Dietary Fructose Enhances the Ability of Low Concentrations of Angiotensin II to Stimulate Proximal Tubule Na+ Reabsorption
Abstract
:1. Introduction
2. Materials and Methods
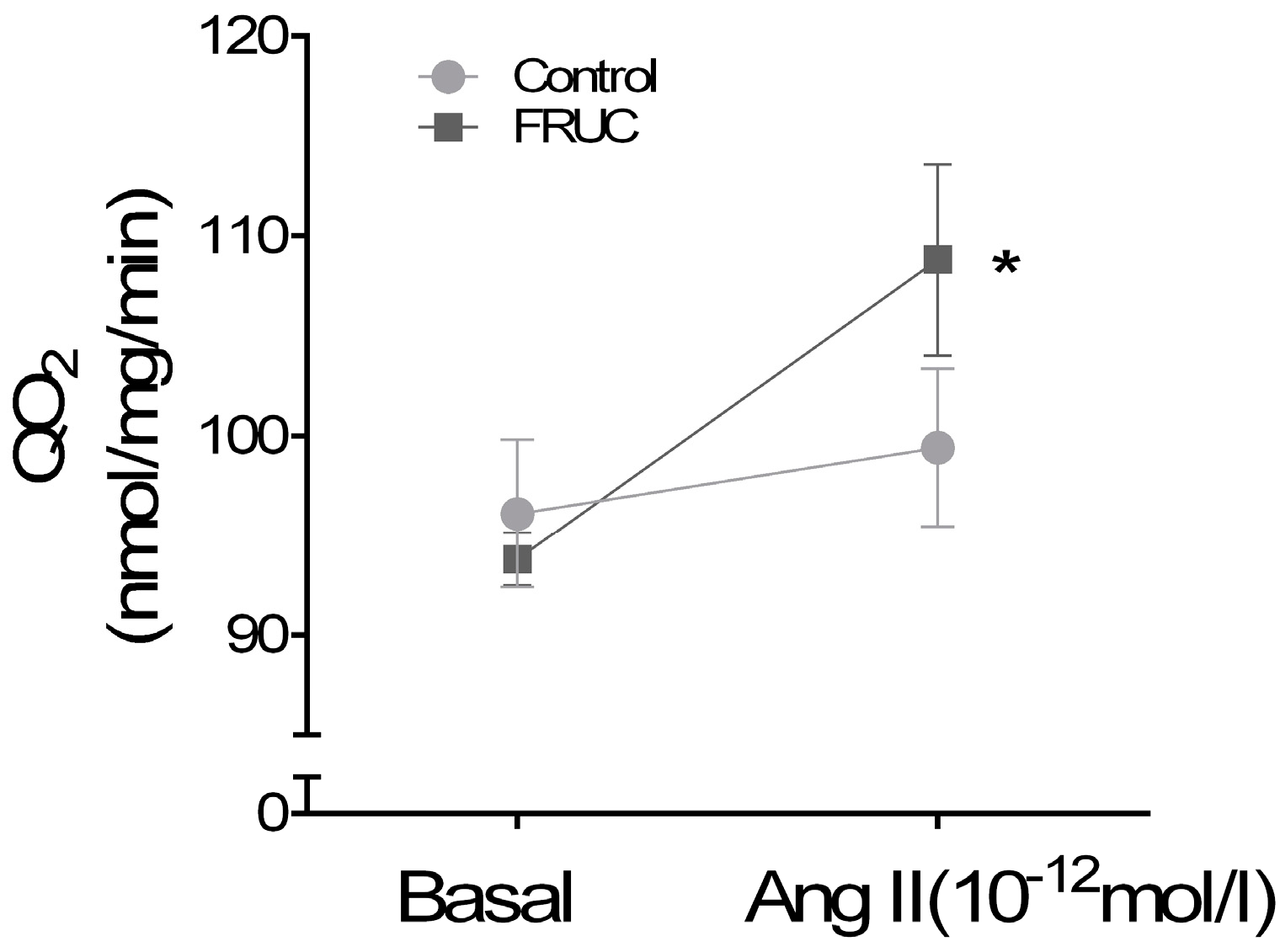
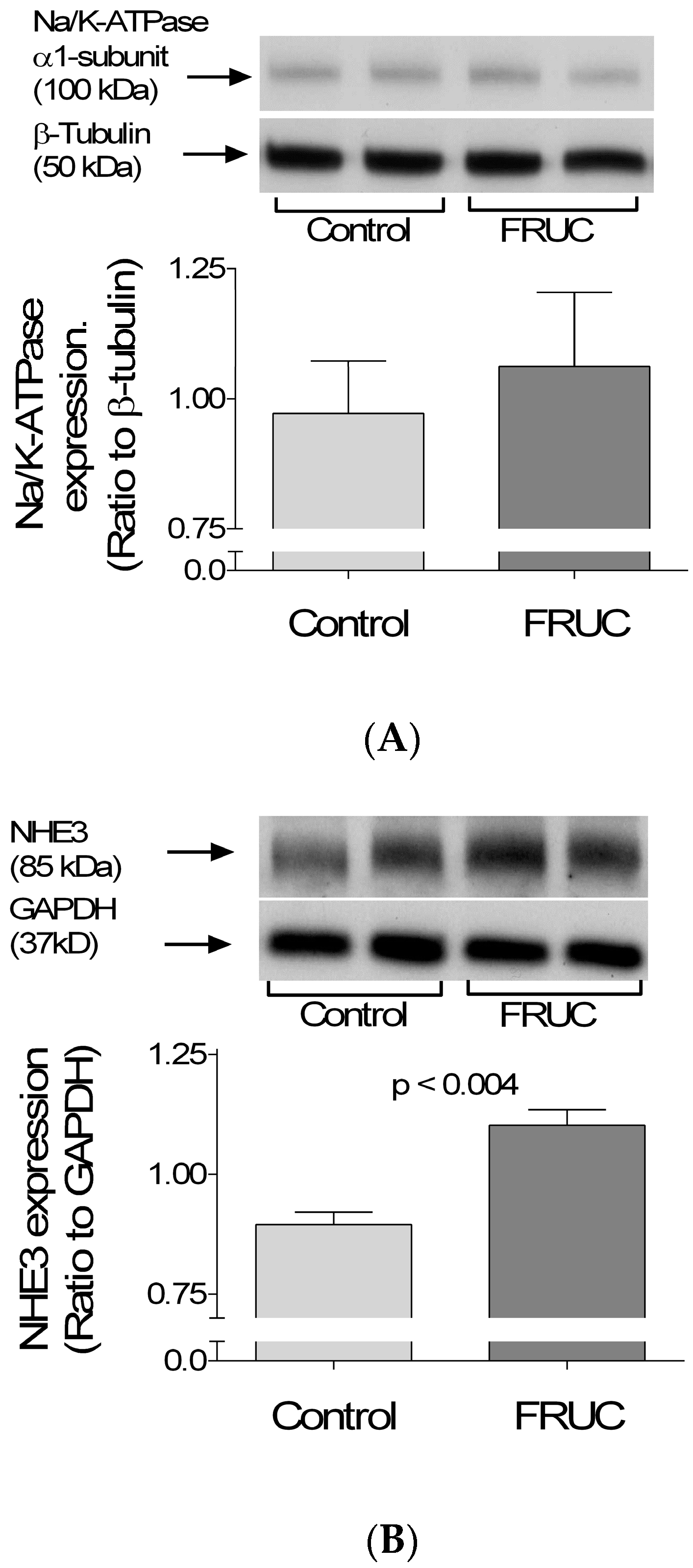
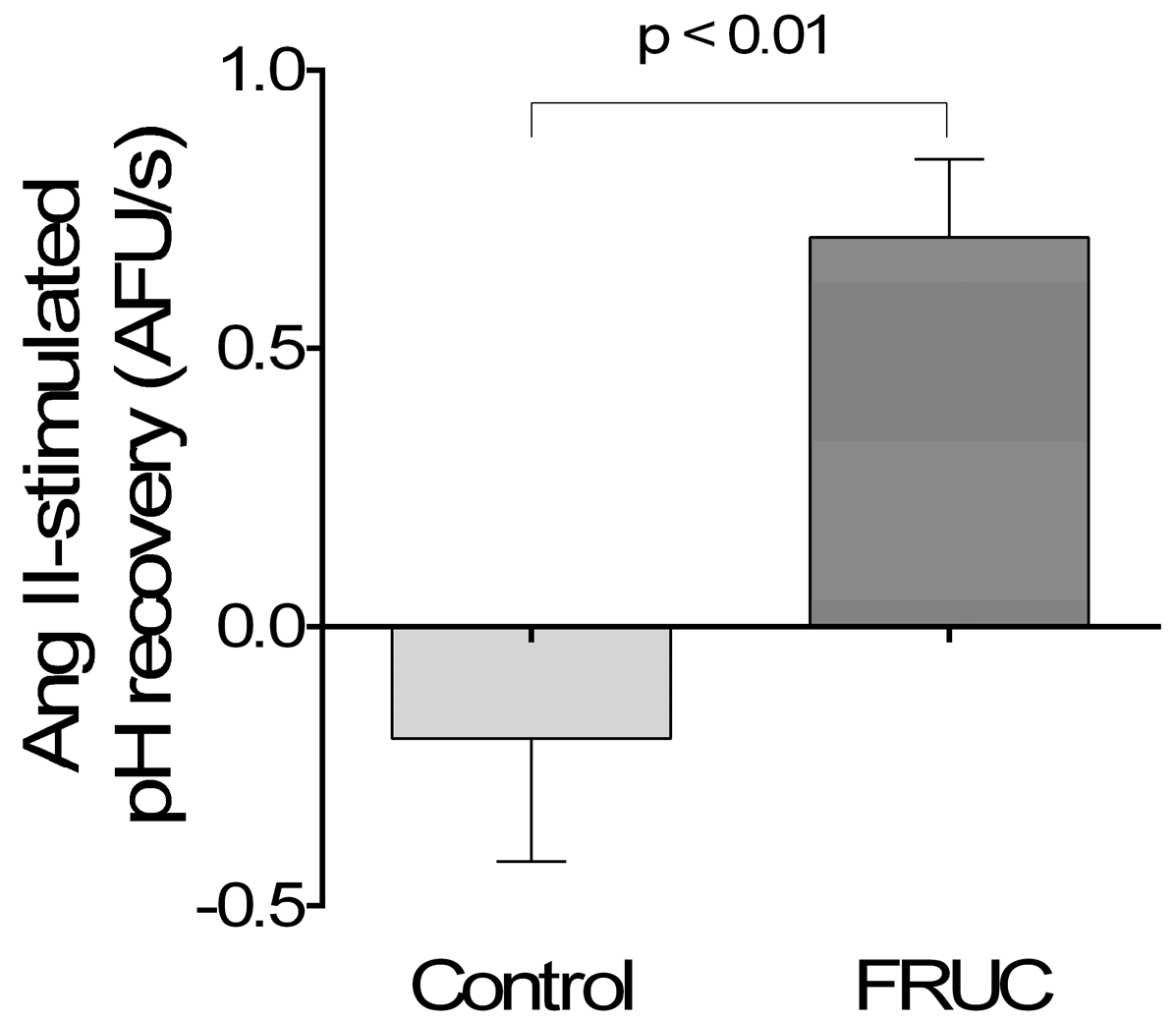
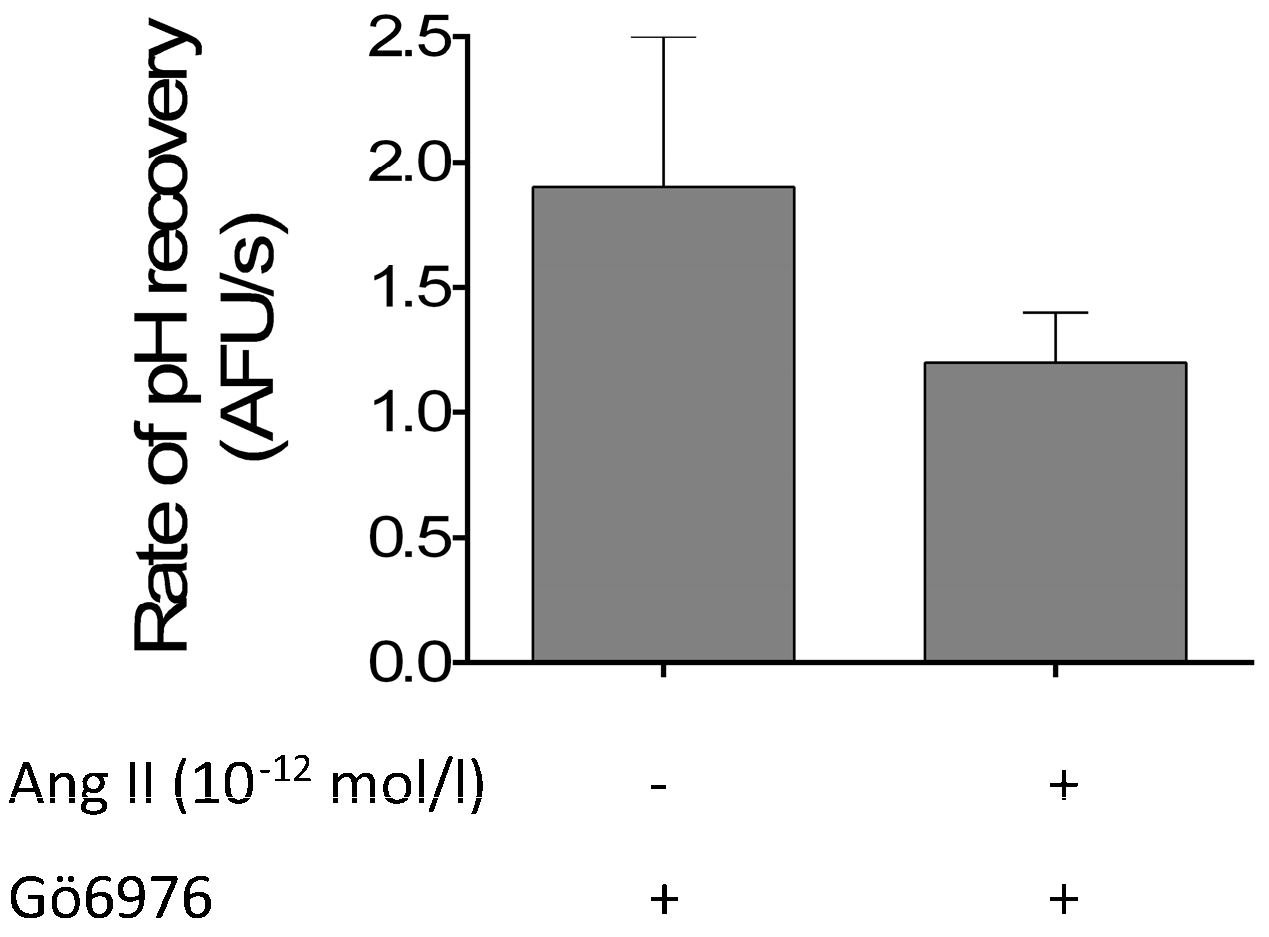
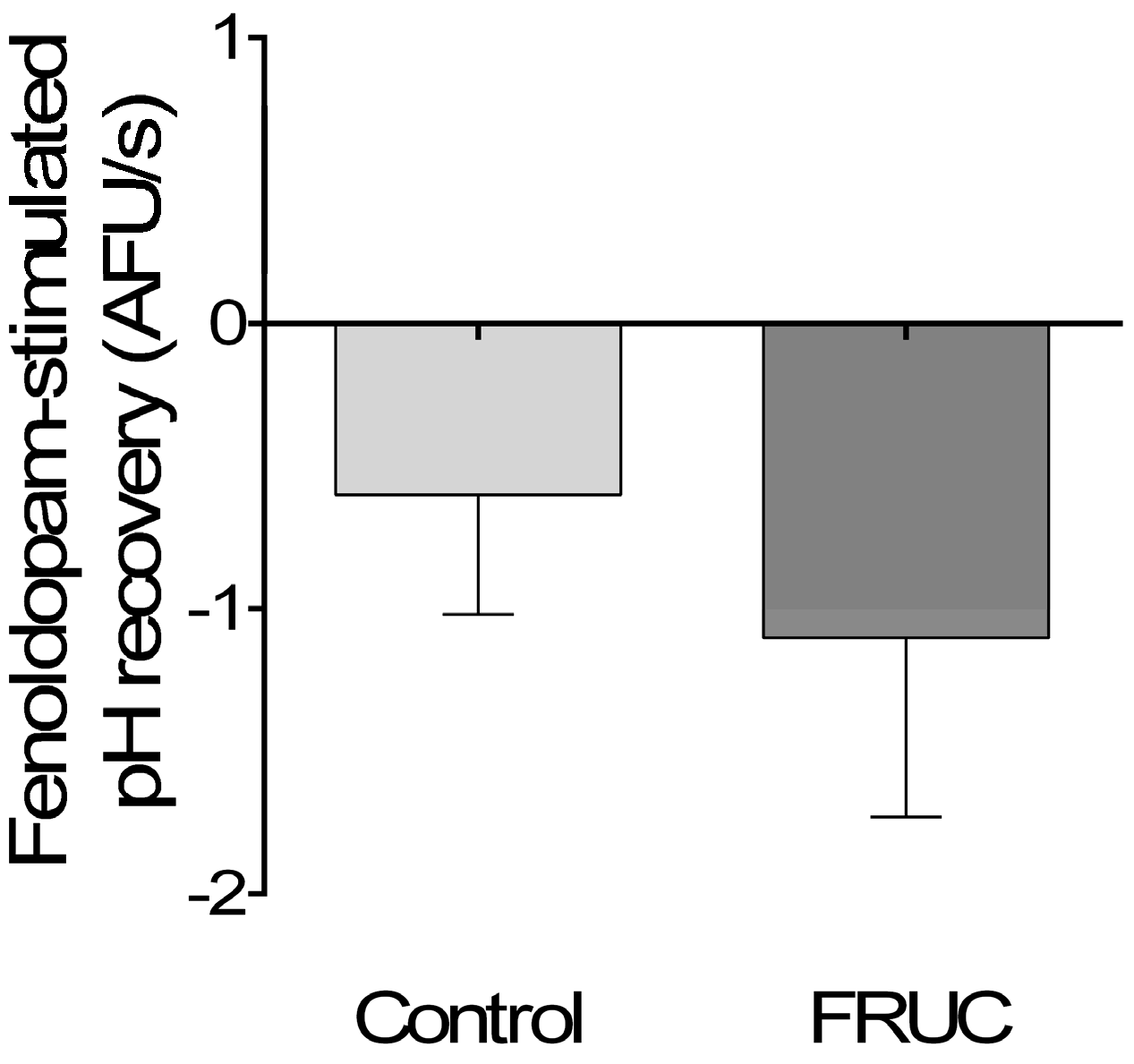
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sluik, D.; Engelen, A.I.; Feskens, E.J. Fructose consumption in the netherlands: The dutch national food consumption survey 2007–2010. Eur. J. Clin. Nutr. 2015, 69, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Jarvinen, R.; Knekt, P.; Heliovaara, M.; Reunanen, A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J. Nutr. 2007, 137, 1447–1454. [Google Scholar] [PubMed]
- Vos, M.B.; Kimmons, J.E.; Gillespie, C.; Welsh, J.; Blanck, H.M. Dietary fructose consumption among us children and adults: The third national health and nutrition examination survey. Medscape J. Med. 2008, 10, 160. [Google Scholar] [PubMed]
- Libuda, L.; Alexy, U.; Buyken, A.E.; Sichert-Hellert, W.; Stehle, P.; Kersting, M. Consumption of sugar-sweetened beverages and its association with nutrient intakes and diet quality in german children and adolescents. Br. J. Nutr. 2009, 101, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Kit, B.K.; Carroll, M.D.; Park, S. Consumption of Sugar Drinks in the United States, 2005–2008; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2011; pp. 1–8. [Google Scholar]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J.; American Heart Association Nutrition Committee of the Council on Nutrition; Physical Activity; Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: A scientific statement from the american heart association. Circulation 2009, 120, 1011–1020. [Google Scholar] [PubMed]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased fructose associates with elevated blood pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The effect of fructose on renal biology and disease. J. Am. Soc. Nephrol. 2010, 21, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA J. Am. Med. Assoc. 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- Nguyen, S.; Choi, H.K.; Lustig, R.H.; Hsu, C.Y. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J. Pediatr. 