. Pak. Med. Assoc. 1996, 46, 77–80. [Google Scholar] [PubMed]
- Yeum, K.J.; Ferland, G.; Patry, J.; Russell, R.M. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J. Am. Coll. Nutr. 1998, 17, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Cogan, P.F.; Kearney, P.J.; Morrissey, P.A. Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur. J. Clin. Nutr. 1999, 53, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Morrison, C.M.; Pagano, I.; Li, X.; Halm, B.M.; Soon, R.; Custer, L.J. Coenzyme Q10, carotenoid, tocopherol, and retinol levels in cord plasma from multiethnic subjects in Hawaii. Free Radic. Res. 2013, 47, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.S. Monitoring maternal beta carotene and retinol consumption may decrease the incidence of neurodevelopmental disorders in offspring. Clin. Med. Insights Reprod. Health 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, E.; Kim, Y.K.; Wassef, L.; Shete, V.; Quadro, L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim. Biophys. Acta 2012, 1821, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Picone, S.; Ritieni, A.; Fabiano, A.; Troise, A.D.; Graziani, G.; Paolillo, P.; Li Volti, G.; D’Orazio, N.; Galvano, F.; Gazzolo, D. Arterial cord blood lutein levels in preterm and term healthy newborns are sex and gestational age dependent. Clin. Biochem. 2012, 45, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- De Barros Silva, S.S.; Rondó, P.H.; Erzinger, G.S. Beta-carotene concentrations in maternal and cord blood of smokers and non-smokers. Early Hum. Dev. 2005, 81, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Chen, S.F.; Hsieh, T.T.; Lo, L.M.; Li, M.J.; Yeh, Y.L. The associations between labor and delivery mode and maternal and placental oxidative stress. Reprod. Toxicol. 2011, 31, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.J.; Kim, Y.H.; Cho, M.K.; Kim, J.W.; Kim, J.W.; Byun, Y.J.; Song, T.B. Comparison of oxidative stress markers in umbilical cord blood after vaginal and cesarean delivery. Obstet. Gynecol. Sci. 2014, 57, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Macias, C.; Schweigert, F.J. Changes in the concentration of carotenoids, vitamin A, alpha-tocopherol and total lipids in human milk throughout early lactation. Ann. Nutr. Metab. 2001, 45, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Canfield, L.M.; Clandinin, M.T.; Davies, D.P.; Fernandez, M.C.; Jackson, J.; Hawkes, J.; Goldman, W.J.; Pramuk, K.; Reyes, H.; Sablan, B.; et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur. J. Nutr. 2003, 42, 133–141. [Google Scholar] [PubMed]
- Lipkie, T.E.; Morrow, A.L.; Jouni, Z.E.; McMahon, R.J.; Ferruzzi, M.G. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS ONE 2015, 10, e0127729. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, F.J.; Bathe, K.; Chen, F.; Büscher, U.; Dudenhausen, J.W. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur. J. Nutr. 2004, 43, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Neilson, E.M.; Yap, H.H.; Baier, M.; Canfield, L.M. Methods of nutritional biochemistry quantitation of and inter/intra-individual variability in major carotenoids of mature human milk. J. Nutr. Biochem. 1994, 5, 551–556. [Google Scholar] [CrossRef]
