The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review
Abstract
:1. Introduction
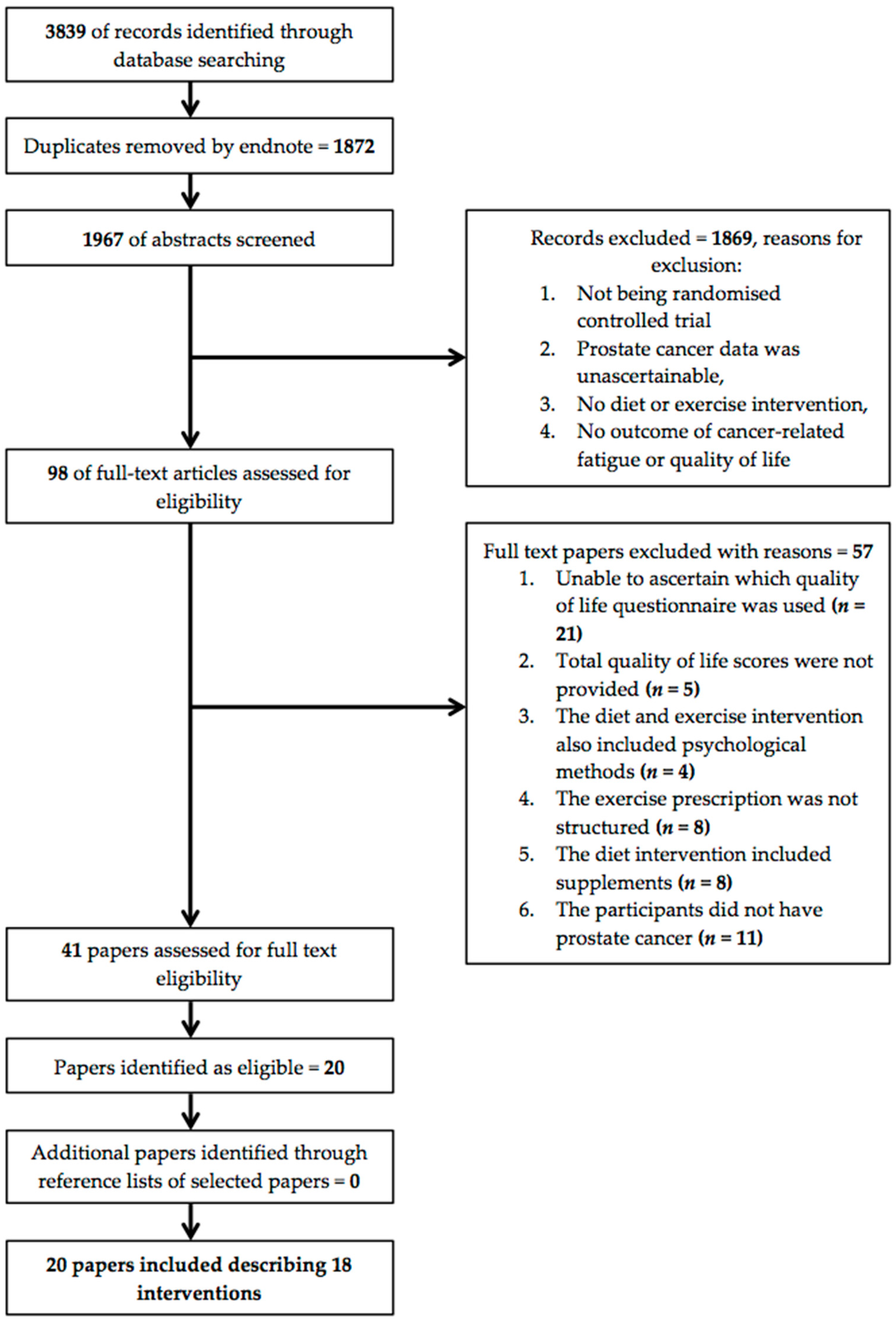
2. Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Data Extraction and Quality Assessment
3. Results
3.1. Study Design and Research Quality
3.2. Quality Assessment
3.3. Study Populations
3.4. Measures of Cancer-Related Fatigue
3.5. Measures of Quality of Life
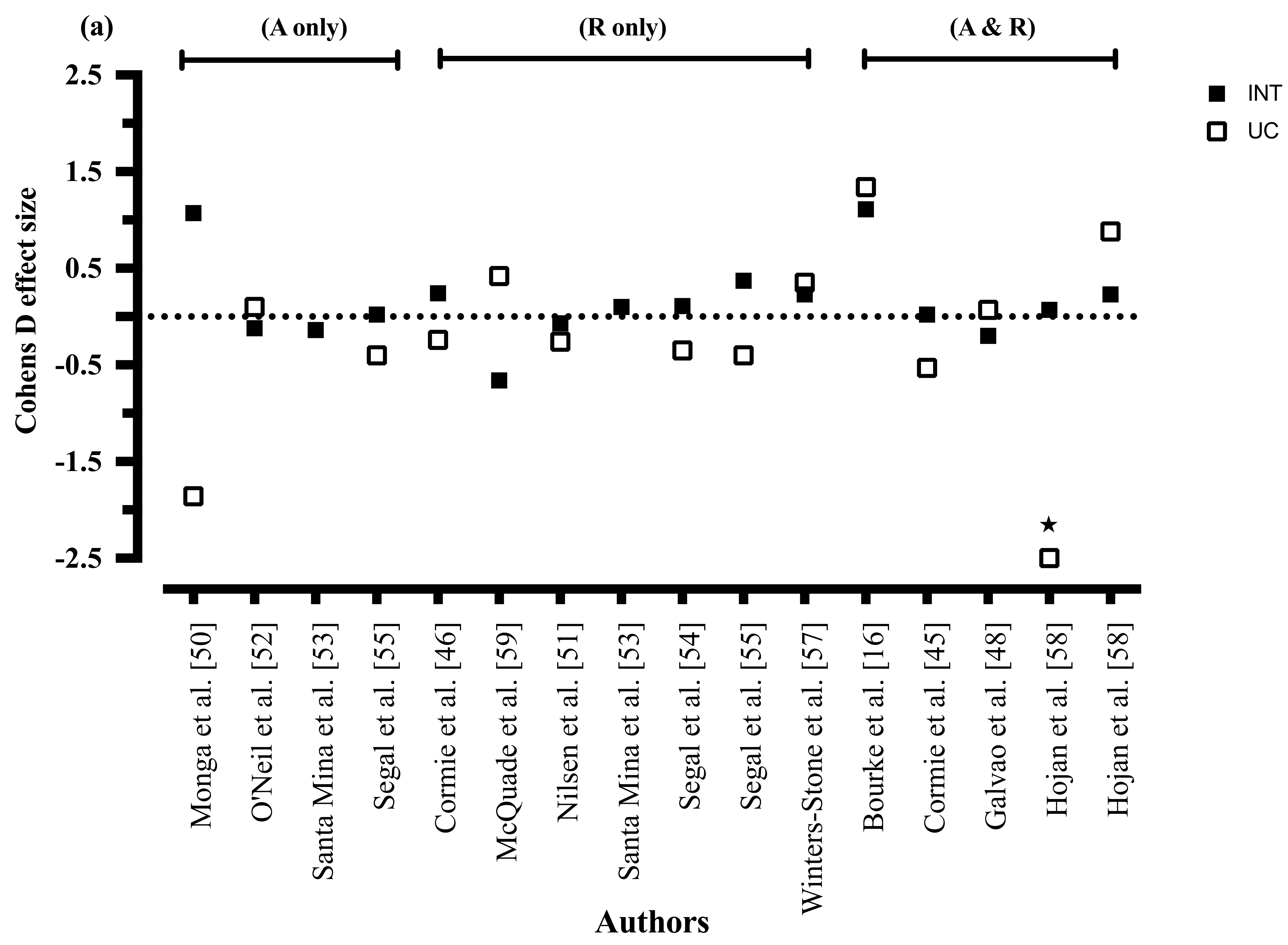
3.6. Intervention Characteristics
3.6.1. Diet only Interventions
3.6.2. Combined Diet and Exercise Interventions
3.6.3. Exercise Only Interventions
3.7. Dropout, Attendance, and Adverse Events
3.8. Reported Findings
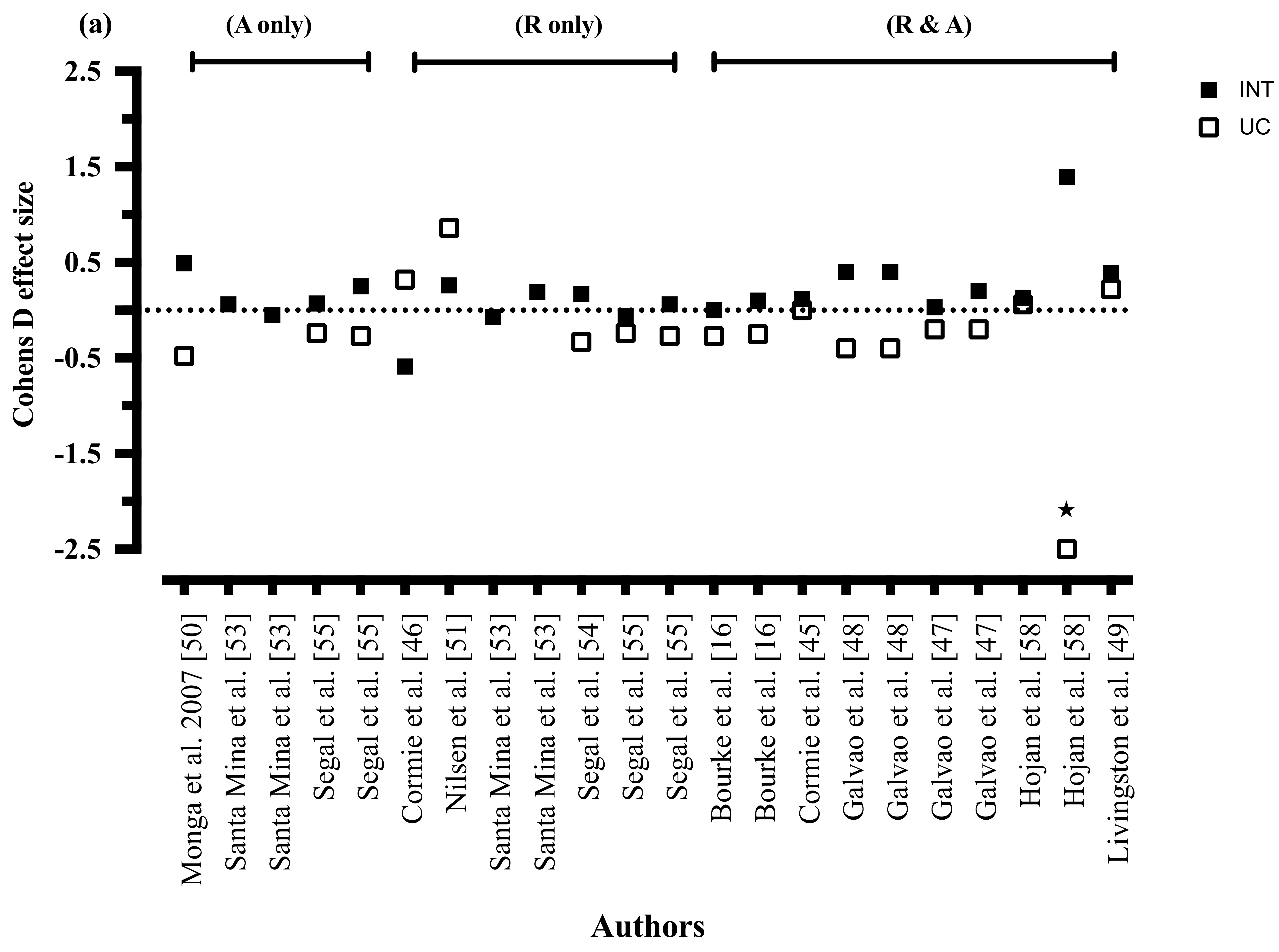
3.8.1. Cancer-Related Fatigue
