Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway
Abstract
:1. Introduction
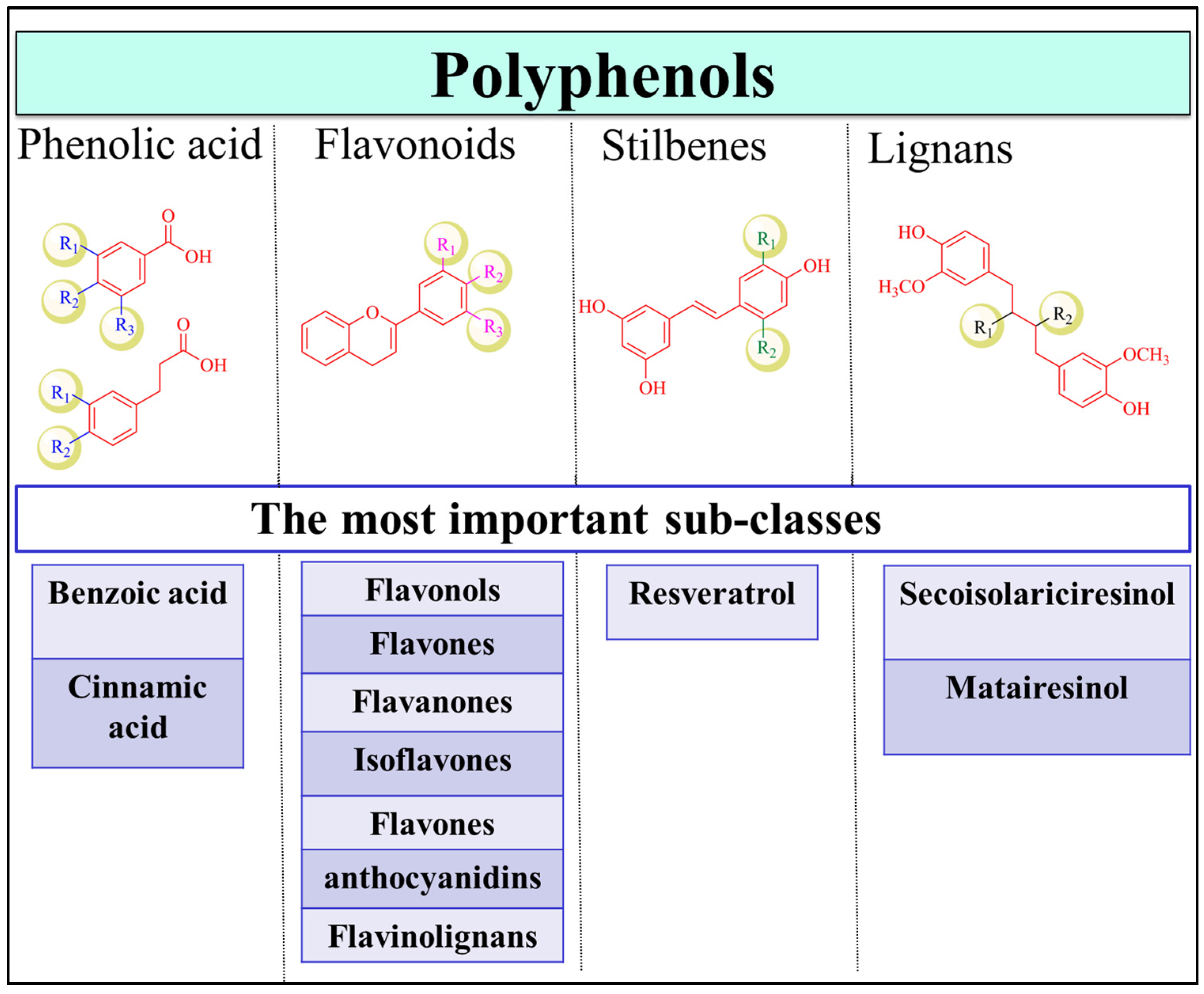
2. Dietary Polyphenols and Health Benefits
Polyphenols in Cancer Prevention and Treatment
3. Polyphenols Role in Reversing EMT Mediated Cancer Metastasis
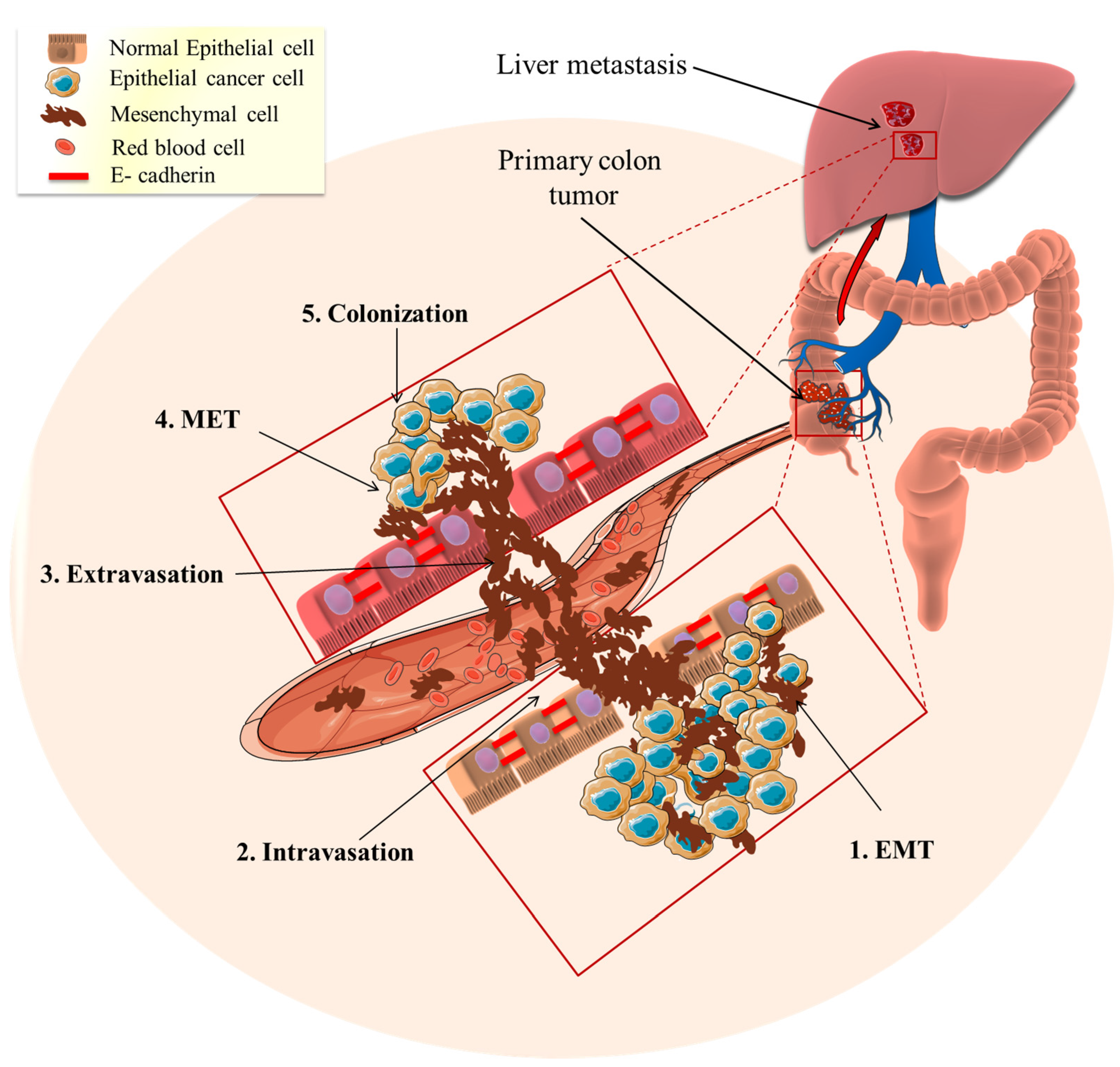
3.1. Metastasis
3.2. EMT Role in Mediating Cancer Metastasis
3.3. Polyphenols Effectiveness in Reversing Metastasis
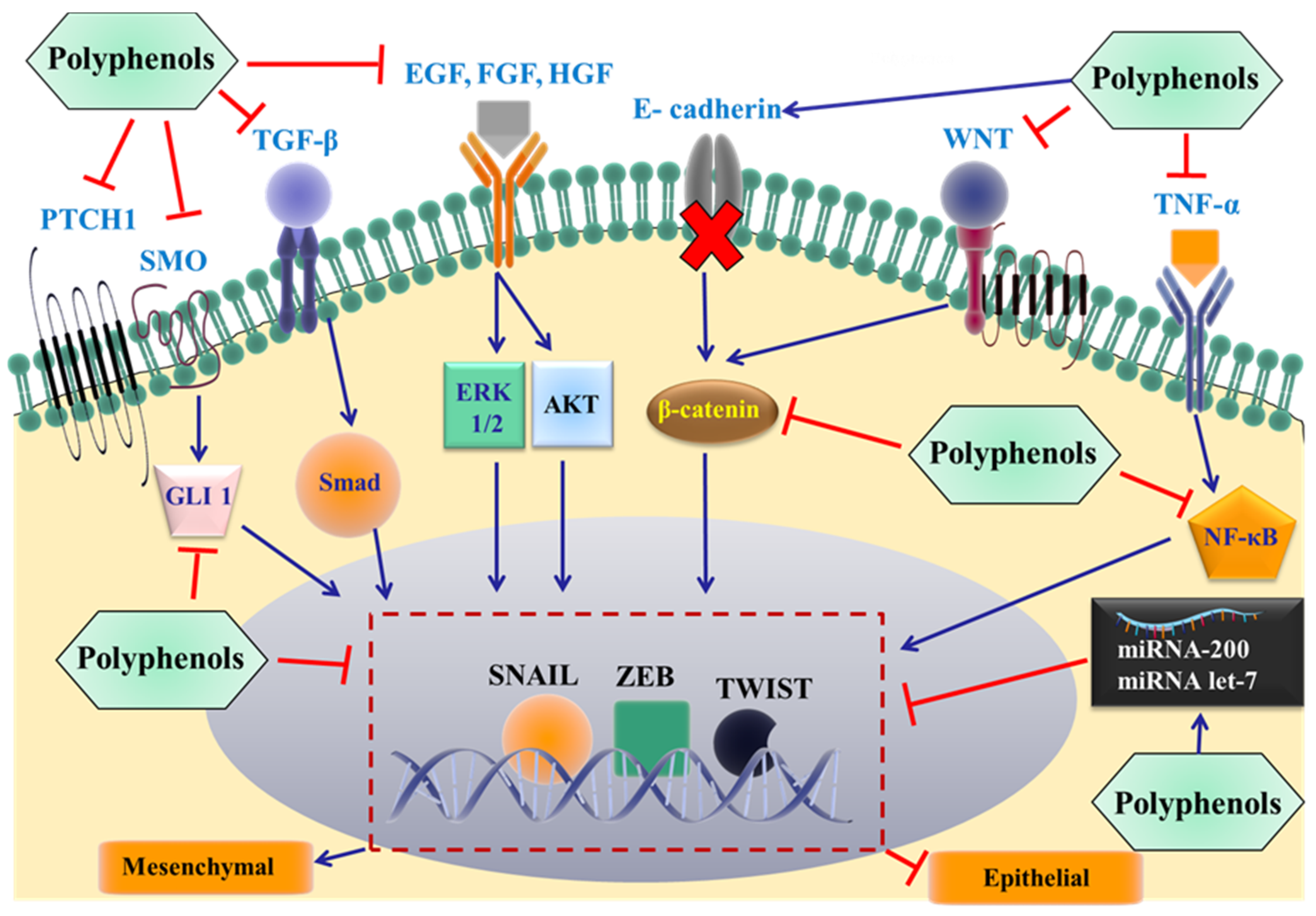
3.4. Polyphenols Reverse Metastasis by Targeting EMT
3.4.1. Flavonoids
3.4.2. Stilbenes
3.4.3. Phenolic Acids
3.4.4. Lignans
4. Future Directions
4.1. Effects of Polyphenols on Specific Proteins in the EMT Pathway
Studying the Impact of Polyphenols on EMT-Related miRNAs
4.2. Whole Extract/Combining Polyphenols Studies
4.3. Use of Doses or Concentrations That Resemble Those Consumed from Dietary Sources
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.N.; Lee, W.S.; Kim, M.J.; Yun, J.W.; Jung, J.H.; Yi, S.M.; Jeong, J.-H.; Kim, H.J.; Choi, Y.H.; Kim, G.S. The inhibitory effect of anthocyanins on Akt on invasion and epithelial-mesenchymal transition is not associated with the anti-EGFR effect of the anthocyanins. Int. J. Oncol. 2014, 44, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Chun, K.-S. The promise of dried fruits in cancer chemoprevention. Asian Pac. J. Cancer Prev. 2014, 15, 3343–3352. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930-n/a. [Google Scholar] [CrossRef] [PubMed]
- Illam, S.P.; Narayanankutty, A.; Mathew, S.E.; Valsalakumari, R.; Jacob, R.M.; Raghavamenon, A.C. Epithelial Mesenchymal Transition in Cancer Progression: Preventive Phytochemicals. Recent Pat. Anti-Cancer Drug Discov. 2017. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J.; Debnath, T. Role of phytochemicals in the inhibition of epithelial-mesenchymal transition in cancer metastasis. Food Funct. 2016, 7, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzynska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Advances in nutrition (Bethesda, Md.) 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stevigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in pseudomonas aeruginosa Pao1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, Y.; Luo, S.; Wickremasinghe, R.; Yamanishi, T. Special issue on tea. Food Rev. Int. 1995, 11, 371–542. [Google Scholar]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.T. Polyphenolic chemistry of tea and coffee: A century of progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304S–312S. [Google Scholar] [PubMed]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [PubMed]
- Cho, Y.; Schiller, N.; Kahng, H.; Oh, K. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.A.; Lee, S.G.; Lee, S.-K.; Chun, O.K. Dietary polyphenols, berries, and age-related bone loss: A review based on human, animal, and cell studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Hong, J.; Yang, G.Y.; Liao, J.; Yang, C.S. Inhibition of carcinogenesis by polyphenols: Evidence from laboratory investigations. Am. J. Clin. Nutr. 2005, 81, 284S–291S. [Google Scholar] [PubMed]
- Who, J.; Consultation, F.E. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Crespy, V.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998, 426, 331–336. [Google Scholar] [CrossRef]
- Farber, E. The multistep nature of cancer development. Cancer Res. 1984, 44, 4217–4223. [Google Scholar] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signaling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Marques, M.P.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P. Plant polyphenols as chemopreventive agents for lung cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017. In Nutrition and Physical Activities; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxid. Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Kuntz, S.; Brendel, M.D.; Daniel, H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000, 60, 3823–3831. [Google Scholar] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Asad, S.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life Sci. 2000, 50, 167–171. [Google Scholar]