- De Azeredo, V.B.; Trugo, N.M.F. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition 2008, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Lien, E.L.; White, S.J.; Bruns, N.J.; Kuhlman, C.F. Major carotenoids in mature human milk: Longitudinal and diurnal patterns. J. Nutr. Biochem. 1998, 9, 2–7. [Google Scholar] [CrossRef]
- Jackson, J.G.; Zimmer, J.P. Lutein and zeaxanthin in human milk independently and significantly differ among women from Japan, Mexico, and the United Kingdom. Nutr. Res. 2007, 27, 449–453. [Google Scholar] [CrossRef]
- Jewell, V.C.; Mayes, C.B.D.; Tubman, T.R.J.; Northrop-Clewes, C.A.; Thurnham, D.I. A comparison of lutein and zeaxanthin concentrations in formula and human milk samples from Northern Ireland mothers. Eur. J. Clin. Nutr. 2004, 58, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Castellazzi, A.M.; Pietri, A.; Roggi, C.; Turconi, G. Lutein concentration in human milk during early lactation and its relationship with dietary lutein intake. Public Health Nutr. 2009, 12, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Haftel, L.; Berkovich, Z.; Reifen, R. Elevated milk β-carotene and lycopene after carrot and tomato paste supplementation. Nutrition 2015, 31, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, J.; Noda, K.; Uchikawa, T.; Maruyama, I.; Shimomura, H.; Miyahara, M. Effect of maternal Chlorella supplementation on carotenoid concentration in breast milk at early lactation. Int. J. Food Sci. Nutr. 2014, 65, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Meneses, F.; Trugo, N.M.F. Retinol, β-carotene, and lutein + zeaxanthin in the milk of Brazilian nursing women: Associations with plasma concentrations and influences of maternal characteristics. Nutr. Res. 2005, 25, 443–451. [Google Scholar] [CrossRef]
- Sherry, C.L.; Oliver, J.S.; Renzi, L.M.; Marriage, B.J. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J. Nutr. 2014, 144, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Database. Beta-Carotene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280489 (accessed on 22 September 2016).
- PubChem Compound Database. Zeaxanthin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280899 (accessed on 22 September 2016).
- PubChem Compound Database. Lutein. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281243 (accessed on 22 September 2016).
- PubChem Compound Database. Lycopene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/446925 (accessed on 22 September 2016).
- PubChem Compound Database. Beta-Cryptoxanthin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281235 (accessed on 22 September 2016).
- Shennan, D.B.; Peaker, M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [PubMed]
- Schweigert, F.J. Effect of gestation and lactation on lipoprotein pattern and composition in dairy cows. J. Anim. Physiol. Anim. Nutr. 1990, 63, 75–83. [Google Scholar] [CrossRef]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Bettler, J.; Zimmer, J.P.; Neuringer, M.; Derusso, P.A. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010, 49, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Meissner, K.; Nelle, M.; Lenhartz, H.; Leichsenring, M. Carotenoid supply in breast-fed and formula-fed neonates. Eur. J. Pediatr. 2000, 159, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.P.; Hammond, B.R. Possible influences of lutein and zeaxanthin on the developing retina. Clin. Ophthalmol. 2007, 1, 25–35. [Google Scholar] [PubMed]