2009, 154, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Tomida, T.; Matsui, H.; Ito, T.; Okumura, K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension 2002, 40, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Tapia, E.; Jimenez, A.; Bautista, P.; Cristobal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F423–F429. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Gill, V.; Parai, S.; Gadag, V. Fructose-induced hypertension in wistar-kyoto rats: Interaction with moderately high dietary salt. Can. J. Physiol. Pharmacol. 2007, 85, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.D.; Hong, N.J.; Hye Khan, M.A.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates NA/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef] [PubMed]
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5, e13162. [Google Scholar] [CrossRef] [PubMed]
- Beierwaltes, W.; Ismail, A.; Szandzik, D.; Garvin, J.; Ortiz, P. A fructose enriched diet (20%) induces salt-sensitive hypertension and prevents salt-induced decrease in plasma renin. FASEB J. 2015, 29, S960. [Google Scholar]
- Thomas, D.; Harris, P.J.; Morgan, T.O. Age-related changes in angiotensin II-stimulated proximal tubule fluid reabsorption in the spontaneously hypertensive rat. J. Hypertens. Suppl. 1988, 6, S449–S451. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.L.; Harris, P.J.; Eitle, E. Increased proximal tubule NHE-3 and H+-atpase activities in spontaneously hypertensive rats. J. Hypertens. 2000, 18, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Control of sodium excretion by angiotensin II: Intrarenal mechanisms and blood pressure regulation. Am. J. Physiol. 1986, 250, R960–R972. [Google Scholar] [PubMed]
- Gurley, S.B.; Riquier-Brison, A.D.; Schnermann, J.; Sparks, M.A.; Allen, A.M.; Haase, V.H.; Snouwaert, J.N.; Le, T.H.; McDonough, A.A.; Koller, B.H.; et al. At1a angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011, 13, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bertorello, A.M. Diacylglycerol activation of protein kinase C results in a dual effect on Na+,K(+)-ATPase activity from intact renal proximal tubule cells. J. Cell Sci. 1992, 101 Pt 2, 343–347. [Google Scholar] [PubMed]
- Bertorello, A.M.; Katz, A.I. Short-term regulation of renal Na-K-ATPase activity: Physiological relevance and cellular mechanisms. Am. J. Physiol. 1993, 265, F743–F755. [Google Scholar] [PubMed]
- Liu, F.Y.; Cogan, M.G. Role of protein kinase C in proximal bicarbonate absorption and angiotensin signaling. Am. J. Physiol. 1990, 258, F927–F933. [Google Scholar] [PubMed]