- Wessner, B.; Strasser, E.M.; Koitz, N.; Schmuckenschlager, C.; Unger-Manhart, N.; Roth, E. Green tea polyphenol administration partly ameliorates chemotherapy-induced side effects in the small intestine of mice. J. Nutr. 2007, 137, 634–640. [Google Scholar] [PubMed]
- Harper, C.E.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Lamartiniere, C.A. Epigallocatechin-3-gallate suppresses early stage, but not late stage prostate cancer in tramp mice: Mechanisms of action. Prostate 2007, 67, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.E.; Cheng, A.L.; Lin, J.K.; Kuo, M.L. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem. Toxicol. 2000, 38, 991–995. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-J.; Yen, G.-C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Daniel, K.G.; Kuhn, D.J.; Kazi, A.; Bhuiyan, M.; Li, L.; Wang, Z.; Wan, S.B.; Lam, W.H.; Chan, T.H. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004, 9, 2618–2631. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, P.A.; Walle, T. Inhibition of benzo [a] pyrene-activating enzymes and DNA binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis 2006, 27, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, M.; Zhang, F.; Hu, Y.; Xu, X.; Li, Y.; Liu, K.; Ma, X.; Tian, X.; Yao, J. Dietary flavonoid genistein induces NRF2 and Phase II detoxification gene expression via ERKS and PKC pathways and protects against oxidative stress in CACO-2 cells. Mol. Nutr. Food Res. 2013, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.; Hirakawa, Y.; Nakano, Y.; Yoshida, H. Effects of epigallocatechin 3-O-gallate on cellular antioxidative system in HepG2 cells. J. Nutr. Sci. Vitaminol. 2002, 48, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Roots, I. In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem. Biophys. Res. Commun. 2003, 303, 902–907. [Google Scholar] [CrossRef]
- Howells, L.M.; Mitra, A.; Manson, M.M. Comparison of oxaliplatin-and curcumin-mediated antiproliferative effects in colorectal cell lines. Int. J. Cancer 2007, 121, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Efimova, T.; Eckert, R.L. Green tea polyphenol stimulates a Ras, Mekk1, Mek3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J. Biol. Chem. 2002, 277, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, Y.; Nedeljkovic-Kurepa, A.; Sarkar, F.H. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003, 22, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wu, J.; Dunn, T.; Yi, J.; Tong, X.; Zhang, D. Inhibition of cyclooxygenase-2 activity in head and neck cancer cells by genistein. Cancer Lett. 2004, 211, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-L.; Kuo, Y.-M.; Lee, Y.-R.; Yang, S.-F.; Chen, W.-R.; Lee, H.-J. Apple polyphenol induces cell apoptosis, cell cycle arrest at G2/m phase, and mitotic catastrophe in human bladder transitional carcinoma cells. J. Funct. Foods 2015, 14, 384–394. [Google Scholar] [CrossRef]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappa B and Akt activation in human cervical cancer cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, A.; Urdaci, M.C.; Pinchuk, I.V.; Lopez-Moratalla, N.; Martinez-Irujo, J.J. Flavonoids induce apoptosis in human leukemia u937 cells through caspase-and caspase-calpain-dependent pathways. Nutr. Cancer 2004, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yumnam, S.; Hong, G.E.; Raha, S.; Saralamma, V.V.; Lee, H.J.