- Chan, G.M.; Chan, M.M.; Gellermann, W.; Ermakov, I.; Ermakova, M.; Bhosale, P.; Bernstein, P.; Rau, C. Resonance Raman spectroscopy and the preterm infant carotenoid status. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Capeding, R.; Gepanayao, C.P.; Calimon, N.; Lebumfacil, J.; Davis, A.M.; Stouffer, N.; Harris, B.J. Lutein-fortified infant formula fed to healthy term infants: Evaluation of growth effects and safety. Nutr. J. 2010, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Neuringer, M.; Johnson, E.E.; Kuchan, M.J.; Pereira, S.L.; Johnson, E.J.; Erdman, J.W. Effect of carotenoid supplemented formula on carotenoid bioaccumulation in tissues of infant rhesus macaques: A pilot study focused on lutein. Nutrients 2017, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Lipkie, T.E.; Banavara, D.; Shah, B.; Morrow, A.L.; McMahon, R.J.; Jouni, Z.E.; Ferruzzi, M.G. Caco-2 accumulation of lutein is greater from human milk than from infant formula despite similar bioaccessibility. Mol. Nutr. Food Res. 2014, 58, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Longini, M.; Marzocchi, B.; Picardi, A.; Bellieni, C.V.; Proietti, F.; Rodriguez, A.; Turrisi, G.; Buonocore, G. Effects of lutein on oxidative stress in the term newborn: A pilot study. Neonatology 2010, 97, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, E.; Nolan, J.; Howard, A.; Beatty, S. Macular pigment and its contribution to vision. Nutrients 2013, 5, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 642, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Khachik, F.; Carvalho, L.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp. Eye Res. 2001, 72, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.L.; Hammond, B.R. Nutritional influences on visual development and function. Prog. Retin. Eye Res. 2011, 30, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.; Possin, D.; Vajzovic, L.; Toth, C.A. Histologic development of the human fovea from midgestation to maturity. Am. J. Ophthalmol. 2012, 154, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Sharifzadeh, M.; Liu, A.; Ermakov, I.; Nelson, K.; Sheng, X.; Panish, C.; Carlstrom, B.; Hoffman, R.O.; Gellermann, W. Blue-light reflectance imaging of macular pigment in infants and children. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Neuringer, M.; Bone, R.A.; Jeffrey, B.; Bettler, J.; Zimmer, J.P.; Wallace, P.; DeRusso, P.A. Lutein in breastmilk and infant formula: Effects on serum lutein, macular pigment and visual function. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1707. [Google Scholar]
- Liu, Z.; Meyers, K.J.; Johnson, E.J.; Snodderly, M.; Tinker, L.; Wallace, R.; Sarto, G.; Mares, J.A. Exposure to lutein in infancy via breast milk and later life macular pigment optical density. Investig. Ophthalmol. Vis. Sci. 2015, 56, 192. [Google Scholar]
- Hardy, P.; Dumont, I.; Bhattacharya, M.; Hou, X.; Lachapelle, P.; Varma, D.R.; Chemtob, S. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: A basis for ischemic retinopathy. Cardiovasc. Res. 2000, 47, 489–509. [Google Scholar] [CrossRef]