3.8.2. Mode of Exercise
3.8.3. Exercise Intensity
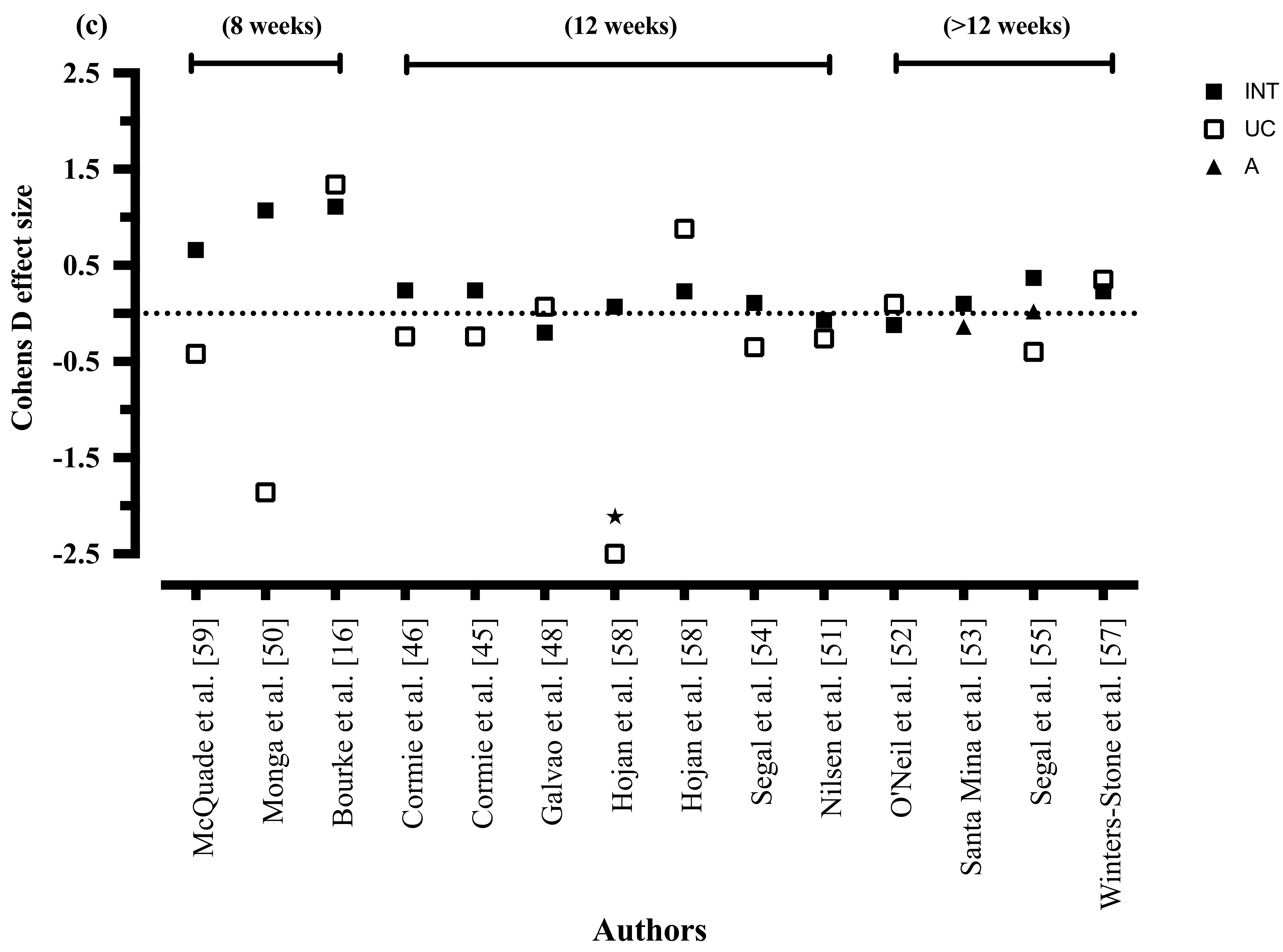
3.8.4. Exercise Duration
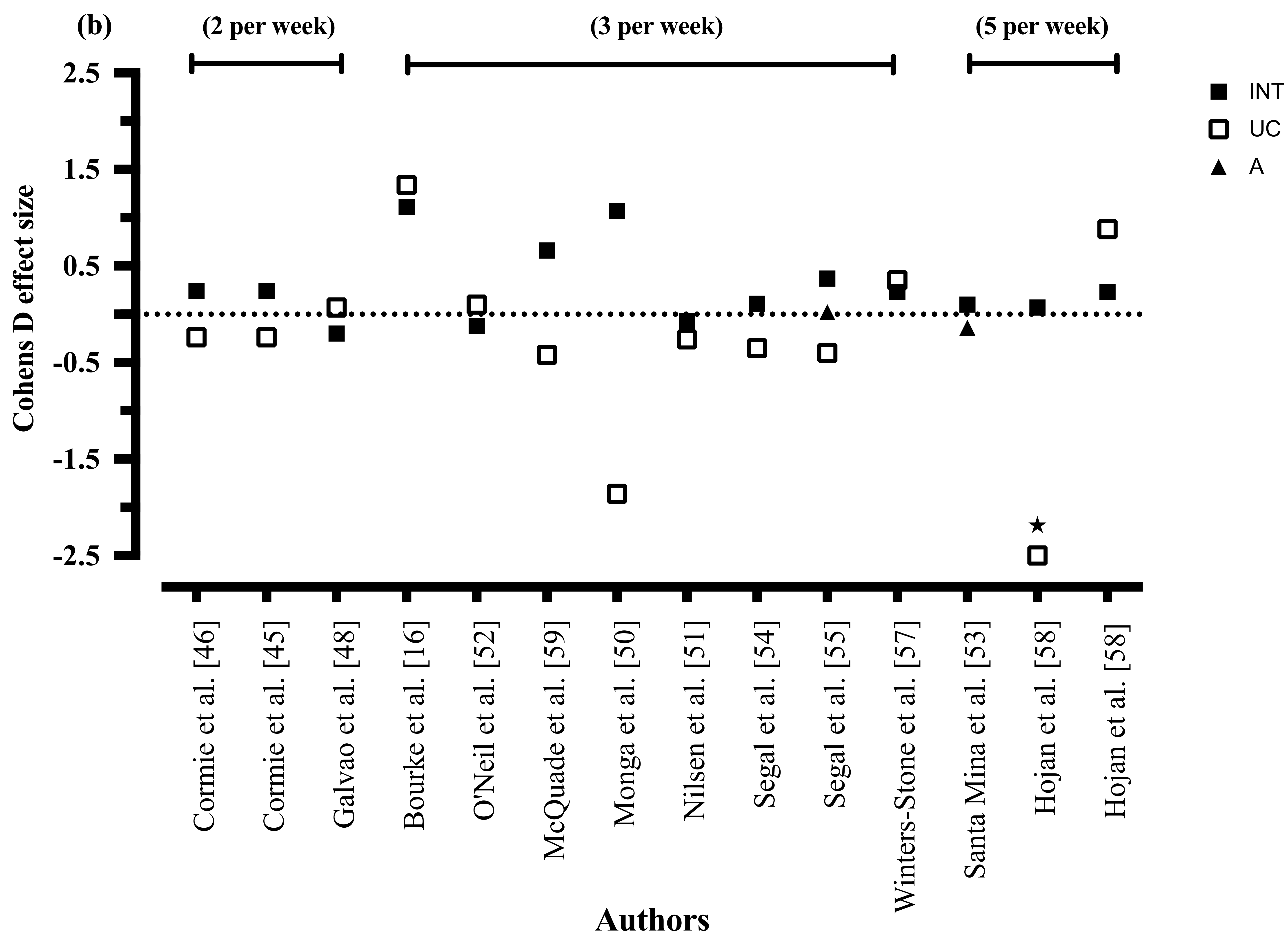
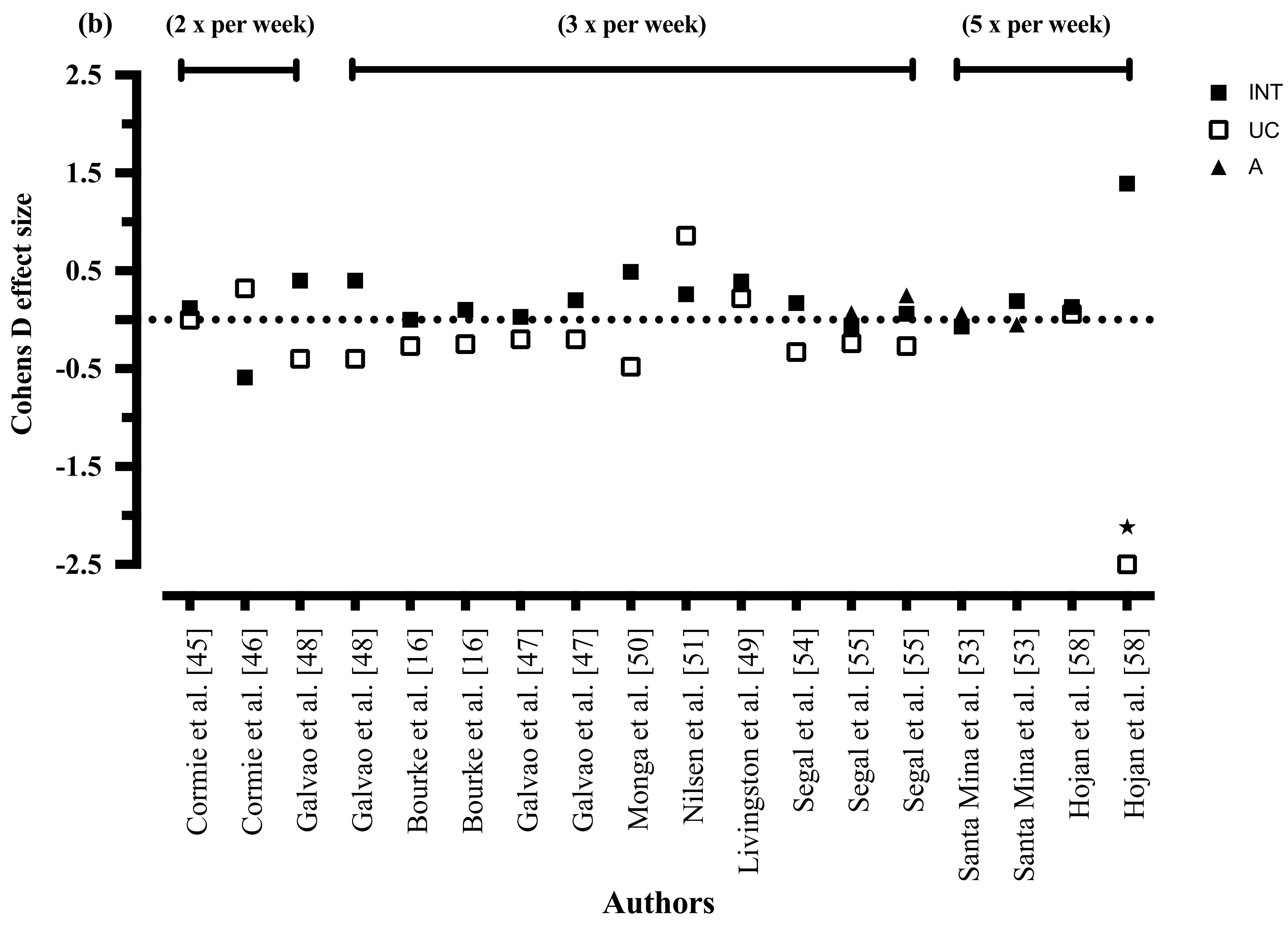
3.8.5. Exercise Frequency
3.8.6. Quality of Life
3.8.7. Exercise Mode
3.8.8. Exercise Intensity
3.8.9. Exercise Duration
3.8.10. Exercise Frequency
4. Discussion
4.1. Nutrition Therapy
4.2. Combined Nutrition Therapy and Exercise
4.3. Exercise
4.4. Future Directions for Nutrition Therapy
4.5. Recommendations
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Australian Institute of Health and Welfare. Cancer in Australia: Actual incidence and mortality data from 1982 to 2007 and projections to 2010. Asia-Pac. J. Clin. Oncol. 2011, 7, 325–338. [Google Scholar]
- Australian Institute of Health and Welfare. Cancer in Australia: Actual incidence data from 1991 to 2009 and mortality data from 1991 to 2010 with projections to 2012. Asia-Pac. J. Clin. Oncol. 2013, 9, 199–213. [Google Scholar]
- Australian Institute of Health and Welfare. Cancer survival and prevalence in Australia: Period estimates from 1982 to 2010. Asia-Pac. J. Clin. Oncol. 2013, 9, 29–39. [Google Scholar]
- World Cancer Research Fund International. Diet, Nutrition, Physical Activity, and Prostate Cancer; American Institute for Cancer Research: Arlington, VA, USA, 2014. [Google Scholar]
- Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM): Prostate Cancer; AIHW: Canberra, Australia, 2016.
