Type 1 Diabetes and Non-Alcoholic Fatty Liver Disease: When Should We Be Concerned? A Nationwide Study in Brazil †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of Metabolic Syndrome and Aminotransferases
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Prevalence of Metabolic Syndrome
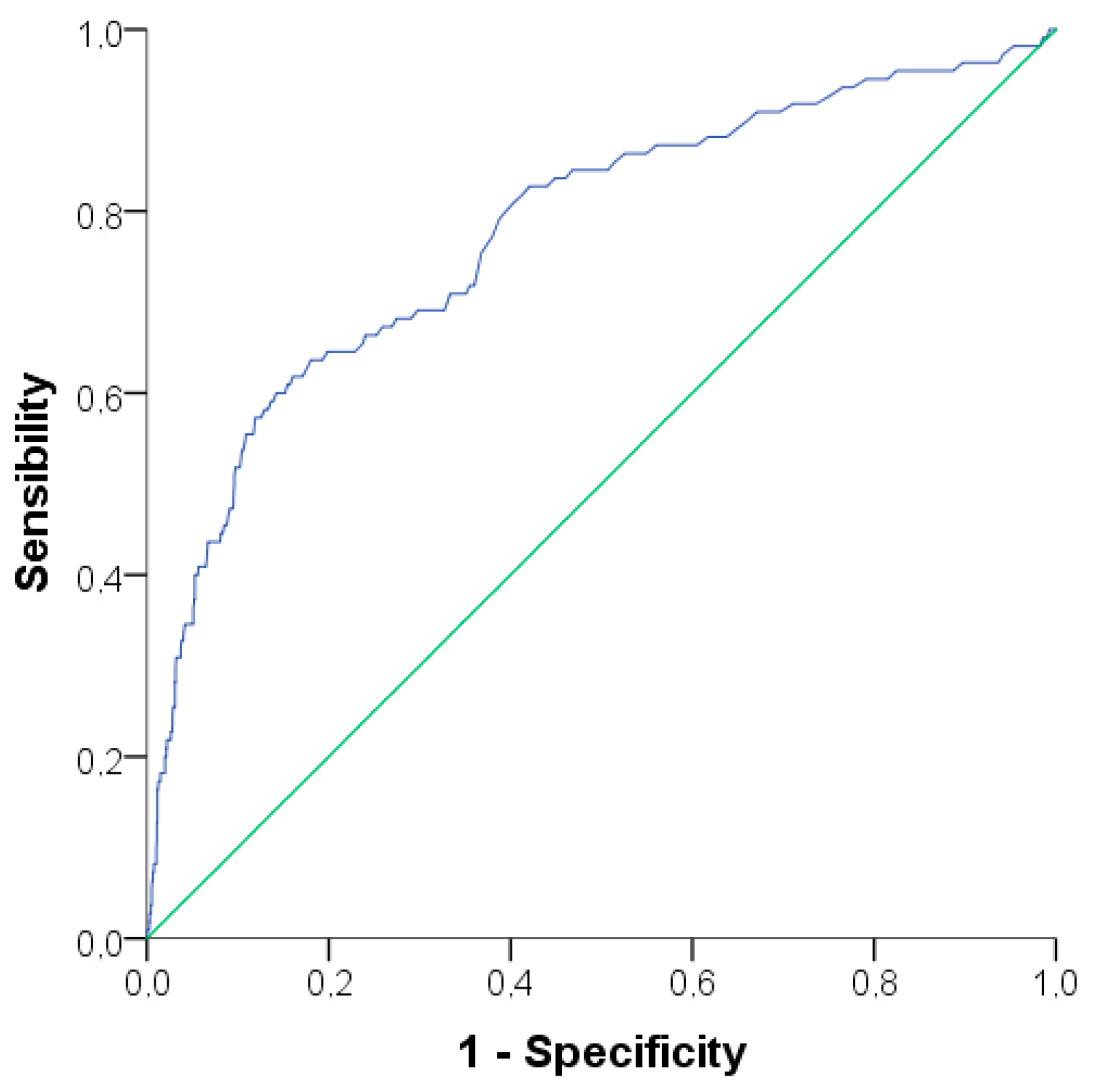
3.2. Evaluation of Alanine Aminotransferase Levels
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Negrato, C.A.; Lauris, J.R.P.; Saggioro, I.B.; Corradini, M.C.M.; Borges, P.R.; Crês, M.C.; Junior, A.L.; Guedes, M.F.S.; Gomes, M.B. Increasing incidence of type 1 diabetes between 1986 and 2015 in Bauru, Brazil. Diabetes Res. Clin. Pract. 2017, 127, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Group, D.P. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet. Med. 2006, 23, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.; Dahlquist, G.; Gyürüs, E.; Green, A.; Soltész, G.; Group, T.E.S. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Group, E.A.S. Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000, 355, 873–876. [Google Scholar]
- Cleland, S.J.; Fisher, B.M.; Colhoun, H.M.; Sattar, N.; Petrie, J.R. Insulin resistance in type 1 diabetes: What is ‘double diabetes’ and what are the risks? Diabetologia 2013, 56, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Miller, R.G.; Costacou, T.; Fried, L.; Kelsey, S.; Evans, R.W.; Orchard, T.J. Adiposity and mortality in type 1 diabetes. Int. J. Obes. 2009, 33, 796–805. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 8 July 2017).
- Manyanga, T.; Sellers, E.A.; Wicklow, B.A.; Doupe, M.; Fransoo, R. Not as skinny as we used to think: Body mass index in children and adolescents at diagnosis of type 1 diabetes mellitus. J. Diabetes Complicat. 2016, 30, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, M. Obesity and dyslipidemia-An urgent matter in youth from the general population and in type 1 diabetic patients. Arch. Endocrinol. Metab. 2015, 59, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, C.; Volkening, L.K.; Diaz, M.; Laffel, L.M. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr. Diabetes 2015, 16, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.A.; Negrato, C.A.; Cobas, R.; Matheus, A.; Tannus, L.; Palma, C.S.; Japiassu, L.; Carneiro, J.R.; Rodacki, M.; Zajdenverg, L.; et al. Relationship between adherence to diet, glycemic control and cardiovascular risk factors in patients with type 1 diabetes: A nationwide survey in Brazil. Nutr. J. 2014, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Minges, K.E.; Whittemore, R.; Weinzimer, S.A.; Irwin, M.L.; Redeker, N.S.; Grey, M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: The T1D Exchange Clinic Registry. Diabetes Res. Clin. Pract. 