- Du, Z.; Ferguson, W.; Wang, T. Role of PKC and calcium in modulation of effects of angiotensin II on sodium transport in proximal tubule. Am. J. Physiol. Ren. Physiol. 2003, 284, F688–F692. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.N.; Katovich, M.J. Effect of chronic losartan potassium treatment on fructose-induced hypertension. Life Sci. 1994, 55, PL139–PL144. [Google Scholar] [CrossRef]
- Kamari, Y.; Harari, A.; Shaish, A.; Peleg, E.; Sharabi, Y.; Harats, D.; Grossman, E. Effect of telmisartan, angiotensin II receptor antagonist, on metabolic profile in fructose-induced hypertensive, hyperinsulinemic, hyperlipidemic rats. Hypertens. Res. 2008, 31, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.N.; Katovich, M.J. Effect of acute and chronic losartan treatment on glucose tolerance and insulin sensitivity in fructose-fed rats. Am. J. Hypertens. 1996, 9, 662–668. [Google Scholar] [CrossRef]
- Navarro-Cid, J.; Maeso, R.; Perez-Vizcaino, F.; Cachofeiro, V.; Ruilope, L.M.; Tamargo, J.; Lahera, V. Effects of losartan on blood pressure, metabolic alterations, and vascular reactivity in the fructose-induced hypertensive rat. Hypertension 1995, 26, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J. Regulation of proximal tubule function by angiotensin. Clin. Exp. Pharmacol. Physiol. 1992, 19, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Reilly, A.M.; Harris, P.J.; Williams, D.A. Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am. J. Physiol. 1995, 269, F374–F380. [Google Scholar] [PubMed]
- Harris, P.J.; Navar, L.G.; Ploth, D.W. Evidence for angiotensin-stimulated proximal tubular fluid reabsorption in normotensive and hypertensive rats: Effect of acute administration of captopril. Clin. Sci. 1984, 66, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, M.A.; Perriello, V.A.; Marsden, C.D.; Jones, N.F. The influence of the experimental conditions on the renal response to angiotensin in the rat. Experientia 1967, 23, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, M.A.; Jones, N.F.; Marsden, C.D. Effect of angiotensin on renal function in the rat. Am. J. Physiol. 1967, 212, 1153–1157. [Google Scholar] [PubMed]