- Australian Cancer Network. Clincal Practice Guidelines for the Management of Locally Advanced and Metastatic Prostate Cancer; Cancer Council Australia and Australian Cancer Network: Sydney, Australia, 2010. [Google Scholar]
- Larkin, D.; Lopez, V.; Aromataris, E. Managing cancer—Related fatigue in men with prostate cancer: A systematic review of non—Pharmacological interventions. Int. J. Nurs. Pract. 2014, 20, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, E.C.; van der Vorst, M.J.; Blauwhoff-Buskermolen, S.; Verheul, H.M. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013, 18, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S. Pathophysiology of cancer-related fatigue. Clin. J. Oncol. Nurs. 2008, 12, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Newton, R.U.; Tunn, U.W.; Gruca, D. Integrating diet and exercise into care of prostate cancer patients on androgen deprivation therapy. Res. Rep. Urol. 2016, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Langston, B.; Armes, J.; Levy, A.; Tidey, E.; Ream, E. The prevalence and severity of fatigue in men with prostate cancer: A systematic review of the literature. Support. Care Cancer 2013, 21, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Storey, D.J.; McLaren, D.B.; Atkinson, M.A.; Butcher, I.; Frew, L.C.; Smyth, J.F.; Sharpe, M. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann. Oncol. 2012, 23, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhao, F.; Fisch, M.J.; O’Mara, A.M.; Cella, C.; Mendoza, T.R.; Cleeland, C.S. Prevalence and characteristics of moderate to severe fatigue: A multicenter study in cancer patients and survivors. Cancer 2014, 120, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Drummond, F.J.; Kinnear, H.; O’Leary, E.; Donnelly; Gavin, A.; Sharp, L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J. Cancer Surviv. 2015, 9, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.P.; King, M.T.; Egger, S.; Berry, M.P.; Stricker, P.D.; Cozzi, P.; Ward, J.; O’Connell, D.L.; Armstrong, B.K. Quality of life three years after diagnosis of localised prostate cancer: Population based cohort study. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Doll, H.; Crank, H.; Daley, A.; Rosario, D.; Saxton, J.M. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: A feasibility study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.; Saxton, J.M.; Rosario, D.J. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur. Urol. 2014, 65, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Barrera, S.; Demark-Wahnefried, W. Nutrition during and after cancer therapy. Oncology 2009, 23, 15–21. [Google Scholar] [PubMed]
- Robien, K.; Demark-Wahnefried, W.; Rock, C.L. Evidence-based nutrition guidelines for cancer survivors: Current guidelines, knowledge gaps, and future research directions. J. Am. Diet Assoc. 2011, 111, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [PubMed]
- Skolarus, T.A.; Wolf, A.M.; Erb, N.L.; Brooks, D.D.; Rivers, B.M.; Underwood, W.; Salner, A.L.; Zelefsky, M.J.; Aragon-Ching, J.B.; Slovin, S.F.; et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J. Clin. 2014, 64, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Lamkin, D.M. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013, 30, S48–S57. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J. Cancer Surviv. 2014, 8, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet components associated with perceived fatigue in breast cancer survivors. Eur. J. Cancer Care 2013, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Stobaus, N.; Muller, M.J.; Kupferling, S.; Schulzke, J.D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Galvao, D.A.; Brug, J.; Chinapaw, M.J.; Newton, R.U. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat. Rev. 2014, 40, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.M.; Schwenke, D.C.; Epstein, D.R. Exercise preferences among men with prostate cancer receiving androgen-deprivation therapy. Oncol. Nurs. Forum 2013, 40, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.W.; Shepherd, D.; Krageloh, C.U.; Ryan, C.; Masters, J.; Shepard, G.; MacLeod, R. Predictors of physical activity and quality of life in New Zealand prostate cancer survivors undergoing androgen-deprivation therapy. N. Z. Med. J. 2010, 123, 20–29. [Google Scholar] [PubMed]
- Chipperfield, K.; Brooker, J.; Fletcher, J.; Burney, S. The impact of physical activity on psychosocial outcomes in men receiving androgen deprivation therapy for prostate cancer: A systematic review. Health Psychol. 2014, 33, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.R.; Livingston, P.M.; Fraser, S.F. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: A systematic review. J. Clin. Oncol. 2014, 32, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, V.; Singh, B.; Kaur, S.; Prokop, L.J.; Kaushik, D. The effects of exercise on fatigue, quality of life, and psychological function for men with prostate cancer: Systematic review and meta-analysis. Eur. Urol. Focus 2016, 2, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Culos-Reed, S.N.; Robinson, J.W.; Lau, H.; Stephenson, L.; Keats, M.; Norris, S.; Kline, G.; Faris, P. Physical activity for men receiving androgen deprivation therapy for prostate cancer: Benefits from a 16-week intervention. Support. Care Cancer 2010, 18, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Mina, D.S.; Connor, M.K.; Alibhai, S.M.; Toren, P.; Guglietti, C.; Matthew, A.G.; Trachtenberg, J.; Ritvo, P. Exercise effects on adipokines and the IGF axis in men with prostate cancer treated with androgen deprivation: A randomized study. Can. Urol. Assoc. J. 2013, 7, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Teleni, L.; Chan, R.J.; Chan, A.; Isenring, E.A.; Vela, I.; Inder, W.J.; McCarthy, A.L. Exercise improves quality of life in androgen deprivation therapy-treated prostate cancer: Systematic review of randomised controlled trials. Endocr.-Relat. Cancer 2016, 23, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Dennett, A.M.; Peiris, C.L.; Shields, N.; Prendergast, L.A.; Taylor, N.F. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: A systematic review and meta-regression. J. Physiother. 2016, 62, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Barsevick, A.M.; Irwin, M.R.; Hinds, P.; Miller, A.; Berger, A.; Jacobsen, P.; Ancoli-Israel, S.; Reeve, B.B.; Mustian, L.; O’Mara, A.; et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J. Natl. Cancer Inst. 2013, 105, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, H.; McNeill, G.; Haseen, F.; N’Dow, J.; Craig, L.C.; Heys, S.D. The Effect of Dietary and Exercise Interventions on Body Weight in Prostate Cancer Patients: A Systematic Review. Nutr. Cancer 2015, 67, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. (Lond. Engl.) 