- Dillon, J.; Zheng, L.; Merriam, J.C.; Gaillard, E.R. Transmission of light to the aging human retina: Possible implications for age related macular degeneration. Exp. Eye Res. 2004, 79, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1978, 17, 601–607. [Google Scholar]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investig. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar]
- Jewell, V.C.; Northrop-Clewes, C.A.; Tubman, R.; Thurnham, D.I. Nutritional factors and visual function in premature infants. Proc. Nutr. Soc. 2001, 60, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Mechanistic evidence for eye diseases and carotenoids. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 445–472. ISBN 12-978-0-203-02664-9. [Google Scholar]
- Li, B.; Ahmed, F.; Bernstein, P.S. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch. Biochem. Biophys. 2010, 504, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Fung, F.K.; Fu, Z.J.; Wong, D.; Chan, H.H.; Lo, A.C. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: In vivo and in vitro studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5976–5984. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Sasaki, M.; Noriko Takahashi, N.; Mamoru Kamoshita, M.; Seiji Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, D.V.; Ojima, I.; Geng, X.; Adler, A.J. Tubulins in the primate retina: Evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorg. Med. Chem. 2001, 9, 1967–1976. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Balashov, N.A.; Tsong, E.D.; Rando, R.R. Retinal tubulin binds macular carotenoids. Investig. Ophthamol. Vis. Sci. 1997, 38, 167–175. [Google Scholar]
- Stahl, W.; Nicolai, S.; Briviba, K.; Hanusch, M.; Broszeit, G.; Peters, M.; Martin, H.D.; Sies, H. Biological activities of natural and synthetic carotenoids: Induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 1997, 18, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R., Jr. Possible role for dietary lutein and zeaxanthin in visual development. Nutr. Rev. 2008, 66, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.H.M.; Gilbert, C.E.; Spector, T.D.; Mellerio, J.; Van Kuijk, F.J.; Beatty, S.; Fitzke, F.; Marshall, J.; Hammond, C.J. Central retinal thickness is positively correlated with macular pigment optical density. Exp. Eye Res. 2006, 82, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.R.; Feeney-Burns, L.; Peterson, L.H.; Klein, M.L.; Neuringer, M. Diet-related macular anomalies in monkeys. Investig. Ophthalmol. Vis. Sci. 1980, 19, 857–863. [Google Scholar]
- Leung, I.Y.; Sandstrom, M.M.; Zucker, C.L.; Neuringer, M.; Snodderly, D.M. Nutritional manipulation of primate retinas, II: Effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3244–3256. [Google Scholar] [CrossRef] [PubMed]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef] [PubMed]
- Sale, A.; Berardi, N.; Maffei, L. Enrich the environment to empower the brain. Trends Neurosci. 2009, 32, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Neuringer, M.; Snodderly, D.M.; Schalch, W.; Johnson, E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Schalch, W.; Johnson, E.J. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr. Neurosci. 