Personalised Interventions—A Precision Approach for the Next Generation of Dietary Intervention Studies
Abstract
:1. Diet, Dietary Efficacy, and Dietary Advice
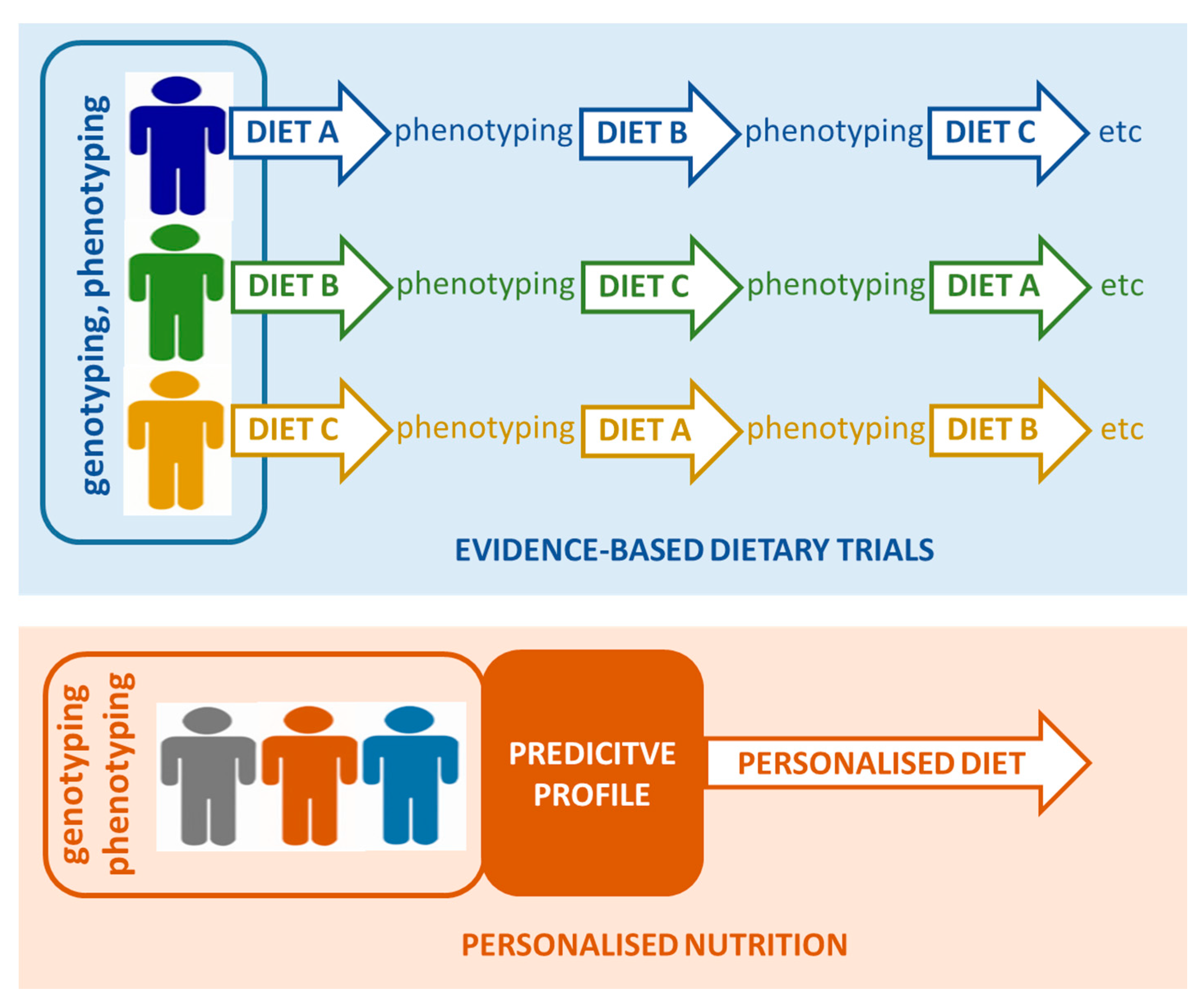
2. Precision Medicine and Precision Nutrition
3. Adaptation of Precision Nutrition Approaches in Future Studies
3.1. Phenotyping of Individuals to Enable Precision Nutrition
3.2. Nutrigenetics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Etherton, T.D.; Carlson, J.; Gardner, C. Recent discoveries in inclusive food-based approaches and dietary patterns for reduction in risk for cardiovascular disease. Curr. Opin. Lipidol. 2002, 13, 397–407. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research Policy and Action for Cancer Prevention. Food, Nutrition, and Physical activity: A Global Perspective; AICR: Washington, DC, USA, 2009. [Google Scholar]
- De Roos, B. Personalised nutrition: Ready for practice? Proc. Nutr. Soc. 2013, 72, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone Diets for Weight Loss and Heart Disease Risk Reduction: A Randomized Trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Schmeichel, B.J. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. J. Exp. Psychol. Gen. 2007, 136, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Choosing Health: Making Healthy Choices Easier; Public Health White Paper; Department of Health: London, UK, 2004.
- Bouwman, L.I.; Koelen, M.A. Communication on personalised nutrition: Individual-Environment interaction. Genes Nutr. 2007, 2, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, C.B.; Walsh, M.C.; Gibney, M.J.; Gibney, E.R.; Brennan, L. Can metabotyping help deliver the promise of personalised nutrition? Proc. Nutr. Soc. 2016, 75, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.C.; McMonagle, J.; Shaw, D.I.; Gulseth, H.L.; Helal, O.; Saris, W.H.; Paniagua, J.A.; Golabek-Leszczynska, I.; Defoort, C.; Williams, C.M.; et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: A European randomized dietary intervention study. Int. J. Obes. 2011, 35, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomas-Barberan, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [PubMed]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2012, 56, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Liu, J.; Woo, J. Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women. PLoS ONE 2014, 9, e87861. [Google Scholar] [CrossRef] [PubMed]