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Cormie, P.; Galvao, D.A.; Spry, N.; Joseph, D.; Chee, R.; Taaffe, D.R.; Chambers, S.K.; Newton, R.U. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015, 115, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Newton, R.U.; Spry, N.; Joseph, D.; Taaffe, D.R.; Galvao, D.A. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013, 16, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Galvao, D.A.; Spry, N.; Denham, J.; Taaffe, D.R.; Cormie, P.; Joseph, D.; Lamb, D.S.; Chambers, S.K.; Newton, R.U. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur. Urol. 2014, 65, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Galvao, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J. Clin. Oncol. 2010, 28, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Livingston, P.M.; Craike, M.J.; Salmon, J.; Courneya, K.S.; Gaskin, C.J.; Fraser, S.F.; Mohebbi, M.; Broadbent, S.; Botti, M.; Kent, B.; et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: A multicenter cluster randomized controlled trial (ENGAGE). Cancer 2015, 121, 2646–2654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monga, U.; Garber, S.L.; Thornby, J.; Vallbona, C.; Kerrigan, A.L.; Monga, T.N.; Zimmermann, K.P. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch. Phys. Med. Rehabil. 2007, 88, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.S.; Raastad, T.; Skovlund, E.; Courneya, K.S.; Langberg, C.W.; Lilleby, W.; Fosså, S.D.; Thorsen, L. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. (Stockh. Swed.) 2015, 54, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.F.; Haseen, F.; Murray, L.J.; O’Sullivan, J.M.; Cantwell, M.M. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J. Cancer Surviv. Res. Pract. 2015, 9, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Santa Mina, D.; Alibhai, S.M.; Matthew, A.G.; Guglietti, C.L.; Pirbaglou, M.; Trachtenberg, J.; Ritvo, P. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J. Aging Phys. Act. 2013, 21, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Malone, S.C.; Parliament, M.B.; Scott, C.G.; Venner, P.M.; Quinney, H.A.; Jones, L.W.; et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2003, 21, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Sigal, R.J.; Kenny, G.P.; Prud’Homme, D.G.; Malone, S.C.; Wells, G.A.; Scott, C.G.; Slovinec D’Angelo, M.E. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J. Clin. Oncol. 2009, 27, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Vitolins, M.Z.; Griffin, L.; Tomlinson, W.V.; Vuky, J.; Adams, P.T.; Moose, D.; Frizzell, B.; Lesser, G.J.; Naughton, M.; Radford, J.E., Jr.; et al. Randomized trial to assess the impact of venlafaxine and soy protein on hot flashes and quality of life in men with prostate cancer. J. Clin. Oncol. 2013, 31, 4092–4098. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Dobek, J.C.; Bennett, J.A.; Dieckmann, N.F.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: Evidence from a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hojan, K.; Kwiatkowska-Borowczyk, E.; Leporowska, E.; Górecki, M.; Ozga-Majchrzak, O.; Milecki, T.; Milecki, P. Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high-risk prostate cancer patients during radiotherapy: A prospective, randomized clinical study. Eur. J. Phys. Rehabil. Med. 2016, 52, 489–501. [Google Scholar] [PubMed]
- McQuade, J.L.; Prinsloo, S.; Chang, D.Z.; Spelman, A.; Wei, Q.; Basen-Engquist, K.; Harrison, C.; Zhang, Z.; Kuban, D.; Lee, A.; et al. Qigong/tai chi for sleep and fatigue in prostate cancer patients undergoing radiotherapy: A randomized controlled trial. Psycho-Oncology 2016. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Newton, R.U.; Chinapaw, M.J.; Taaffe, D.R.; Spry, N.A.; Denham, J.W.; Joseph, D.J.; Lamb, D.S.; Brug, J.; Galvao, D.A. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer 2015, 121, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Ros, W.J.G.; Chinapaw, M.J.M.; Brug, J.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Mediators of the resistance and aerobic exercise intervention effect on physical and general health in men undergoing androgen deprivation therapy for prostate cancer. Cancer 2014, 120, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Truong, P.T.; Gaul, C.A.; McDonald, R.E.; Petersen, R.B.; Jones, S.O.; Alexander, A.S.; Lim, J.T.; Ludgate, C. Prospective evaluation of a 12-week walking exercise program and its effect on fatigue in prostate cancer patients undergoing radical external beam radiotherapy. Am. J. Clin. Oncol. 2011, 34, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Sen, A.; Han-Markey, T.L.; Harris, R.E. Examination of the association of diet and persistent cancer-related fatigue: A pilot study. Oncol. Nurs. Forum 2013, 40, E41–E49. [Google Scholar] [CrossRef] [PubMed]
- Maschke, J.; Kruk, U.; Kastrati, K.; Kleeberg, J.; Buchholz, D.; Erickson, N.; Huebner, J. Nutritional care of cancer patients: A survey on patients’ needs and medical care in reality. Int. J. Clin. Oncol. 2017, 22, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.W.; MacLeod, R.D. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: A systematic review. J. Pain Symptom Manag. 2012, 43, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.P.; Murray, D.M.; Hwang, B.S.; Gouin, J.P.; Thayer, S.F.; Sollers, J.J.; Shapiro, C.L.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology 2011, 36, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, R.D.; Vigano, A.; Trutschnigg, B.; Hornby, L.; Lucar, E.; Bacon, S.L.; Morais, J.A. Cancer-related fatigue: The impact of skeletal muscle mass and strength in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2010, 1, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvao, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Turner, D.; Newton, R.U. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: A comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009, 12, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Ishizaki, F.; Saito, T.; Nishiyama, T.; Kawasaki, T.; Takahashi, K. Decrease in lean body mass in men with prostate cancer receiving androgen deprivation therapy: Mechanism and biomarkers. Urology 2013, 81, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Camila, M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: Long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012, 96, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Day, J.M.; Katz, M.L.; Herndon, J.E.; Bittoni, M.A.; Oliveri, J.M.; Donohue, K.; Paskett, E.D. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psycho-Oncology 2009, 18, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, V.; Germani, A.; Capuzzo Dolcetta, E.; Donini, L.M.; Del Balzo, V. The New Modern Mediterranean Diet Italian Pyramid. Ann. Ig. 2016, 28, 179–186. [Google Scholar] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Chiva-Blanch, G.; Ros, E.; Martinez-Gonzalez, M.A.; Covas, M.I.; Lamuela-Raventos, R.M.; Salas-Savado, J.; Fiol, M.; et al. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdrich, S.; Bishop, K.S.; Karunasinghe, N.; Han, D.Y.; Ferguson, L.R. A pilot study to investigate if New Zealand men with prostate cancer benefit from a Mediterranean-style diet. PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Bhindi, B.; Kulkarni, G.S.; Finelli, A.; Alibhai, S.M.; Hamilton, R.J.; Toi, A.; van der Kwast, T.H.; Evans, A.; Hersey, K.; Jewett, M.A.; et al. Obesity Is Associated with Risk of Progression for Low-risk Prostate Cancers Managed Expectantly. Eur. Urol. 2014, 66, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.A.; Small, B.J.; Andrykowski, M.A.; Munster, P.; Jacobsen, P.B. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007, 26, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Reinertsen, K.V.; Cvancarova, M.; Loge, J.H.; Edvardsen, H.; Wist, E.; Fossa, S.D. Predictors and course of chronic fatigue in long-term breast cancer survivors. J. Cancer Surviv. 2010, 4, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Bennett, J.A.; Nail, L.; Schwartz, A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol. Nurs. Forum 2008, 35, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Choi, I.J.; Ryu, K.H.; Park, B.J.; Kim, Y.W.; Kim, H.B.; Kim, J.S. The effect of abdominal visceral fat, circulating inflammatory cytokines, and leptin levels on reflux esophagitis. J. Neurogastroenterol. Motil. 2015, 21, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergi, R.; Kirby, K.C.; Fabhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Ambrosius, W.; Messier, S.P.; Miller, G.D.; Penninx, B.W.; Loeser, R.F.; Palla, S.; Bleecker, E.; Pahor, M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: A randomized controlled clinical trial. Am. J. Clin. Nutr. 2004, 79, 544–551. [Google Scholar] [PubMed]
- Nicklas, B.J.; You, T.; Pahor, M. Behavioural treatments for chronic systemic inflammation: Effects of dietary weight loss and exercise training. Can. Med. Assoc. J. 2005, 172, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Nicklas, B.J. Reductions in Plasma Cytokine Levels with Weight Loss Improve Insulin Sensitivity in Overweight and Obese Postmenopausal Women. Diabetes Care 2004, 27, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Margella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of Inflammatory Cytokine Concentrations and Improvement of Endothelial Functions in Obese Women after Weight Loss over One Year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef] [PubMed]

| Criteria | Description |
|---|---|
| Participants | Men with a histologically confirmed diagnosis of prostate cancer (including all stages of, and treatments for, prostate cancer) |
| Intervention(s) | Any structured diet only intervention |
| Any exercise only protocol detailing frequency, intensity, time and type of exercise | |
| Any combined diet and exercise protocol, for any duration (with the exception of a single bout of exercise comparing pre- and post-exercise acute fatigue measures) | |
| Comparison(s) | Comparison group receiving diet, exercise, or a combined diet and exercise of a lesser intensity, or a control group not receiving the intervention at any time point during the trial |
| Outcome(s) | Changes in cancer-related fatigue and quality of life |
| Author | 1. Randomisation | 2. Treatment Allocation | 3. Group Similarity at Baseline, or Adjustment in Analysis | 4. Eligibility Criteria Specified | 5. Point Estimates and Measures of Variability | 6. Intention to Treat Analysis | Total Score | |
|---|---|---|---|---|---|---|---|---|
| 1 | Bourke, et al. [16] | Y | Y | Y | Y | Y | Y | 100% |
| 2 | Bourke, et al. [17] | Y | Y | Y | Y | N | N | 66% |
| 3 | Cormie, et al. [46] | Y | Y | Ya | Y | Y | Y | 100% |
| 4 | Cormie, et al. [45] | Y | Y | Y | Y | Y | Y | 100% |
| 5a | Galvão, et al. [48] | Y | Y | Y | Y | Y | Y | 100% |
| 5b | Buffart, et al. [61] b | |||||||
| 6a | Galvão, et al. [47] | Y | Y | Y | Y | Y | Y | 100% |
| 6b | Buffart, et al. [60] b | |||||||
| 7 | Hojan, et al. [58] | Y | Y | Y | Y | Y | N | 83% |
| 8 | Livingston, et al. [49] | Y | N | Y | Y | Y | N | 66% |
| 9 | McQuade, et al. [59] | Y | Y | U | Y | N | N | 50% |
| 10 | Monga, et al. [50] | Y | U | Y | Y | Y | N | 66% |
| 11 | Nilsen, et al. [51] | Y | Y | Y | Y | Y | Y | 100% |
| 12 | O’Neil, et al. [52] | Y | Y | Y | Y | Y | N | 83% |
| 13 | Santa Mina, et al. [53] | Y | Y | Y | Y | Y | Y | 100% |
| 14 | Segal, et al. [54] | Y | Y | Y | Y | Y | Y | 100% |
| 15 | Segal, et al. [55] | Y | Y | Y a | Y | Y | Y | 100% |
| 16 | Truong, et al. [62] | N | N | Y | Y | N | U | 33% |
| 17 | Vitolins, et al. [56] | Y | Y a | U | Y | N | Y | 66% |
| 18 | Winters-Stone, et al. [57] | Y | Y | Y | Y | N | Y | 83% |
| Number of papers scoring a point/total papers | 17/18 | 15/18 | 16/18 | 18/18 | 14/18 | 11/18 | ||
| Author (Year) Country | Study Design | Participants (Mean Age ± SD Range (Years) | Prostate Cancer Treatment (Treatment Duration ± SD Range (Months)) | Control Group | Dropout Number | Exercise | Nutrition Therapy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode | Time/Intensity | Frequency | Duration | Intervention Details (Frequency, Delivery, Diet) | |||||||
| Combined Diet and Exercise Interventions | |||||||||||
| Bourke, et al. [16] United Kingdom | RCT | I = 25 (71.3; 6.4) | ADT (30 ± 31) | Y = usual care | I = 4 | A and R | A: 55–80% APMHR | 3 times per week | 12 weeks | Fortnightly small group nutrition seminars, with nutrition handout pack. Diet composition of low SF, refined CHO, moderate EtOH, high fibre, fruit and vegetables. | |
| C = 25 (72.2; 7.7) | C = 3 | R: progressive resistance load of 2–4 sets of 8–12 repetitions of upper and lower body muscle groups (unknown amount of exercises) | |||||||||
| Bourke, et al. [17] United Kingdom | RCT | I = 50 (71; 6) | ADT (33 ± 33) | Y = usual care | I = 7 | A and R | A: 55–80% APMHR | 3 times per week | 12 weeks | Fortnightly small group nutrition seminars, with nutrition handout pack. Diet composition of low SF, refined CHO, moderate EtOH, high fibre, fruit and vegetables. | |
| C = 50 (71; 8) | C = 8 | R: progressive resistance load of 2–4 sets of 8–12 repetitions of upper and lower body muscle groups (unknown amount of exercises) | |||||||||
| O’Neil, et al. [52] United Kingdom | RCT | I = 47 (69.7; 6.8) | ADT (26.4 ± 32.4) | Y = usual care | I = 1 | A | Moderate: intensity NM | 3 times per week | 24 weeks | Baseline consult to individually meet ≥5 servings of vegetables and fruits/day, 30–35% of total energy from fat/day, ≤10% energy from SF/day, 10% energy from PUFA/day, limited consumption of processed meats, 25–35 g fibre/day, limited EtOH, and intake of Na+, and/or sugar. | |