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H.; et al. Flavonoids of korean citrus aurantium L. induce apoptosis via intrinsic pathway in human hepatoblastoma HepG2 cells. Phytother. Res. PTR 2015, 29, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Bernd, A.; Sempere, J.M.; Diaz-Alperi, J.; Ramirez, A. The curcuma antioxidants: Pharmacological effects and prospects for future clinical use. A review. Arch. Gerontol. Geriatr. 2002, 34, 37–46. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. The role of nutraceuticals in the regulation of Wnt and hedgehog signaling in cancer. Cancer Metastasis Rev. 2010, 29, 383–394. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. High intake of specific carotenoids and flavonoids does not reduce the risk of bladder cancer. Nutr. Cancer 1999, 35, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C.; Bueno De Mesquita, H.B.; Feskens, E.J.; Kromhout, D. Dietary catechins and epithelial cancer incidence: The zutphen elderly study. Int. J. Cancer 2001, 92, 298–302. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nature Reviews Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kohn, E.C. Development and prevention of metastasis. Anticancer Res. 1992, 13, 2553–2559. [Google Scholar]
- Ali, S.; Lazennec, G. Chemokines: Novel targets for breast cancer metastasis. Cancer Metastasis Rev. 2007, 26, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.-Y.; Yang, S.-C.; Hong, T.-M.; Yuan, A.; Chen, J.J.; Yu, C.-J.; Chang, Y.-L.; Lee, Y.-C.; Peck, K.; Wu, C.-W. Collapsin response mediator protein-1 and the invasion and metastasis of cancer cells. J. Natl. Cancer Inst. 2001, 93, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Orr, F.W.; Wang, H.H.; Lafrenie, R.M.; Scherbarth, S.; Nance, D.M. Interactions between cancer cells and the endothelium in metastasis. J. Pathol. 2000, 190, 310–329. [Google Scholar] [CrossRef]
- Clark, E.A.; Golub, T.R.; Lander, E.S.; Hynes, R.O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000, 406, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Mikami, S.; Mizuno, R.; Kosaka, T.; Saya, H.; Oya, M.; Okada, Y. Expression of TNF-α and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. Int. J. Cancer 2015, 136, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: Mmp-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Slaney, C.Y.; Rautela, J.; Parker, B.S. The emerging role of immunosurveillance in dictating metastatic spread in breast cancer. Cancer Res. 2013, 73, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.A.; Zhang, W.; Yang, D.; Iqbal, S.; El-Khouiery, A.; Nagashima, F.; Lurje, G.; Labonte, M.; Wilson, P.; Sherrod, A.; et al. Gender-specific genomic profiling in metastatic colorectal cancer patients treated with 5-fluorouracil and oxaliplatin. Pharmacogenomics 2011, 12, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Massague, J. Genetic determinants of cancer metastasis. Nat. Rev. Genet. 2007, 8, 341–352. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Metastatic Cancer; National institute of cancer at the National institute of Health: Bethesda, MD, USA, 2017.
- Liang, X. EMT: New signals from the invasive front. Oral Oncol. 2011, 47, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial—Mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, ß-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Law, B.K.; Chytil, A.M.; Brown, K.A.; Aakre, M.E.; Moses, H.L. Activation of the Erk pathway is required for TGF-β1-induced EMT in vitro. Neoplasia 2004, 6, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Boyer, B.; Vallés, A.M.; Edme, N. Induction and regulation of epithelial—Mesenchymal transitions. Biochem. Pharmacol. 2000, 60, 1091–1099. [Google Scholar] [CrossRef]