2017, 126, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Minges, K.E.; Whittemore, R.; Grey, M. Overweight and obesity in youth with type 1 diabetes. Annu. Rev. Nurs. Res. 2013, 31, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Cleland, S.J. Cardiovascular risk in double diabetes mellitus—When two worlds collide. Nat. Rev. Endocrinol. 2012, 8, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Hermosillo, A.; Ramírez-Rentería, C.; Mendoza-Zubieta, V.; Molina-Ayala, M.A. Utility of the waist-to-height ratio, waist circumference and body mass index in the screening of metabolic syndrome in adult patients with type 1 diabetes mellitus. Diabetol. Metab. Syndr. 2014, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Merger, S.R.; Kerner, W.; Stadler, M.; Zeyfang, A.; Jehle, P.; Müller-Korbsch, M.; Holl, R.W.; DPV Initiative; German BMBF Competence Network Diabetes Mellitus. Prevalence and comorbidities of double diabetes. Diabetes Res. Clin. Pract. 2016, 119, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Kantartzis, K.; Häring, H.U. Causes and metabolic consequences of Fatty liver. Endocr. Rev. 2008, 29, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Diagnosis of non-alcoholic fatty liver disease (NAFLD). Diabetologia 2016, 59, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar]
- Gomes, M.B.; Negrato, C.A. Adherence to insulin therapeutic regimens in patients with type 1 diabetes. A nationwide survey in Brazil. Diabetes Res. Clin. Pract. 2016, 120, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, J.; Zhang, X.; Liu, S.; Ge, Y. Association of the serum uric acid level with liver histology in biopsy-proven non-alcoholic fatty liver disease. Biomed. Rep. 2016, 5, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Hyperuricaemia and non-alcoholic fatty liver disease (NAFLD): A relationship with implications for vascular risk? Curr. Vasc. Pharmacol. 