- Kuroki, M.T.; Fink, G.D.; Osborn, J.W. Comparison of arterial pressure and plasma ANG II responses to three methods of subcutaneous ANG II administration. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H670–H679. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Nakata, M.; Dezaki, K.; Lu, M.; Gantulga, D.; Yamamoto, K.; Shimada, K.; Kario, K.; Yada, T. Ghrelin counteracts salt-induced hypertension via promoting diuresis and renal nitric oxide production in dahl rats. Endocr. J. 2013, 60, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Seikaly, M.G.; Arant, B.S., Jr.; Seney, F.D., Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J. Clin. Investig. 1990, 86, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Braam, B.; Mitchell, K.D.; Fox, J.; Navar, L.G. Proximal tubular secretion of angiotensin II in rats. Am. J. Physiol. 1993, 264, F891–F898. [Google Scholar] [PubMed]
- Navar, L.G.; Harrison-Bernard, L.M.; Wang, C.T.; Cervenka, L.; Mitchell, K.D. Concentrations and actions of intraluminal angiotensin II. J. Am. Soc. Nephrol. 1999, 10 (Suppl. 11), S189–S195. [Google Scholar] [PubMed]
- Nishiyama, A.; Seth, D.M.; Navar, L.G. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension 2002, 39, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.E.; Liard, J.F.; Cowley, A.W., Jr. Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am. J. Physiol. 1990, 259, H1629–H1636. [Google Scholar] [PubMed]
- Tank, J.E.; Moe, O.W.; Henrich, W.L. Abnormal regulation of proximal tubule renin mRNA in the Dahl/Rapp salt-sensitive rat. Kidney Int. 1998, 54, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Stuart, D.; Hillas, E.; Gociman, B.R.; Ramkumar, N.; Lalouel, J.M.; Kohan, D.E. Overexpression of mouse angiotensinogen in renal proximal tubule causes salt-sensitive hypertension in mice. Am. J. Hypertens. 2012, 25, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Ho, H.; Donnelly, R.; Reaven, G.M. Salt-sensitive and carbohydrate-sensitive rodent hypertension: Evidence of strain differences. Blood Press. 1994, 3, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Cavarape, A.; Novello, M.; Giacchetti, G.; Sechi, L.A. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003, 64, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Boini, K.M.; Friedrich, B.; Metzger, M.; Just, L.; Osswald, H.; Wulff, P.; Kuhl, D.; Vallon, V.; Lang, F. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R935–R944. [Google Scholar] [CrossRef] [PubMed]