- Hazim, S.; Curtis, P.J.; Schar, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostertag, L.M.; Kroon, P.A.; Wood, S.; Horgan, G.W.; Cienfuegos-Jovellanos, E.; Saha, S.; Duthie, G.G.; de Roos, B. Flavan-3-ol-enriched dark chocolate and white chocolate improve acute measures of platelet function in a gender-specific way—A randomized-controlled human intervention trial. Mol. Nutr. Food Res. 2013, 57, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Gardeux, V.; Achour, I.; Li, J.; Maienschein-Cline, M.; Li, H.; Pesce, L.; Parinandi, G.; Bahroos, N.; Winn, R.; Foster, I.; et al. ‘N-of-1-pathways’ unveils personal deregulated mechanisms from a single pair of RNA-Seq samples: Towards precision medicine. J. Am. Med. Inform. Assoc. 2014, 21, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Schissler, A.G.; Gardeux, V.; Li, Q.; Achour, I.; Li, H.; Piegorsch, W.W.; Lussier, Y.A. Dynamic changes of RNA-sequencing expression for precision medicine: N-of-1-pathways Mahalanobis distance within pathways of single subjects predicts breast cancer survival. Bioinformatics 2015, 31, i293–i302. [Google Scholar] [CrossRef] [PubMed]
- Punja, S.; Bukutu, C.; Shamseer, L.; Sampson, M.; Hartling, L.; Urichuk, L.; Vohra, S. N-of-1 trials are a tapestry of heterogeneity. J. Clin. Epidemiol. 2016, 76, 47–56. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Quinn, F.; Vieira, R.; O’Brien, N.; White, M.; Johnston, D.W.; Sniehotta, F.F. The state of the art and future opportunities for using longitudinal n-of-1 methods in health behaviour research: A systematic literature overview. Health Psychol. Rev. 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Mias, G.I.; Li-Pook-Than, J.; Jiang, L.; Lam, H.Y.; Chen, R.; Miriami, E.; Karczewski, K.J.; Hariharan, M.; Dewey, F.E.; et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 2012, 148, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Li-Pook-Than, J.; Snyder, M. iPOP goes the world: Integrated personalized Omics profiling and the road toward improved health care. Chem. Biol. 2013, 20, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Stanberry, L.; Mias, G.I.; Haynes, W.; Higdon, R.; Snyder, M.; Kolker, E. Integrative analysis of longitudinal metabolomics data from a personal multi-omics profile. Metabolites 2013, 3, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of personalized nutrition on health-related behaviour change: Evidence from the Food4Me European randomized controlled trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J.; Goetz, L.H. Single-Subject studies in translational nutrition research. Ann. Rev. Nutr. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Flint, H.J.; Johnstone, A.M.; Lappi, J.; Poutanen, K.; Dewulf, E.; Delzenne, N.; de Vos, W.M.; Salonen, A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE 2014, 9, e90702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Ouden, H.; Pellis, L.; Rutten, G.E.; Geerars-van Vonderen, I.K.; Rubingh, C.M.; van Ommen, B.; van Erk, M.J.; Beulens, J.W. Metabolomic biomarkers for personalised glucose lowering drugs treatment in type 2 diabetes. Metabolomics 2016, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 2016, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Rotroff, D.M.; Corum, D.G.; Motsinger-Reif, A.; Fiehn, O.; Bottrel, N.; Drevets, W.C.; Singh, J.; Salvadore, G.; Kaddurah-Daouk, R. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: New mechanistic insights for rapid acting antidepressants. Transl. Psychiatr. 2016, 6, e894. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Gibney, M.J.; Brennan, L. Dietary intake patterns are reflected in metabolomic profiles: Potential role in dietary assessment studies. Am. J. Clin. Nutr. 2011, 93, 314–321. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, C.B.; Walsh, M.C.; Nugent, A.P.; McNulty, B.; Walton, J.; Flynn, A.; Gibney, M.J.; Gibney, E.R.; Brennan, L. Use of metabotyping for the delivery of personalised nutrition. Mol. Nutr. Food Res. 2015, 59, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Vasan, R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006, 113, 2335–2362. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, G.; Aggett, P.; Brighenti, F.; Delzenne, N.; Frayn, K.; Nieuwenhuizen, A.; Pannemans, D.; Theis, S.; Tuijtelaars, S.; Vessby, B. PASSCLAIM—Body weight regulation, insulin sensitivity and diabetes risk. Eur. J. Nutr. 2004, 43 (Suppl. 2), II7–II46. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Aro, A.; Den Hond, E.; German, J.B.; Griffin, B.A.; ten Meer, H.U.; Mutanen, M.; Pannemans, D.; Stahl, W. PASSCLAIM—Diet-Related cardiovascular disease. Eur. J. Nutr. 2003, 42 (Suppl. 1), I6–I27. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B. Proteomic analysis of human plasma and blood cells in nutritional studies: Development of biomarkers to aid disease prevention. Expert Rev. Proteom. 2008, 5, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Constitution of the World Health Organisation. WHO, 2006. Available online: http://www.who.int/governance/eb/who_constitution_en.pdf (accessed on 21 July 2017).