2011, 9, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: A cross-sectional study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, C.; Li, X.; Sun, L.; Zhu, X.; Zhao, C.; Zhang, Z.; Yang, Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2015, 100, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Llauradó, G.; Sevastianova, K.; Sädevirta, S.; Hakkarainen, A.; Lundbom, N.; Orho-Melander, M.; Groop, P.H.; Forsblom, C.; Yki-Järvinen, H. Liver fat content and hepatic insulin sensitivity in overweight patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 2015, 100, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Regnell, S.E.; Lernmark, Å. Hepatic steatosis in type 1 diabetes. Rev. Diabet. Stud. 2011, 8, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Sanyal, A.J.; Zhang, S.; Hartman, M.L.; Bue-Valleskey, J.M.; Hoogwerf, B.J.; Haupt, A. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes. Metab. 2017. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Pedro, L.; Guiu, B.; Duvillard, L.; Bouillet, B.; Jooste, V.; Habchi, M.; Crevisy, E.; Fourmont, C.; Buffier, P.; et al. Type 1 diabetes is not associated with an increased prevalence of hepatic steatosis. Diabet. Med. 2015, 32, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
| Adults | Adolescents | |||||
|---|---|---|---|---|---|---|
| MS+ | MS− | p-Value | MS+ | MS− | p-Value | |
| N (%) | 445 (32.3) | 931 (67.7) | 24 (8.4) | 262 (91.6) | ||
| Clinical parameters | ||||||
| Age, years | 36.3 ± 11.5 | 31.4 ± 10.5 | <0.001 | 16.9 ± 1.3 | 15.7 ± 1.7 | <0.001 |
| Female gender, n (%) | 304 (68.3) | 478 (51.3) | <0.001 | 18 (75.0) | 118 (45.0) | 0.005 |
| Diabetes duration, years | 19.2 ± 9.7 | 16.0 ± 8.9 | <0.001 | 9.1 ± 4.9 | 7.6 ± 4.2 | 0.11 |
| Years of education | 12.2 ± 4.3 | 12.7 ± 3.8 | 0.02 | 11.0 ± 2.8 | 10.4 ± 2.3 | 0.27 |
| WC (centimeters) | 94.2 ± 9.6 | 79.1 ± 9.0 | <0.001 | 89.4 ± 7.1 | 75.3 ± 8.3 | <0.001 |
| BMI (kg/m2) | 27.8 ± 4.2 | 23.1 ± 3.2 | <0.001 | 27.0 ± 3.4 | 21.3 ± 3.0 | <0.001 |
| SBP (mmHg) | 129.4 ± 16.8 | 120.9 ± 15.1 | <0.001 | 120.3 ± 10.9 | 112.2 ± 11.7 | 0.002 |
| DBP (mmHg) | 79.7 ± 10.1 | 74.5 ± 9.7 | <0.001 | 76.3 ± 8.3 | 69.1 ± 9.1 | <0.001 |
| Hypertension, n (%) | 175 (39.3) | 117 (12.6) | <0.001 | 0 | 1 (0.4) | 1.0 |
| Dyslipidemia, n (%) | 149 (33.5) | 182 (19.5) | <0.001 | 3 (12.5) | 21 (8.0) | 0.44 |
| Obesity, n (%) | 98 (22.0) | 30 (3.2) | <0.001 | 5 (20.8) | 8 (3.1) | <0.001 |
| Overweight, n (%) | 228 (51.2) | 189 (20.3) | <0.001 | 10 (41.7) | 36 (13.7) | <0.001 |
| Smoking, n (%) | 21 (4.7) | 55 (5.9) | 0.37 | 1 (4.2) | 11 (4.2) | 1.0 |
| Metformin use, n (%) | 105 (23.1) | 70 (7.5) | <0.001 | 12 (50.0) | 15 (5.7) | <0.001 |