- Boesch, D.M.; Garvin, J.L. Age-dependent activation of PKC isoforms by angiotensin II in the proximal nephron. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R861–R867. [Google Scholar] [PubMed]
- Gonzalez-Vicente, A.; Garvin, J.L. Angiotensin II-induced hypertension increases plasma membrane Na pump activity by enhancing Na entry in rat thick ascending limbs. Am. J. Physiol. Ren. Physiol. 2013, 305, F1306–F1314. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Cabral, P.D.; Garvin, J.L. Resveratrol increases nitric oxide production in the rat thick ascending limb via Ca2+/calmodulin. PLoS ONE 2014, 9, e110487. [Google Scholar] [CrossRef] [PubMed]
- Garvin, J.L. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in rat proximal straight tubules. J. Am. Soc. Nephrol. 1991, 1, 1146–1152. [Google Scholar] [PubMed]
- Toop, C.R.; Gentili, S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: A systematic review and meta-analysis. Nutrients 2016, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Magyar, C.E.; Zhang, Y.; Holstein-Rathlou, N.H.; McDonough, A.A. Downstream shift in sodium pump activity along the nephron during acute hypertension. J. Am. Soc. Nephrol. 2001, 12, 2231–2240. [Google Scholar] [PubMed]
- Yingst, D.R.; Massey, K.J.; Rossi, N.F.; Mohanty, M.J.; Mattingly, R.R. Angiotensin II directly stimulates activity and alters the phosphorylation of Na-K-ATPase in rat proximal tubule with a rapid time course. Am. J. Physiol. Ren. Physiol. 2004, 287, F713–F721. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.S.; Johns, E.J. The receptor subtype mediating the action of angiotensin II on intracellular sodium in rat proximal tubules. Br. J. Pharmacol. 1998, 124, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Leite, G.D.; Crajoinas, R.O.; Neri, E.A.; Bezerra, C.N.; Girardi, A.C.; Reboucas, N.A.; Malnic, G. Fructose acutely stimulates NHE3 activity in kidney proximal tubule. Kidney Blood Press. Res. 2012, 36, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Navarro, S.L.; Diep, P.; Thomas, W.K.; Razmpoosh, E.C.; Schwarz, Y.; Wang, C.Y.; Kratz, M.; Neuhouser, M.L.; Lampe, J.W. Comparison and validation of 2 analytical methods for measurement of urinary sucrose and fructose excretion. Nutr. Res. 2013, 33, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Luceri, C.; Caderni, G.; Lodovici, M.; Spagnesi, M.T.; Monserrat, C.; Lancioni, L.; Dolara, P. Urinary excretion of sucrose and fructose as a predictor of sucrose intake in dietary intervention studies. Cancer Epidemiol. Prev. Biomark. 1996, 5, 167–171. [Google Scholar]