- Huber, M.; Knottnerus, J.A.; Green, L.; van der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; van der Meer, J.W.; et al. How should we define health? BMJ 2011, 343, d4163. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Duthie, G.G. Role of dietary pro-oxidants in the maintenance of health and resilience to oxidative stress. Mol. Nutr. Food Res. 2015, 59, 1229–1248. [Google Scholar] [CrossRef] [PubMed]
- Kardinaal, A.F.; van Erk, M.J.; Dutman, A.E.; Stroeve, J.H.; van de Steeg, E.; Bijlsma, S.; Kooistra, T.; van Ommen, B.; Wopereis, S. Quantifying phenotypic flexibility as the response to a high-fat challenge test in different states of metabolic health. FASEB J. 2015, 29, 4600–4613. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, T.J.; Kremer, B.H.; Marcondes, R.M.; Hoevenaars, F.P.; Weber, P.; Hoeller, U.; van Ommen, B.; Wopereis, S. The impact of micronutrient status on health: Correlation network analysis to understand the role of micronutrients in metabolic-inflammatory processes regulating homeostasis and phenotypic flexibility. Genes Nutr. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. One (small) step towards precision nutrition by use of metabolomics. Lancet Diabetes Endocrinol. 2017, 5, 154–155. [Google Scholar] [CrossRef]
- Srinivasan, B.; Lee, S.; Erickson, D.; Mehta, S. Precision nutrition—Review of methods for point-of-care assessment of nutritional status. Curr. Opin. Biotechnol. 2017, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Brennan, L. The role of metabolomics in determination of new dietary biomarkers. Proc. Nutr. Soc. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.; Michielsen, C.J.R.; Rundle, M.; Frost, G.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Gibney, M.J.; Brennan, L. Demonstration of the utility of biomarkers for dietary intake assessment; proline betaine as an example. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.; Walsh, M.C.; Gibney, M.J.; Brennan, L.; Gibney, E.R. Personalised nutrition: The role of new dietary assessment methods. Proc. Nutr. Soc. 2016, 75, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.; Rosing, H.; Schellens, J.H.; Beijnen, J.H. Procedures and practices for the validation of bioanalytical methods using dried blood spots: A review. Bioanalysis 2014, 6, 2481–2514. [Google Scholar] [CrossRef] [PubMed]
- Lacher, D.A.; Berman, L.E.; Chen, T.C.; Porter, K.S. Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein cholesterol, and C-reactive protein. Clin. Chim. Acta 2013, 422, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Cardon, L.R.; Harris, T. Precision medicine, genomics and drug discovery. Hum. Mol. Genet. 2016, 25, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- Caslake, M.J.; Miles, E.A.; Kofler, B.M.; Lietz, G.; Curtis, P.; Armah, C.K.; Kimber, A.C.; Grew, J.P.; Farrell, L.; Stannard, J.; et al. Effect of sex and genotype on cardiovascular biomarker response to fish oils: The FINGEN Study. Am. J. Clin. Nutr. 2008, 88, 618–629. [Google Scholar] [PubMed]
- Wilson, C.P.; Ward, M.; McNulty, H.; Strain, J.J.; Trouton, T.G.; Horigan, G.; Purvis, J.; Scott, J.M. Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: A 4-y follow-up. Am. J. Clin. Nutr. 2012, 95, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Coltell, O.; Mattingley, G.; Sorli, J.V.; Ordovas, J.M. Utilizing nutritional genomics to tailor diets for the prevention of cardiovascular disease: A guide for upcoming studies and implementations. Expert Rev. Mol. Diagn. 2017, 17, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.D.; Paoletti, X.; Burzykowski, T.; Buyse, M. Precision medicine needs randomized clinical trials. Nat. Rev. Clin. Oncol. 2017, 14, 317–323. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Roos, B.; Brennan, L. Personalised Interventions—A Precision Approach for the Next Generation of Dietary Intervention Studies. Nutrients 2017, 9, 847. https://doi.org/10.3390/nu9080847
De Roos B, Brennan L. Personalised Interventions—A Precision Approach for the Next Generation of Dietary Intervention Studies. Nutrients. 2017; 9(8):847. https://doi.org/10.3390/nu9080847
Chicago/Turabian StyleDe Roos, Baukje, and Lorraine Brennan. 2017. "Personalised Interventions—A Precision Approach for the Next Generation of Dietary Intervention Studies" Nutrients 9, no. 8: 847. https://doi.org/10.3390/nu9080847