2016, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Iannaccone, A.; Scott, T.M.; Kritchevsky, S.B.; Jennings, B.J.; Carboni, G.; Forma, G.; Satterfield, S.; Harris, T.; Johnson, K.C.; et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing 2014, 43, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Walk, A.M.; Khan, N.A.; Barnett, S.M.; Raine, L.B.; Kramer, A.F.; Cohen, N.J.; Moulton, C.J.; Renzi-Hammond, L.M.; Hammond, B.R.; Hillman, C.H. From neuro-pigments to neural efficiency: The relationship between retinal carotenoids and behavioral and neuroelectric indices of cognitive control in childhood. Int. J. Psychophysiol. 2017, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A. Relationship between Serum and Brain Carotenoids, Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr.; Smith, J.W.; Kuchan, M.J.; Mohn, M.S.; Johnson, E.J.; Rubakhin, S.S.; Wang, L.; Sweedler, J.V.; Neuringer, M. Lutein and Brain Function. Foods 2015, 4, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Effects of carotenoids and retinoids on gap junctional communication. Biofactors 2001, 15, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, J.; Manivasagam, T.; Justin, A.; Essa, M.M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. 2016, 19, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Li, B.; Bernstein, P.S.; Vishwanathan, R.; Johnson, M.A.; Poon, L.; Johnson, E.J. Relationship between concentrations of lutein and StARD3 among pediatric and geriatric human brain tissue. PLoS ONE 2016, 11, e0155488. [Google Scholar]
- Naberhuis, J.K.; Lai, C.S. Enhanced delivery of lipophilic nutrients to the infant brain via high density lipoprotein. Med. Hypotheses 2015, 85, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef]
- Perrone, S.; Tei, M.; Longini, M.; Santacroce, A.; Turrisi, G.; Proietti, F.; Felici, C.; Picardi, A.; Bazzini, F.; Vasarri, P.; et al. Lipid and protein oxidation in newborn infants after lutein administration. Oxid. Med. Cell. Longev. 2014, 2014, 781454. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Tirone, C.; Persichilli, S.; Gervasoni, J.; Zuppi, C.; Barone, G.; Zecca, E. Lutein absorption in premature infants. Eur. J. Clin. Nutr. 2010, 64, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Giannantonio, C.; Romagnoli, C.; Vento, G.; Gervasoni, J.; Persichilli, S.; Zuppi, C.; Cota, F. Effects of lutein supplementation on biological antioxidant status in preterm infants: A randomized clinical trial. J. Matern. Fetal Neonatal Med. 2013, 26, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; Mackey, A.D.; Dimmit, R.A.; Hartmann, E.E.; et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Guardione, R.; Bonetti, P.; Priolo, C.; Maestri, A.; Mansoldo, C.; Mostert, M.; Anselmetti, G.; Sardei, D.; Bellettato, M.; et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: A multicenter randomized controlled trial. Am. J. Perinatol. 2013, 30, 25–32. [Google Scholar] [CrossRef] [PubMed]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar]
- Fang, J.L.; Sorita, A.; Carey, W.A. Interventions to Prevent Retinopathy of Prematurity: A Meta-analysis. Pediatrics 2016, 137, e20153387. [Google Scholar] [CrossRef] [PubMed]