- Willis, B.C.; Borok, Z. TGF-β-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.R.; Zhau, H.E.; Odero-Marah, V.A.; Osunkoya, A.O.; Kimbro, K.S.; Tighiouart, M.; Liu, T.; Simons, J.W.; O’Regan, R.M. Insulin-like growth factor-I–dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008, 68, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-W.; Hsu, S.-C.; Xia, W.; Cao, X.; Shih, J.-Y.; Wei, Y.; Abbruzzese, J.L.; Hortobagyi, G.N.; Hung, M.-C. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of Twist gene expression. Cancer Res. 2007, 67, 9066–9076. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, Z.; Sarkar, S.H.; Li, Y.; Banerjee, S.; Saliganan, A.; Kim, H.R.C.; Cher, M.L.; Sarkar, F.H. Platelet-derived growth factor-d overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells 2008, 26, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Puig, I.; Caretti, E.; Bonaventure, J.; Nelles, L.; Van Roy, F.; Dargemont, C.; De Herreros, A.G.; Bellacosa, A.; Larue, L. Activation of NF-κb by Akt upregulates snail expression and induces epithelium mesenchyme transition. Oncogene 2007, 26, 7445–7456. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; Van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; Larue, L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar] [PubMed]
- Kim, K.; Lu, Z.; Hay, E.D. Direct evidence for a role of β-catenin/lef-1 signaling pathway in induction of EMT. Cell Biol. Int. 2002, 26, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-T.; Wu, J.-R.; Chang, T.Y.; Cheng, C.-C.; Wu, W.-S. The transcriptional factor snail simultaneously triggers cell cycle arrest and migration of human hepatoma HepG2. J. Biomed. Sci. 2008, 15, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Marin, F.; Cubillo, E.; Stark, H.-J.; Fusenig, N.; Nieto, M.A.; Cano, A. Snail and E47 repressors of E-cadherin induce distinct invasive and angiogenic properties in vivo. J. Cell Sci. 2004, 117, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Spaderna, S.; Schmalhofer, O.; Wahlbuhl, M.; Dimmler, A.; Bauer, K.; Sultan, A.; Hlubek, F.; Jung, A.; Strand, D.; Eger, A. The transcriptional repressor Zeb1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008, 68, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the mesenchymal–epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, F.H.; Rosário, M.; Birchmeier, W. Balancing cell adhesion and wnt signaling, the key role of β-catenin. Curr. Opin. Genet. Dev. 2006, 16, 51–59. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Eastman, Q.; Grosschedl, R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 1999, 11, 233–240. [Google Scholar] [CrossRef]
- Roose, J.; Huls, G.; Van Beest, M.; Moerer, P.; Van Der Horn, K.; Goldschmeding, R.; Logtenberg, T.; Clevers, H. Synergy between tumor suppressor APC and the β-catenin-TCF4 target TCF1. Science 1999, 285, 1923–1926. [Google Scholar] [CrossRef] [PubMed]
- Van De Wetering, M.; Sancho, E.; Verweij, C.; De Lau, W.; Oving, I.; Hurlstone, A.; Van Der Horn, K.; Batlle, E.; Coudreuse, D.; Haramis, A.-P. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002, 111, 241–250. [Google Scholar] [CrossRef]
- Davidson, G.; Wu, W.; Shen, J.; Bilic, J.; Fenger, U.; Stannek, P.; Glinka, A.; Niehrs, C. Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 2005, 438, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Orsulic, S.; Huber, O.; Aberle, H.; Arnold, S.; Kemler, R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 1999, 112, 1237–1245. [Google Scholar] [PubMed]