- Hallfrisch, J.; Ellwood, K.; Michaelis, O.E.T.; Reiser, S.; Prather, E.S. Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J. Am. Coll. Nutr. 1986, 5, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, G.V.; Welsh, J.D. Urinary fructose excretion after fructose loadin. Before and after portal-systemic shunting. Arch. Surg. 1968, 96, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Reed, M.J.; Azhar, S.; Reaven, G.M. Expression of the major isoenzyme of protein kinase-C in skeletal muscle, nPKC theta, varies with muscle type and in response to fructose-induced insulin resistance. Endocrinology 1994, 135, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Chang, H.; Azhar, S.; Reaven, G.M. Tissue-dependent activation of protein kinase C in fructose-induced insulin resistance. Endocrine 1995, 3, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Burch, H.B.; Cole, B.; Choi, S.; Alvey, T.R.; Dence, C. Diversity of effects of fructose loads on different parts of the nephron. Int. J. Biochem. 1980, 12, 37–40. [Google Scholar] [CrossRef]
- Burch, H.B.; Narins, R.G.; Chu, C.; Fagioli, S.; Choi, S.; McCarthy, W.; Lowry, O.H. Distribution along the rat nephron of three enzymes of gluconeogenesis in acidosis and starvation. Am. J. Physiol. 1978, 235, F246–F253. [Google Scholar] [PubMed]
- Diggle, C.P.; Shires, M.; Leitch, D.; Brooke, D.; Carr, I.M.; Markham, A.F.; Hayward, B.E.; Asipu, A.; Bonthron, D.T. Ketohexokinase: Expression and localization of the principal fructose-metabolizing enzyme. J. Histochem. Cytochem. 2009, 57, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, R.; Pedemonte, C.H. Contrary to rat-type, human-type Na,K-ATPase is phosphorylated at the same amino acid by hormones that produce opposite effects on enzyme activity. J. Am. Soc. Nephrol. 2006, 17, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yingst, D.R.; Araghi, A.; Doci, T.M.; Mattingly, R.; Beierwaltes, W.H. Decreased renal perfusion rapidly increases plasma membrane Na-K-ATPase in rat cortex by an angiotensin II-dependent mechanism. Am. J. Physiol. Ren. Physiol. 2009, 297, F1324–F1329. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Hoog, J.O.; Nairn, A.C.; Greengard, P.; Aperia, A. Regulation of rat Na(+)-K(+)-ATPase activity by PKC is modulated by state of phosphorylation of Ser-943 by PKA. Am. J. Physiol. 1997, 273, C1981–C1986. [Google Scholar] [PubMed]