| Statin use, n (%) | 155 (34.0) | 194 (20.8) | <0.001 | 4 (16.7) | 6 (2.3) | 0.006 |
| Fibrate use, n (%) | 8 (1.8) | 5 (0.5) | 0.03 | - | - | - |
| ACEi or AT2 blocker use, n (%) | 201 (44.1) | 226 (24.3) | <0.001 | 2 (8.3) | 19 (7.3) | 0.68 |
| Total daily insulin (U/kg) | 0.8 ± 0.3 | 0.9 ± 0.4 | 0.02 | 0.9 ± 0.4 | 1.1 ± 0.4 | 0.048 |
| Family history of T2D, n (%) | 151 (33.9) | 232 (24.9) | 0.001 | 5 (20.8) | 28 (10.7) | 0.172 |
| Family history of obesity, n (%) | 130 (28.5) | 196 (21.1) | 0.001 | 6 (25.0) | 58 (22.1) | 0.798 |
| Diet adherence ≥ 80%, n (%) | 212 (47.6) | 510 (54.7) | 0.059 | 10 (50.0) | 119 (50.9) | 1.0 |
| Physical exercise, yes n (%) | 192 (43.1) | 477 (51.2) | 0.004 | 13 (54.2) | 174 (66.4) | 0.264 |
| Acanthosis nigricans, n (%) | 46 (10.3) | 15 (1.6) | <0.001 | 3 (12.5) | 4 (1.5) | 0.015 |
| Laboratorial parameters | ||||||
| ALT (U/L) | 16.2 ± 12.6 | 13.1 ± 9.3 | <0.001 | 13.0 ± 10.0 | 12.8 ± 9.4 | 0.94 |
| AST (U/L) | 23.7 ± 19.5 | 19.4 ± 12.2 | <0.001 | 17.3 ± 14.3 | 19.8 ± 12.8 | 0.38 |
| Albumin (mg/dL) | 3.9 ± 0.7 | 3.9 ± 0.5 | 0.62 | 3.9 ± 0.5 | 4.0 ± 0.6 | 0.21 |
| GGT (U/L) | 21 (21) | 18 (13) | <0.001 | 19 (17.5) | 16 (9) | 0.41 |
| FPG (mg/dL) | 171.0 (132.5) | 166.5 (155.2) | 0.99 | 152.5 (166.7) | 200.0 (161.7) | 0.13 |
| HbA1c (%) | 8.9 ± 1.9 | 8.8 ± 2.1 | 0.96 | 9.5 ± 2.5 | 9.7 ± 2.4 | 0.73 |
| HbA1c (mmol) | 73.3 ± 20.7 | 73.3 ± 22.8 | 0.96 | 80.9 ± 27.9 | 82.8 ± 26.6 | 0.73 |
| RCP (mg/dL) | 0.7 ± 1.5 | 0.4 ± 1.0 | <0.001 | 0.4 ± 0.5 | 0.3 ± 0.6 | 0.17 |
| GFR (ml/min) | 75.5 ± 25.6 | 84.0 ± 25.8 | <0.001 | 102.5 ± 26.2 | 115.7 ± 30.8 | 0.03 |
| TC (mg/dL) | 198.8 ± 64.3 | 184.2 ± 41.4 | <0.001 | 184.6 ± 55.5 | 185.3 ± 57.1 | 0.95 |
| LDL-c (mg/dL) | 117.3 ± 47.1 | 107.2 ± 34.5 | <0.001 | 111.7 ± 41.0 | 106.7 ± 37.3 | 0.54 |
| HDL-c (mg/dL) | 53.8 ± 20.9 | 58.1 ± 17.6 | <0.001 | 46.3 ± 13.0 | 55.7 ± 16.6 | 0.007 |
| Triglycerides (mg/dL) | 80 (95.5) | 107 (51) | <0.001 | 88.5 (131) | 84 (59) | 0.15 |
| Uric acid (mg/dL) | 5.6 ± 2.1 | 4.9 ± 1.7 | <0.001 | 5.0 ± 1.8 | 4.9 ± 1.4 | 0.78 |
| Variable | B | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Age (years) | 0.044 | 1.04 | 1.03–1.06 | <0.001 |
| Female gender | 0.967 | 2.63 | 2.02–3.42 | <0.001 |
| Years of education | 0.010 | 1.01 | 0.98–1.04 | 0.531 |
| Diabetes duration (years) | 0.007 | 1.01 | 0.99–1.02 | 0.364 |
| Acid uric levels (mg/dL) | 0.265 | 1.30 | 1.22–1.40 | <0.001 |
| Practice of physical exercise, yes | −0.171 | 0.84 | 0.66–1.07 | 0.165 |