- Chang, Y.-W.; Su, Y.-J.; Hsiao, M.; Wei, K.-C.; Lin, W.-H.; Liang, C.-J.; Chen, S.-C.; Lee, J.-L. Diverse targets of β-catenin during the epithelial–mesenchymal transition define cancer stem cells and predict disease relapse. Cancer Res. 2015, 75, 3398–3410. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Baribault, H.; Kemler, R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989, 8, 1711. [Google Scholar] [PubMed]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial—Mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, V.; De Craene, B.; Berx, G.; Behrens, J. Snail promotes Wnt target gene expression and interacts with β-catenin. Oncogene 2008, 27, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.I.; Li, X.-Y.; Ota, I.; Fearon, E.R.; Weiss, S.J. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 2005, 280, 11740–11748. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.-C. Dual regulation of snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Zucchini-Pascal, N.; Peyre, L.; Rahmani, R. Crosstalk between beta-catenin and snail in the induction of epithelial to mesenchymal transition in hepatocarcinoma: Role of the Erk1/2 pathway. Int. J. Mol. Sci. 2013, 14, 20768–20792. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, H.; Ren, G. Epithelial-mesenchymal transition and drug resistance in breast cancer (review). Int. J. Oncol. 2015, 47, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.L.; Heckford, S.E.; Skerker, J.M.; Worland, P.; Torri, J.A.; Thompson, E.W.; Byers, S.W.; Gelmann, E.P. Loss of epithelial markers and acquisition of vimentin expression in adriamycin-and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992, 52, 5190–5197. [Google Scholar] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S. Targeting epithelial–mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [PubMed]
- Zhou, Q.; Bennett, L.L.; Zhou, S. Multifaceted ability of naturally occurring polyphenols against metastatic cancer. Clin. Exp. Pharmacol. Physiol. 2016, 43, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Miura, Y.; Yagasaki, K. Antiproliferative and anti-invasive effect of piceatannol, a polyphenol present in grapes and wine, against hepatoma AH109A cells. BioMed Res. Int. 2012, 2012, 672416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jaganath, I.B.; Wang, S.M.; Sekaran, S.D. Antimetastatic effects of phyllanthus on human lung (A549) and breast (MCF-7) cancer cell lines. PLoS ONE 2011, 6, e20994. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Bettini, S.; Tinelli, A.; Valli, L.; Storelli, C.; Leo, S.; Santino, A.; Maffia, M. Antitumor activity of the dietary diterpene carnosol against a panel of human cancer cell lines. Food Funct. 2014, 5, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Chung, I.M.; Kim, M.Y.; Park, K.D.; Park, W.W.; Moon, H.I. Inhibition of lung metastasis in mice by oligonol. Phytother. Res. 2009, 23, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Yang, S.F.; Peng, C.Y.; Chou, M.Y.; Chang, Y.C. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J. Oral Pathol. Med. 2007, 36, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Hu, Y.; Guo, T.; Wang, H.-F.; Zhang, X.-P.; He, W.-J.; Tan, H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 2006, 27, 1447. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, R.L.H.; Cardelli, J.A. The green tea catechins, (−)-epigallocatechin-3-gallate (EGCG) and (−)-epicatechin-3-gallate (ECG), inhibit HGF//Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene 2006, 25, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.A.; Burke, P.; Coleman, D.T.; Bigelow, R.L.; Steffan, J.J.; Carroll, J.L.; Williams, B.J.; Cardelli, J.A. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-met and epidermal growth factor receptor inhibitors in non–small cell lung cancer cells. Clin. Cancer Res. 2009, 15, 4885–4894. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Nerlich, A.G.; Iancu, C.M.; Cilli, M.; Schleicher, E.; Vené, R.; Dell’Eva, R.; Jochum, M.; Albini, A.; Pfeffer, U. The chemopreventive polyphenol curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell. Physiol. Biochem. 