- Efendiev, R.; Bertorello, A.M.; Zandomeni, R.; Cinelli, A.R.; Pedemonte, C.H. Agonist-dependent regulation of renal Na+,K+-ATPase activity is modulated by intracellular sodium concentration. J. Biol. Chem. 2002, 277, 11489–11496. [Google Scholar] [CrossRef] [PubMed]
- Sanada, H.; Jose, P.A.; Hazen-Martin, D.; Yu, P.Y.; Xu, J.; Bruns, D.E.; Phipps, J.; Carey, R.M.; Felder, R.A. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension 1999, 33, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Eisner, G.M.; Felder, R.A. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr. Opin. Nephrol. Hypertens. 2002, 11, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, Z.; Hopfer, U.; Asico, L.D.; Eisner, G.M.; Felder, R.A.; Jose, P.A. Rat strain effects of AT1 receptor activation on D1 dopamine receptors in immortalized renal proximal tubule cells. Hypertension 2005, 46, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Jose, P.A. Dopamine receptors: Important antihypertensive counterbalance against hypertensive factors. Hypertension 2011, 57, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Spicarova, Z.; Zelenin, S.; Holtback, U.; Scott, L.; Aperia, A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2008, 295, F1110–F1116. [Google Scholar] [CrossRef] [PubMed]

| Bicarbonate- Buffered Physiological Saline | HEPES- Buffered Physiological Saline | K-Free HEPES-Buffered Solution | Acid Pulse Buffer | 4X Reaction Media | 4X Reaction Media with Ouabain | ||
|---|---|---|---|---|---|---|---|
| NaHCO3 | (mmol/L) | 25.0 | - | - | - | - | - |
| HEPES | " | - | 10.0 | 10.0 | 10.0 | - | - |
| Imidazole | " | - | - | - | - | 200.0 | 200.0 |
| NaCl | " | 114.0 | 130.0 | 130.0 | 120.0 | 320.0 | 320.0 |
| KCl | " | 4.0 | 4.0 | - | 4.0 | 120.0 | - |
| Na2HPO4 | " | 2.1 | 2.5 | 2.5 | 2.5 | - | - |
| NaH2PO4 | " | 0.4 | - | - | - | - | - |
| Mg SO4 | " | 1.2 | 1.2 | 1.2 | 1.2 | 20.0 | 20.0 |
| Ca(Lactate)2 | " | 2.0 | 2.0 | 2.0 | 2.0 | - | - |
| Na3Citrate | " | 1.0 | 1.0 | 1.0 | 1.0 | - | - |
| DL-alanine | " | 6.0 | 6.0 | 6.0 | 6.0 | - | - |
| Glucose | " | 5.5 | 5.5 | 5.5 | 5.5 | - | - |
| NH4Cl | " | - | - | - | 10.0 | - | - |
| EGTA | " | - | - | - | - | 2.0 | 2.0 |
| Na2ATP | " | - | - | - | - | 20.0 | 20.0 |
| NADH | " | - | - | - | - | 4.0 | 4.0 |
| Ascorbic Acid | " | - | - | - | - | 4.0 | 4.0 |
| PEP | " | - | - | - | - | 40.0 | 40.0 |