| C = 47 (69.9; 7) | C = 2 | ||||||||||
| Diet Only Interventions | |||||||||||
| Vitolins, et al. [56] United States | RCT | N = 78 | Orchiectomy, LHRH, Antiandrogen, Radiation | N | Group 1 = 39 | 12 weeks | Participants randomly assigned to received one of four treatments: (Group 1) placebo pill or 75 mg venlafixine once daily with 20 g soy protein containing 160 mg isoflavones, (Group 2) 75 mg venlafaxine or placebo once daily without soy protein155 | ||||
| (Group 1) = 30 (67; Range 47–81) | |||||||||||
| (Group 2) = 30 (67; Range 47–82) | Group 2 = 39 | ||||||||||
| (Group 3) = 30 (71; Range 54–85) | |||||||||||
| (Group 4) = 30 (69; Range 46–91) | |||||||||||
| Exercise Only Interventions | |||||||||||
| Cormie, et al. [45] Australia | RCT | I = 32 (69.9; 6.5) | ADT I = 6.2; 1.6 (days) | Y = usual care | I = 1 | R and A | A: 20–30 min 70–85% estimated HRmax | 2 times per week | 12 weeks | ||
| C = 31 (67.1; 7.5) | C = 5.6: 2.0 (days) | C = 7 | R: Progressive resistance load, 8 exercises 2–4 sets of 6–12 repetitions, 60–85% 1RM | ||||||||
| Cormie, et al. [46] Australia | RCT | I = 10 (73.1; 7.5) | ADT, radiation (NA) | Y = usual care | I = 2 | R | Progressive resistance load, 8 exercises 2–4 sets of 8–12 repetitions, 60–85% 1RM | 2 times per week | 12 weeks | ||
| C = 10 (71.2; 6.9) | C = 3 | ||||||||||
| Galvão, et al. [47]; Buffart, et al. [60] Australia | RCT | I = 50 (71.9; 5.6) | ADT and radiation | Y = mailed exercise guidelines | I = 14 | R and A | A: 20–30 min at 70–85% HRmax | 3 times per week | 52 weeks | ||
| I = 12.9; 5.9 | R: Progressive resistance load, 8 exercises 2–4 sets of 12 repetitions (moderate intensity) | ||||||||||
| C = 50 (71.5; 7.2) | C = 11.0; 5.9 | C = 8 | C: modified educational booklet to perform 150 min per week of moderate PA | ||||||||
| Galvão, et al. [48]; Buffart, et al. [61] Australia | RCT | I = 29 (69.5; 7.3) | ADT I = 18.2; 38.5 | Y = usual care | I = 1 | R and A | A: 15–20 min 70–80% HRmax | 2 times per week | 12 weeks | ||
| C = 28 (70.1; 7.3) | C = 10.1; 26.8 | C = 1 | R: Progressive resistance load, 8 exercises 2–4 sets of 6–12 repetitions (moderate intensity) | ||||||||
| Hojan, et al. [58] Poland | RCT | I = 27 (67.4; 8.3) | ADT, and Radiation (NA) | Y = usual care | NA | R and A | A: 30 min 65–70% estimated maximal heart rate (220-age (years)) | 5 times per week | 12 weeks | ||
| C = 27 (69.9; 7.2) | R: 2 sets of 8 repetitions at 70–75% estimated 1RM of upper and lower body muscle groups | ||||||||||
| Livingston, et al. [49] Australia | RCT | I = 54 (66.9; 8.2) | Radical prostatectomy, radiation, ADT (NA) | Y = usual care | I = 7 | R and A | A: 20 mins 40–70% APMHR | 2 supervised, 1 home-based per week | 12 weeks | ||
| C = 93 (64.7; 8.7) | C = 10 | R: progressive resistance load, 4–8 exercises 2 sets of 8–12 repetitions (moderate intensity) | |||||||||
| Unsupervised: body weight and Thera-band exercises | |||||||||||
| McQuade, et al. [59] United States | RCT | I = 26 (65; 5.9) | Radiation (NA) | Y = usual care | I = 5 | R | 40 min of 8–12 sets of 8–12 repetitions of various muscle groups (light intensity) | 3 times per week | 8 weeks | ||
| U = 24 (66; 8.4) | U = 0 | ||||||||||
| Tai chi = 26 (62.2; 7.4) | Tai chi = 5 | ||||||||||
| Monga, et al. [50] United States | RCT | I = 11 (68; 4.2) | Radiation | Y = usual care | N = 9 | A | 30 min at (0.65) × (HRmax − resting HR) + resting HR, with 15–20 min warm-up and cool-down | 3 times per week | 8 weeks | ||
| C = 10 (70.6; 5.3) | |||||||||||
| Nilsen, et al. [51] Norway | RCT | I = 28 (66; 54–76) | ADT I = 17 ± 87 C = 18 ± 8.2 | Y = usual care | I = 6 | R | Progressive resistance load, 9 exercises 2–3 sets of 10 repetitions, 40–90% 1RM | 3 times per week | 16 weeks | ||
| C = 30 (66; 54–76) | Radiation I & C = 3.0 ± 1.3 | C = 3 | |||||||||
| Santa Mina, et al. [53] Canada | RCT | A: 32 (72.1; 8.9) | ADT (NA) | N | A: 13 | A or R | A: 30–60 min 60–80% HRmax | 3–5 times per week | 24 weeks | ||
| R: 34 (70.6; 9.5) | R: 22 | ||||||||||
| C: 1 | R: Progressive resistance load, 11 exercises 2–3 sets, 8–12 repetitions (moderate intensity) | ||||||||||
| Segal, et al. [54] Canada | RCT | I = 82 (68.2; 7.9) | ADT I = (12.5; 18.9) | Y = waiting list | I = 8 | R | Progressive resistance load, 9 exercises, 2 sets of 8–12 repetitions, 60–70% 1RM | 3 times per week | 12 weeks | ||
| C = 73 (67.7; 7.5) | C = (13.4; 22.2) | C = 12 | |||||||||
| Segal, et al. [55] Canada | RCT | A: 40 (66.2; 6.8) | Radiation ± ADT (NA) | Y = usual care | A: 3 | A or R | A: Progressive HR workload of (weeks 1–4) 50–60% VO2peak to (weeks 5–24) 70–75% VO2peak | 3 times per week | 24 weeks | ||
| R: 40 (66.4; 7.6) | R: 7 | R: Progressive resistance load, 11 exercises 2–3 sets, 8–12 repetitions, 60–70% 1RM | |||||||||
| C: 41 (66.3; 7.0) | C: 1 | ||||||||||
| Truong, et al. [62] Canada | Prospective Cohort | I = 50 (67; 6.5) | Radiation ± ADT I = 12; 2.9 | Y = usual care | I = 8 | A | A: 20 min at 60–70% APMHR | 3 times per week | 12 weeks | ||
| C = 30 (69; 6.3) | C = 12; 2.8 | C = 0 | |||||||||
| Winters-Stone, et al. [57] United States | RCT | R = 29 (69.9; 9.3) | ADT ± Radiation I = (39.0; 36.1) | N | I = 3 | R | R: progressive resistance load per % BW, 8 exercises 1–2 sets 8–14 repetitions (moderate intensity) | 3 times per week | 52 weeks | ||
| Stretching = 22 (70.5; 7.8) | C = (28.5; 29.2) | C = 5 | |||||||||
| Author (Year) Country | Measure of Fatigue | Baseline | Outcome Measure ± SD | Δ Fatigue Pre- and Post- Intervention (Mean ± SD (95% CI)) | Δ Fatigue Pre-Intervention Follow-up (Mean ± SD (95% CI)) | |||
|---|---|---|---|---|---|---|---|---|
| Post Intervention | Follow-up | Between-Group | Within-Group | Between-Group | Within-Group | |||
| Bourke, et al. [16] United Kingdom | FACT-F | I = 44 ± 6 | 48 ± 4 | ❖ 43 ± 7 | 5.4 * (0.8, 10.0) | ❖ 3.1 * (−0.3, 6.4) | ||
| C = 42 ± 8 | 48 ± 4 | 40 ± 8 | (p = 0.002) | (p = 0.006) | ||||
| Bourke, et al. [17] United | FACT-F | I = 40.3 ± 8.2 | 45.8 (NA) | 43.5 (NA) | 5.3 * (2.7, 7.9) | ❖ 3.9 * (1.1, 6.8) | ||
| C = 41.4 ± 8.6 | 42.4 (NA) | 41.9 (NA) | (p ≤ 0.001) | (p = 0.007) | ||||
| O’Neil, et al. [52] United Kingdom | MFSI-SF | I = 30.7 ± 14.9 | 29.4 ± 15.5 | 2.8 (−7.8, 2.1) | ||||
| C = 32.8 ± 17.6 | 34.1 ± 19 | (p = 0.26) | ||||||
| Vitolins, et al. [56] United States | ||||||||
| Cormie, et al. [45] Australia | FACT-F | I = 43.7 ± 8.3 | I = 43.8 ± 6.8 | 3.1 * (0.1, 6.2) | I = 0.1 ± 6.6 (p = 0.961) C = −3.4 (6.4) * (p = 0.006) | |||
| C = 44.8 ± 8.5 | C = 41.4 ± 9.5 | (p = 0.042) | ||||||
| Cormie, et al. [46] Australia | MFSI-SF | I = 5.2 ±16.8 | I = 8.8 ± 24.9 | −4.2 (−17.6, 9.2) (p = 0.521) | ||||
| C = 6.0 ± 12.3 | C = 3.8 ± 13.7 | |||||||
| Galvão, et al. [47]; Buffart, et al. [60] Australia | ||||||||