2007, 19, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.; Pandey, M.K.; Nakajima, Y.; Nishida, H.; Konishi, T.; Chaturvedi, M.M.; Aggarwal, B.B. Identification of a novel blocker of Iκbα kinase activation that enhances apoptosis and inhibits proliferation and invasion by suppressing nuclear factor-κb. Mol. Cancer Ther. 2008, 7, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zetter, P.; Bruce, R. Angiogenesis and tumor metastasis. Ann. Rev. Med. 1998, 49, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Donnini, S.; Giachetti, A.; Iñiguez, M.A.; Fresno, M.; Melillo, G.; Ziche, M. Inhibition of hypoxia inducible factor-1α by dihydroxyphenylethanol, a product from olive oil, blocks microsomal prostaglandin-E synthase-1/vascular endothelial growth factor expression and reduces tumor angiogenesis. Clin. Cancer Res. 2010, 16, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Lee, W.S.; Panchanathan, R.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenols from Artemisia annua L. inhibit adhesion and EMT of highly metastatic breast cancer cells MDA-MB-231. Phytother. Res. 2016, 30, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Lee, W.S.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.-M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenol mixtures of euphorbia supina the inhibit invasion and metastasis of highly metastatic breast cancer MDA-MB-231 cells. Oncol. Rep. 2015, 34, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Xu, C.; Song, L.; Huang, L.; Lai, Y.; Wang, Y.; Chen, H.; Gu, D.; Ren, L. Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol. Rep. 2016, 35, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Int. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Kang, J.; Kim, E.; Kim, W.; Seong, K.M.; Youn, H.; Kim, J.W.; Kim, J.; Youn, B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by MIR-34A-mediated suppression of notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013, 288, 27343–27357. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Shen, Y.-A.; Hung, P.-H.; Yu, Y.-B.; Chen, Y.-J. Epigallocathechin gallate, polyphenol present in green tea, inhibits stem-like characteristics and epithelial-mesenchymal transition in nasopharyngeal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tsai, P.H.; Kandaswami, C.C.; Cheng, C.H.; Ke, F.C.; Lee, P.P.; Hwang, J.J.; Lee, M.T. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011, 102, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated snail/E-cadherin expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Valente, C.M.; Tinelli, A.; Siciliano, C.; Lorusso, V.; Acierno, R.; Giovinazzo, G.; Santino, A.; Storelli, C.; Maffia, M. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011, 310, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yuan, Y.; Gan, H.Z.; Peng, Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol. Lett. 2015, 9, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, S.; Chaudhry, P.; Parent, S.; Asselin, É. Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. PLoS ONE 2014, 9, e86987. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; De Domenico, S.; Tinelli, A.; Stanca, A.; Del Mercato, L.L.; Giudetti, A.M.; Simeone, P.; Guazzelli, N.; Lessi, M.; Manzini, C.; et al. Anticancer effects of novel resveratrol analogues on human ovarian cancer cells. Mol. Biosyst. 2017, 13, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. The role of phenolic compounds in the fight against cancer—A review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Niero, E.L.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Hu, M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [PubMed]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/Fak/Rho GTPases signaling pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Jiang, X.; He, L.; Tang, Y.; Yin, G.; Zeng, Q.; Jiang, Z.; Tan, J. Anacardic acid sensitizes prostate cancer cells to radiation therapy by regulating h2ax expression. Int. J. Clin. Exp. Pathol. 2015, 8, 15926–15932. [Google Scholar] [PubMed]