| Presence of acanthosis nigricans, yes | 0.1674 | 5.33 | 2.97–9.58 | <0.001 |
| Positive family history of T2D | 0.044 | 0.75 | 0.79–1.38 | 0.752 |
| Positive family history of obesity | 0.195 | 1.22 | 0.92–1.60 | 0.164 |
| Model | R | Adjusted R2 | Variables | B | 95% CI for B | p-Value |
|---|---|---|---|---|---|---|
| 1 | 0.437 | 0.191 | triglycerides (mg/dL) | 0.06 | 0.05–0.06 | <0.001 |
| 2 | 0.446 | 0.199 | triglycerides (mg/dL) | 0.05 | 0.04–0.06 | <0.001 |
| uric acid (mg/dL) | 0.55 | 0.27–0.83 | <0.001 | |||
| 3 | 0.452 | 0.202 | triglycerides (mg/dL) | 0.05 | 0.04–0.06 | <0.001 |
| uric acid (mg/dL) | 0.54 | 0.26–0.81 | <0.001 | |||
| non-Caucasian ethnicity | 1.52 | 0.61–2.44 | 0.001 | |||
| 4 | 0.456 | 0.206 | triglycerides (mg/dL) | 0.05 | 0.04–0.06 | <0.001 |
| uric acid (mg/dL) | 0.50 | 0.22–0.78 | <0.001 | |||
| non-Caucasian ethnicity | 1.68 | 0.76–2.60 | <0.001 | |||
| age (years) | 0.06 | 0.02–0.09 | 0.004 | |||
| 5 | 0.461 | 0.210 | triglycerides (mg/dL) | 0.05 | 0.05–0.06 | <0.001 |
| uric acid (mg/dL) | 0.38 | 0.09–0.67 | 0.009 | |||
| non-Caucasian ethnicity | 1.66 | 0.75–2.58 | <0.001 | |||
| age (years) | 0.06 | 0.02–0.09 | 0.002 | |||
| male gender | 1.50 | 0.55–2.44 | 0.002 | |||
| 6 | 0.464 | 0.212 | triglycerides (mg/dL) | 0.05 | 0.04–0.06 | <0.001 |
| uric acid (mg/dL) | 0.40 | 0.11–0.69 | 0.007 | |||
| non-Caucasian ethnicity | 1.54 | 0.62–2.46 | 0.001 | |||
| age (years) | 0.07 | 0.03–0.11 | 0.001 | |||
| male gender | 1.55 | 0.60–2.50 | 0.001 | |||
| HbA1c (%) | 0.26 | 0.03–0.48 | 0.023 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, B.S.V.; Conte Santos, D.; Haas Pizarro, M.; Melo, L.G.N.d.; Brito Gomes, M. Type 1 Diabetes and Non-Alcoholic Fatty Liver Disease: When Should We Be Concerned? A Nationwide Study in Brazil. Nutrients 2017, 9, 878. https://doi.org/10.3390/nu9080878
Barros BSV, Conte Santos D, Haas Pizarro M, Melo LGNd, Brito Gomes M. Type 1 Diabetes and Non-Alcoholic Fatty Liver Disease: When Should We Be Concerned? A Nationwide Study in Brazil. Nutrients. 2017; 9(8):878. https://doi.org/10.3390/nu9080878
Chicago/Turabian StyleBarros, Bianca Senger Vasconcelos, Deborah Conte Santos, Marcela Haas Pizarro, Laura Gomes Nunes de Melo, and Marilia Brito Gomes. 2017. "Type 1 Diabetes and Non-Alcoholic Fatty Liver Disease: When Should We Be Concerned? A Nationwide Study in Brazil" Nutrients 9, no. 8: 878. https://doi.org/10.3390/nu9080878