- Wilinska, M.; Borszewska-Kornacka, M.K.; Niemiec, T.; Jakiel, G. Oxidative stress and total antioxidant status in term newborns and their mothers. Ann. Agric. Environ. Med. 2015, 22, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R. Effects of dietary retinoids and carotenoids on immune development. Proc. Nutr. Soc. 2007, 66, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Melo van Lent, D.; Leermakers, E.T.M.; Darweesh, S.K.L.; Moreira, E.M.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; Brusselle, G.G.; et al. The effects of lutein on respiratory health across the life course: A systematic review. Clin. Nutr. ESPEN 2016, 13, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Mulder, K.A.; Innis, S.M.; Rasmussen, B.F.; Wu, B.T.; Richardson, K.J.; Hasman, D. Plasma lutein concentrations are related to dietary intake, but unrelated to dietary saturated fat or cognition in young children. J. Nutr. Sci. 2014, 3, e11. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R. Lutein and cognition in children. J. Nutr. Sci. 2014, 3, e53. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.; Kiefte-de Jong, J.C.; Hofman, A.; Jaddoe, V.W.; Franco, O.H. Lutein intake at the age of 1 year and cardiometabolic health at the age of 6 years: The Generation R Study. Br. J. Nutr. 2015, 114, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Skibsted, L.H.; Sampson, J.; Rice-Evans, C.; Everett, S.A. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997, 418, 91–97. [Google Scholar] [CrossRef]
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar]
- Törnwall, M.E.; Virtamo, J.; Korhonen, P.A.; Virtanen, M.J.; Taylor, P.R.; Albanes, D.; Huttunen, J.K. Effect of alpha-tocopherol and beta-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. Eur. Heart J. 2004, 25, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; Haile, R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Safety, bioavailability and suitability of lutein for the particular nutritional use by infants and young children—Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2008, 823, 1–24. [Google Scholar]
- Wallace, T.C.; Blumberg, J.B.; Johnson, E.J.; Shao, A. Dietary Bioactives: Establishing a Scientific Framework for Recommended Intakes. Adv. Nutr. 2015, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R., Jr.; Renzi, L.M. Carotenoids. Adv. Nutr. 2013, 4, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
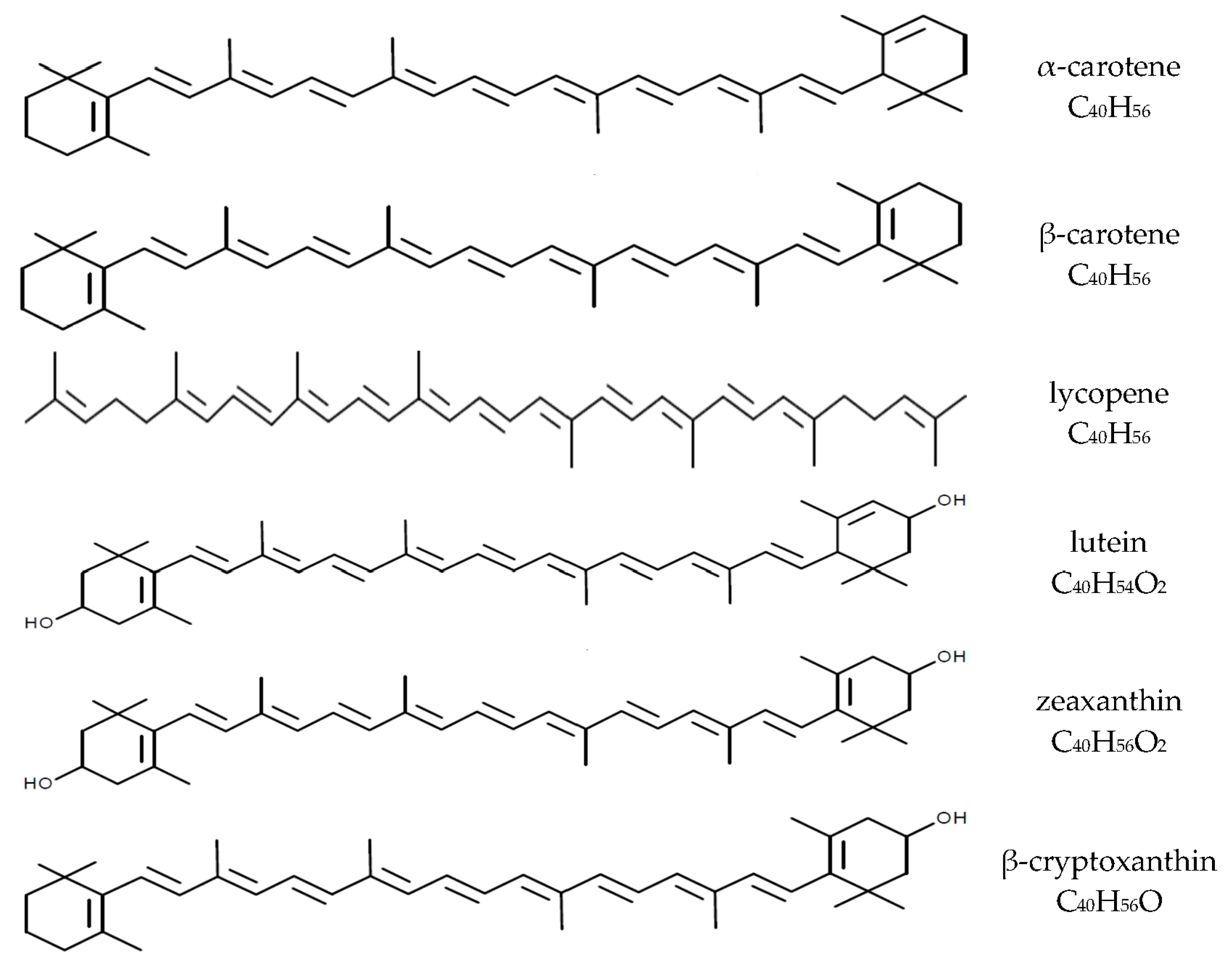
| Pregnancy Period | Study Group | Carotenoids | Source | |
|---|---|---|---|---|
| Type | Intake (µg/day) | |||
| 1 and 2 trimester | USA n = 1290 62.6% households with annual income of >$70,000 75.6% White, 10.4%, Black, 5.4% Hispanic, 4.9% Asian, 3.7% other ethnicity 9.5% smokers | dietary + supplements total β-carotene | 4770.3 ± 2293.0 | [17] |
| dietary β-carotene | 3863.5 ± 2036.5 | |||
| dietary lycopene | 7368.8 ± 3979.7 | |||
| dietary lutein and zeaxanthin | 2686.8 ± 1724.4 | |||
| dietary α-carotene | 878.0 ± 657.7 | |||
| dietary β-cryptoxanthin | 207.3 ± 130.9 | |||
| 1–3 trimester | Poland n = 215 no information about socio-economic group white Caucasian | dietary β-carotene | 4513.0 ± 3908.1 | [29] |
| dietary lycopene | 4419.8 ± 3267.1 | |||
| dietary lutein | 2091.1 ± 1699.9 | |||
| 9–20 Hbd 1 | United Kingdom n = 774 26.6 ± 4.6 years 34% higher socio-economic group no information about ethnicity 40.4% smokers | dietary β-carotene | 937 ± 789–1168 ± 890 | [25] |
| dietary total carotenoids | 1323 ± 999–1843 ± 1125 | |||