- Yang, Y.; Li, Y.; Wang, K.; Wang, Y.; Yin, W.; Li, L. P38/NF-kb-/Snail pathway is involved in caffeic acid-induced inhibition of cancer stem cells-like properties and migratory capacity in malignant human keratinocyte. PLoS ONE 2013, 8, e58915. [Google Scholar]
- Wei, M.G.; Sun, W.; He, W.M.; Ni, L.; Yang, Y.Y. Ferulic acid attenuates TGF-beta1-induced renal cellular fibrosis in NRK-52E cells by inhibiting smad/ILK/snail pathway. Evid.-Based Complement. Altern. Med. 2015, 2015, 619720. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the pi3k/akt, mek/erk and wnt/beta-catenin signaling pathways in b16f10 cells. Int. J. of Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Lin, M.; Garcia, M.; Mulholland, D.; Lilly, M.; Martins-Green, M. Luteolin, ellagic acid and punicic acid are natural products that inhibit prostate cancer metastasis. Carcinogenesis 2014, 35, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Lai, T.Y.; Yang, J.S.; Yang, J.H.; Ma, Y.S.; Weng, S.W.; Lin, H.Y.; Chen, H.Y.; Lin, J.G.; Chung, J.G. Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras. Melanoma Res. 2011, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Milder, I.E.; Arts, I.C.; van de Putte, B.; Venema, D.P.; Hollman, P.C. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Polascik, T.J.; George, S.L.; Switzer, B.R.; Madden, J.F.; Ruffin, M.T.T.; Snyder, D.C.; Owzar, K.; Hars, V.; Albala, D.M.; et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol. Prev. Biomark. 2008, 17, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.; Aluko, R. A review on the anti-carcinogenic and anti-metastatic effects of flax seed lignan secolariciresinol diglucoside (SDG). Discov. Phytomed. 2015, 2, 12–17. [Google Scholar] [CrossRef]
- Li, D.; Yee, J.A.; Thompson, L.U.; Yan, L. Dietary supplementation with secoisolariciresinol diglycoside (SDG) reduces experimental metastasis of melanoma cells in mice. Cancer Lett. 1999, 142, 91–96. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Thompson, L.U. The inhibitory effect of flaxseed on the growth and metastasisof estrogen receptor negative human breast cancer xenograftsis attributed to both its lignan and oil components. Int. J. Cancer 2005, 116, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Pandima Devi, K.; Rajavel, T.; Daglia, M.; Nabavi, S.F.; Bishayee, A.; Nabavi, S.M. Targeting mirnas by polyphenols: Novel therapeutic strategy for cancer. Semin. Cancer Biol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. Mirbase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Ferracin, M.; Liu, C.-G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M. Microrna gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, I.; Miska, E.A. MicroRNA functions in animal development and human disease. Development (Cambridge, England) 2005, 132, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Wilda, M.; Busch, K.; Viehmann, S.; Borkhardt, A. High expression of precursor microrna-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 2004, 39, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microrna targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M. Human microrna genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.Z.; O’Connor, S.M.; van Holst Pellekaan, N.G.; Young, G.P.; James, R.J. Reduced accumulation of specific microRNAs in colorectal neoplasia11note: Susan M. O’connor and Nicholas G. Van Holst Pellekaan contributed equally to this work. Mol. Cancer Res. 2003, 1, 882–891. [Google Scholar] [PubMed]