| Antibody | Provider | Catalog | Source | Blocking | Conditions | ||
|---|---|---|---|---|---|---|---|
| Number | Buffer | Dilution | Buffer | Time | |||
| NHE3 | Abcam | ab95299 | Rabbit | 5% BSA | 1:1000 | 5% BSA | 2 h |
| α1-Na/K-ATPase | Cell Signaling | #3010 | Rabbit | 5% Milk | 1:5000 | 5% Milk | 2 h |
| β-tubulin | Abcam | ab6046 | Rabbit | 5% Milk | 1:10,000 | 5% Milk | 2 h |
| GAPDH-HRP | Abcam | ab9485 | - | 5% BSA | 1:15,000 | 5% BSA | 2 h |
| 2ry anti-Rabbit-HRP | GE Healthcare | NA9340V | Donkey | - | 1:2500 | 5% BSA | 1 h |
| Control (n = 5) | Fructose (n = 6) | Change | T test | ||||
| Mean | SEM | Mean | SEM | ||||
| Caloric Intake | (kcal/24 h) | 69.9 | 6.0 | 65.5 | 3.2 | = | p < 0.51 |
| Weight Gain | (g/24 h) | 9.4 | 1.3 | 9.2 | 2.4 | = | p < 0.94 |
| Fluid Intake | (mL/24 h) | 29.2 | 4.6 | 25.8 | 3.1 | = | p < 0.55 |
| Food Intake | (g/24 h) | 17.4 | 1.5 | 12.0 | 0.5 | ↓ | p < 0.01 |
| Final Weight | (g) | 236 | 7 | 232 | 10 | = | p < 0.72 |
| Systolic BP | (mmHg) | 130 | 11 | 147 | 6 | = | p < 0.18 |
| Control | Fructose | Change | T test | ||||
| Mean | SEM | Mean | SEM | ||||
| pH | 7.42 | 0.01 | 7.38 | 0.03 | = | p < 0.18 | |
| Na | (mmol/L) | 136.6 | 0.6 | 137.5 | 0.5 | = | p < 0.31 |
| K | (mmol/L) | 3.8 | 0.2 | 3.7 | 0.1 | = | p < 0.73 |
| Cl | (mmol/L) | 106.4 | 0.5 | 106.0 | 0.3 | = | p < 0.55 |
| Lactate | (mmol/L) | 1.02 | 0.14 | 1.03 | 0.07 | = | p < 0.98 |
| Insulin | (µg/mL) | 0.38 | 0.14 | 0.36 | 0.15 | = | p < 0.93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Yang, N.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Enhances the Ability of Low Concentrations of Angiotensin II to Stimulate Proximal Tubule Na+ Reabsorption. Nutrients 2017, 9, 885. https://doi.org/10.3390/nu9080885
Gonzalez-Vicente A, Cabral PD, Hong NJ, Asirwatham J, Yang N, Berthiaume JM, Dominici FP, Garvin JL. Dietary Fructose Enhances the Ability of Low Concentrations of Angiotensin II to Stimulate Proximal Tubule Na+ Reabsorption. Nutrients. 2017; 9(8):885. https://doi.org/10.3390/nu9080885
Chicago/Turabian StyleGonzalez-Vicente, Agustin, Pablo D. Cabral, Nancy J. Hong, Jessica Asirwatham, Nianxin Yang, Jessica M. Berthiaume, Fernando P. Dominici, and Jeffrey L. Garvin. 2017. "Dietary Fructose Enhances the Ability of Low Concentrations of Angiotensin II to Stimulate Proximal Tubule Na+ Reabsorption" Nutrients 9, no. 8: 885. https://doi.org/10.3390/nu9080885