| 5–39 Hbd | Japan n = 763 30.0 ± 4.0 years 31.3% households with annual income ≥6,000,000 yen no information about ethnicity 90% European origin, 7% Maori, 3% other ethnicities | dietary β-carotene | 2620.4 ± 1653.0 | [21] |
| dietary α-carotene | 345.3 ± 277.0 | |||
| 4 and 7 month of gestation | New Zealand n = 214 29.3 ± 4.4 years 72% higher socio-economic group | dietary β-carotene | 1887–2510 | [27] |
| 34 Hbd | United Kingdom n = 1149 29.4 ± 5.5 years white Caucasian 45.5% smokers | dietary β-carotene | 2302 ± 1861.6 | [26] |
| β-carotene supplements | 90.88 ± 591.7 | |||
| dietary + supplements total β-carotene | 2394 ± 2001.2 | |||
| Lactation Stage | Study Group | Milk Collection Method | Carotenoid Concentration in Breast Milk (nmol/L) | Source | |||
|---|---|---|---|---|---|---|---|
| β-Carotene | Lutein (L) and/or Zeaxanthin (Z) | Lycopene | β-Cryptoxanthin | ||||
| Colostrum | Germany; n = 21 30 ± 6 (20–39) | the total milk volume of one breast | 423.4 ± 326.6 | 164.0 ± 84.9 L 33.2 ± 84.9 Z | 508.9 ± 421.7 | 238.8 ± 156.1 | [99] |
| Cuba; n = 21 25 (19–30) | 10–12 mL of primarily foremilk (in the morning) | 125.7 ± 6.37 1 | 67.9 ± 44.9 L 2 9.7 ± 6.7 Z | 137.3 ± 86.1 3 | 61.1 ± 66.6 4 | [96] | |
| Italy; n = 21 33.9 ± 4.37 (24–42) | 5–6 mL of milk | - | 280 ± 220 L 5 | - | - | [105] | |
| Transitional milk | Cuba; n = 21 25 (19–30) | 10–12 mL of primarily foremilk (in the morning) | 44.2 ± 34.1 1 | 44.5 ± 36.1 L 2 8.6 ± 5.5 Z | 44.2 ± 34.1 3 | 24.8 ± 22.4 4 | [96] |
| Mature milk | Cuba; n = 21 25 (19–30) | 10–12 mL of primarily foremilk (in the morning) | 36.2 ± 17.2 1 | 27.3 ± 16.4 L 2 7.9 ± 7.7 Z | 18.8 ± 2.7 3 | 16.6 ± 12.7 4 | [96] |
| Germany; n = 21 30 ± 6 (20–39) | the total milk volume of one breast | 78.2 ± 46.2 | 88.1 ± 37.8 L 2 19.5 ± 10.2 Z | 59.8 ± 38.9 3 | 60.6 ± 36.7 4 | [99] | |
| Brazil; n = 49 26.6 ± 6.3 | the total milk volume of one breast | 18.0 ± 2.0 5 | 6.0 ± 1.0 L + Z 5 | - | - | [108] | |
| Italy; n = 21 33.9 ± 4.37 (24–42) | 5–6 mL of milk | - | 110 ± 50 L 5 | - | - | [105] | |
| Study Group | Intervention | Assessed Outcomes | Results | Source |
|---|---|---|---|---|
| n = 77 GA 1 ≤ 34 (30.4 ± 2.3) 1415 ± 457 g Italy | RCT 2 L + Z 3 (0.5 + 0.02 mg/kg/day) vs. placebo |
|
| [168] |
| n = 203 GA ≤ 33 (29.6 ± 0.2) 1244 ± 32 g USA | RCT Formula with L + β-c + Ly (211 + 219 + 143 µg/L) vs. placebo control formula |
|
| [169] |
| n = 114 GA ≤ 32 (28.8 ± 2.4) 1130 ± 330 g Italy | RCT L + Z (0.14 + 0.006 mg/kg/day) vs. placebo |
|
| [23] |
| n = 63 GA ≤ 32 (29.9 ± 1.9) 1331 ± 415 g Italy | RCT L + Z (0.5 + 0.02 mg/kg/day) vs. placebo |
|
| [167] |
| n = 229 GA ≤ 32 (30.1 ± 1.8) 1336 ± 417 Italy | RCT L + Z (0.14 + 0.006 mg/day) |
|
| [170] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, M.A.; Wesołowska, A.; Pawlus, B.; Hamułka, J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients 2017, 9, 838. https://doi.org/10.3390/nu9080838
Zielińska MA, Wesołowska A, Pawlus B, Hamułka J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients. 2017; 9(8):838. https://doi.org/10.3390/nu9080838
Chicago/Turabian StyleZielińska, Monika A., Aleksandra Wesołowska, Beata Pawlus, and Jadwiga Hamułka. 2017. "Health Effects of Carotenoids during Pregnancy and Lactation" Nutrients 9, no. 8: 838. https://doi.org/10.3390/nu9080838