- Chen, C. Micrornas as oncogenes and tumor suppressors. N. Engl. J. Med. 2005, 353, 1768. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A. The regulatory role of microRNAs in EMT and cancer. J. Oncol. 2015, 2015, 865816. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting Zeb1 and Sip1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The mir-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors Zeb1 and Zeb2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Mudduluru, G.; Ceppi, P.; Muppala, S.; Kozlowski, M.; Niklinski, J.; Papotti, M.; Allgayer, H. MicroRNA-30A inhibits epithelial-to-mesenchymal transition by targeting snai1 and is downregulated in non-small cell lung cancer. Int. J. Cancer 2012, 130, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Ell, B.J.; Buffa, F.M.; Ibrahim, T.; Blanco, M.A.; Celià-Terrassa, T.; Mercatali, L.; Khan, Z.; Goodarzi, H.; Hua, Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 2011, 17, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S. MiR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T. A microrna targeting dicer for metastasis control. Cell 2010, 141, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yang, H.; He, L.; Zhao, J.-J.; Coppola, D.; Dalton, W.S.; Cheng, J.Q. MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol. Cell. Biol. 2008, 28, 6773–6784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L. Microrna control of epithelial–mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012, 31, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Deval, C.; Gouranton, E.; Landrier, J.-F.; Scalbert, A.; Morand, C.; Mazur, A. Modulation of mirna expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE 2012, 7, e29837. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; VandenBoom, T.G., 2nd; Kong, D.; Wang, Z.; Ali, S.; Philip, P.A.; Sarkar, F.H. Up-regulation of mir-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer research 2009, 69, 6704–6712. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin. Anticancer Res. 2009, 29, 4025–4032. [Google Scholar] [PubMed]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Suganuma, M.; Fujiki, H. New role of (−)-epicatechin in enhancing the induction of growth inhibition and apoptosis in human lung cancer cells by curcumin. Cancer Prev. Res. 2010, 3, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
| Polyphenol subfamily | Member | Effect on EMT markers or related proteins | Studies type | |
|---|---|---|---|---|
| Flavonoids | EGCG | ↑ E-cadhrin, ↓ Veminten , Snail | In vitro | |
| Quercetin | ↑ E- cadherin ↓ Fibronectin, Vimentin, Twist, Snail, MMP-9, and N-cadherin | In vitro | ||
| Stilbines | Resveratrol | ↑ E-cadherin ↓ N-cadherin and Snail, Vimentin, ZEB 1/2, and TGF-β, | In vitro | |
| Phenolic acids | hydroxybenzoic acid | gallic acid | ↓ MMP-9, TGF-β, IL-6, β-catenin, NFκB, VEGF | In vitro |
| hydroxycinnamic acid | caffeic acid | ↑ E-cadherin ↓ NF-κB/snail, N-cadherin and Vimentin | In vitro | |
| Ferulic | ↑ E-cadherin ↓ P-Smad2/3, Fibronectin, Snail | In vitro & in vivo | ||
| Lignans | Secoisolariciresinol | No data corelate their effect to EMT | ||
| Matairesinol | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amawi, H.; Ashby, C.R., Jr.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. https://doi.org/10.3390/nu9080911
Amawi H, Ashby CR Jr., Samuel T, Peraman R, Tiwari AK. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients. 2017; 9(8):911. https://doi.org/10.3390/nu9080911
Chicago/Turabian StyleAmawi, Haneen, Charles R. Ashby, Jr., Temesgen Samuel, Ramalingam Peraman, and Amit K. Tiwari. 2017. "Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway" Nutrients 9, no. 8: 911. https://doi.org/10.3390/nu9080911