| Galvão, et al. [48]; Buffart, et al. [61] Australia | EORTC-30 (F) | I = 16.8 ± 17 | I = 14.6 ± 13.8 | 10.08 * (−18.33, 1.82) | ||||
| C = 29.7 ± 18.3 | C = 30.6 ± 17.6 | |||||||
| Hojan, et al. [58] Poland | FACT-F | I = 42.7 ± 2.1 | I = 43.9 ± 5.0 | 19.2 ± 4.7 * (p < 0.01) | I = 1.2 ± 4.8 | |||
| C = 42.5 ± 2.5 | C = 24.7 ± 4.5 | C = −17.8 ± 3.7 * (p < 0.01) | ||||||
| EORTC-C30 (F) | I = 27.3 ± 19.7 | I = 30.7 ± 21.4 | 11.2 ± 22.6 * (p < 0.05) | I = 3.4 ± 19.3 | ||||
| C = 28.0 ± 21.9 | C = 42.1 ± 23.6 | C = 14.0 ± 17.8 * (p < 0.05) | ||||||
| Livingston, et al. [49] Australia | ||||||||
| McQuade, et al. [59] United States | BFI | I = 1.47 ± 0.39 | I = 1.65 ± 0.38 | I = 2.38 ± 0.42 | ||||
| C = 1.97 ± 0.34 | C = 1.87 ± 0.33 | C = 1.81 ± 0.35 | ||||||
| Monga, et al. [50] United States | PFS | I = 2.4 ± 2.4 | I = 0.8 ± 1.8 | −4.3 ± 2.1 (p = 0.001) | I = −1.6 ± 2.0 * (p = 0.02) | |||
| C = 1.1 ± 1.9 | C = 3.8 ± 2.2 | C = 2.7 ± 2.2 (p = 0.004) | ||||||
| Nilsen, et al. [51] Norway | EORTC-30 (F) | I = 34.5 ± 15.2 | I = 33.7 ± 16.1 | 2.3 (−5.84, 10.54) | I = −0.8 (−6.41, 4.82) | |||
| C = 36.5 ± 14.9 | C = 33 ± 22.3 | (p = 0.568) | C = −3.5 (−9.74, 2.70) | |||||
| Santa Mina, et al. [53] Canada | FACT-F | A: 42 ± 8.4 | A: 41.4 ±1.4 | A❖: 42.2 ± 1.3 A♦: 42.4 ± 1.4 | p = 0.795❖ | A❖: 0.19 (0.95) A♦: 0.35 (1.27) | ||
| R: 38.1 ± 12.1 | R: 38.7 ± 1.7 | R❖: 35.6 ± 2.2 R♦: 37.9 ± 2.2 | p = 0.767♦ | R❖: 2.06 (1.94) R♦: 2.83 (1.83) | ||||
| Segal, et al. [54] Canada | FACT-F | I = 40.8 ± 10.6 | I = 41.6 ± 10.5 | I = (p = 0.002) | I = 0.8 ± 5.8 C = -2.2 ± 5.8 | |||
| C = 42.5 ± 8.5 | C = 40.3 ± 9.4 | |||||||
| Segal, et al. [55] Canada | FACT-F | A: 44.1 ± 8.7 | A: 44.2 ± 8.9 | A: 2.65 (−0.29 ± 5.58) (p = 0.80) | A: 0.2 (−1.9 ± 2.29) (p = 0.850) | |||
| R: 42.8 ± 8.7 | R: 45.1 ± 9.1 | R: 4.78 * (1.77 ± 7.78) (p = 0.002) | R: 2.33 * (0.13 ± 4.53) (p = 0.040) | |||||
| C: 44.6 ± 8.7 | C: 42.1 ± 8.8 | C: −2.45 * (−4.50, −0.40) (p = 0.020) | ||||||
| Truong, et al. [62] Canada | BFI | I✜ = 6.3 | I✜ = 6.5 | I✜ = 6.2 | (p = 0.40) | |||
| C✜ = 4.7 | C✜ = 9.0 | C✜ = 9.6 | ||||||
| Winters-Stone, et al. [57] United States | SCFS | I = 9.87 ± 4.47 | I = 9.22 ± 3.46 | ♦ I = 8.83 ± 3.19 | p < 0.01 | |||
| C = 9.92 ± 3.58 | C = 9.17 ± 2.98 | C = 9.83 ± 3.66 | ||||||
| Author (Year) Country | Measure of Quality of Life | Baseline | Outcome Measure ± SD | Δ Quality of Life Pre- and Post- Intervention (Mean ± SD (95% CI)) | Δ Quality of Life Pre-Intervention Follow-up (Mean ± SD (95% CI)) | |||
|---|---|---|---|---|---|---|---|---|
| Post Intervention | Follow-up | Between-Group | Within-Group | Between-Group | Within-Group | |||
| Bourke, et al. [16] United Kingdom | FACT-G | I = 91 ± 10 | 91 ± 10 | ❖ 90 ± 13 | 3.6 (−3.9, 11.0) | ❖ 1.8 (−1.5, 8.6) | ||
| C = 89 ± 13 | 86 ± 18 | 87 ± 17 | (p = 0.25) | (p = 0.36) | ||||
| FACT-P | I = 127 ± 13 | 128 ± 14 | ❖ 125 ± 20 | 5.5 (−4.2, 15.3) (p = 0.21) | ❖ 1.0 (−8.6, 10.6) | |||
| C = 125 ± 19 | 121 ± 25 | NA | (p = 0.45) | |||||
| Bourke, et al. [17] United Kingdom | FACT-P | I = 121.8 ± 15.6 | (NA) | (NA) | 8.9 * (3.7, 14.2) | ❖ 3.3 (−2.6, 9.3) | ||
| C = 119.9 ± 21.3 | (p = 0.001) | (p = 0.55) | ||||||
| FACT-P | I = 33.7 ± 7.4 | I = 118 ± 21.1 | 2.8 (−1.3, 6.9) | |||||
| C = 34.2 ± 7.54 | C = 117.5 ± 22.6 | (p = 0.2) | ||||||
| O’Neil, et al. [52] United Kingdom | ||||||||
| Vitolins, et al. [56] United States | FACT-P | I = 112.5 ± 6.0 (p = 0.048) * | ||||||
| C = 103.8 ± 6.2 | ||||||||
| FACT-G | I = 81.9 ± 4.3 (p = 0.025) | |||||||
| C = 74.1 ± 4.5 | ||||||||
| Cormie, et al. [45] Australia | SF-36 | I = GH: 53.6 ± 9.1 | I = GH: 54.4 ± 10.4 | 1.0 (−2.3, 4.3) (p = 0.554) | I = 0.8 ± 6.4 (p = 0.468) | |||
| C = GH: 52.8 ± 7.8 | C = GH: 52.8 ± 8.5 | C = 0.0 ± 6.7 (p = 0.998) | ||||||
| Cormie, et al. [46] Australia | SF-36 | I = GH: 45.6 ± 10 | I = GH: 41.7 ± 8.6 | 1.9 (−4.1, 7.9) | ||||
| C = GH: 42.4 ± 8.6 | C = GH: 44.5 ± 9.8 | (p = 0.508) | ||||||
| Galvão, et al. [47]; Buffart, et al. [60] Australia | SF-36 | I = GH: 51.5 ± 9.9 | I = GH: 51.3 ± 9.5 | I = GH: 49.2 ± 12.5 | 1.3 (−0.5, 3.1) | −1.7 (−4.7, 1.2) | ||
| C = GH: 49.8 ± 8.3 | C = GH: 48.5 ± 10.0 | C = GH: 49.3 ± 9.9 | (p = 0.167) | (p = 0.242) | ||||
| EORTC-C30 | I = GH: 77.3 ± 16.7 | I = GH: 79.1 ± 13.6 | I = GH: 76.9 ± 16 | 7.39 * (2.06, 12.73) | 2.87 (−1.00–6.73) | |||
| C = GH: 78.5 ± 16.0 | C = GH: 75.8 ± 22.4 | C = GH: 75.0 ± 17.8 | ||||||
| Galvão, et al. [48]; Buffart, et al. [61] Australia | SF-36 | I = GH: 66.0 ± 23.1 | I = GH: 71.4 ± 17.5 | 12.9 * (1.9, 23.9) | ||||
| C = GH: 67.3 ± 23.1 | C = GH: 60.2 ± 26.7 | (p = 0.022) | ||||||
| Hojan, et al. [58] Poland | EORTC-30 | I = GH: 53.7 ± 18.2 | I = GH: 55.4 ± 19.9 | 0.32 ± 18.9 | I = GH: 1.7 ± 27.7 | |||
| C = GH: 54.2 ± 23 | C = GH: 55.1 ± 17.7 | C = GH: 0.9 ± 21.1 | ||||||
| FACT-G | I = 70.7 ± 2.1 | I = 72.3 ± 6.3 | 17.8 ± 5.9 * (p < 0.05) | I = 1.6 ± 4.8 | ||||
| C = 70.0 ± 1.9 | C = 54.4 ± 3.9 | C = −15.6 ± 2.9 * (p < 0.01) | ||||||
| Livingston, et al. [49] Australia | EORTC-30 | I = GH: 75.9 ± 17.4 | I = GH: 80.3 ± 14.7 | 2.2 (−2.6, 6.9) | ||||
| C = GH: 77.5 ± 16.0 | C = GH: 80.0 ± 15.9 | (p = 0.37) | ||||||
| McQuade, et al. [59] United States | ||||||||
| Monga, et al. [50] United States | FACT-P | I = 138.5 ± 24.1 | I = 145.9 ± 18.3 | 13.8 * ± 10.1 | I = 7.4 * ± 10.4 (p = 0.04) | |||
| C = 144.5 ± 9.2 | C = 138.1 ± 12.7 | (p = 0.006) | C = −6.4 ± 9.8 (p = 0.07) | |||||
| Nilsen, et al. [51] Norway | EORTC-30 | I = GH: 76.5 ± 17.3 | I = GH: 79.6 ± 17 | −6.9 (−13.9, 0.1) | I = 3.1 (−1.12, 7.29) C = 12.2 (6.54, 17.93) | |||
| C = GH: 66.7 ± 19.6 | C = GH: 78.9 ± 20.7 | (p = 0.054) | ||||||
| Santa Mina, et al. [53] Canada | FACT-P | A: 123.9 ± 17.3 | A: 124.4 ± 3.1 | A❖: 124.2 ± 3.1 A♦: 125.5 ± 3.0 | p = 0.796❖ | A❖: 0.52 (2.48) A♦: 1.61 (2.86) | ||
| R: 119.3 ± 19.6 | R: 118.6 ± 3.4 | R❖: 117.4 ± 4.1 R♦: 119 ± 4.4 | p = 0.207♦ | R❖: 2.68 (3.83) R♦: 6.11 (4.72) | ||||
| PORPUS | A: 67.3 ± 11.5 | A: 67.0 ± 1.2 | A❖: 65.8 ± 2.1 A♦: 67.2 ± 2.0 | p = 0.434❖ | A❖: −2.35 (1.82) A♦: −0.33 (1.93) | |||
| R: 62.2 ± 10.4 | R: 63.2 ± 1.9 | R❖: 62.3 ± 2.2 R♦: 64.5 ± 2.8 | p = 0.021♦ | R❖: −0.22 (1.83) R♦: 8.09 * (2.45) | ||||
| Segal, et al. [54] Canada | FACT-P | I = 118.2 ± 16.7 | I = 120.2 ± 15.9 | I = (p = 0.001) | I = 2.0 ± 9.1 * C = −3.3 ± 10.2 | |||
| C = 120.9 ± 13.6 | C = 117.6 ± 14.9 | |||||||
| Segal, et al. [55] Canada | FACT-P | A: 37.5 ± 6.4 | A: 37.8 ± 6.5 | A: 1.44 (−0.8, 3.68) (p = 0.088) | A: 0.31 (−1.29, 1.90) (p = 0.703) | |||
| R: 37.4 ± 6.4 | R: 37.7 ± 6.7 | R: 1.40 | R: 0.27 | |||||
| C: 37.1 ± 6.4 | C: 36.0 ± 6.4 | (−0.89, 3.7) (p = 0.22) | (−1.41, 1.95) (p = 0.750) C: −1.13 (−2.70, 0.43) (p = 0.154) | |||||
| FACT-G | A: 89.5 ± 13 | 91.8 ± 13.1 | A: 2.35 (−0.06 ± 4.77) (p = 0.055) | A: 2.52 (−0.85–5.9) (p = 0.141) | ||||
| R: 91.8 ± 13.1 | 92.4 ± 13.4 | R: 4.17 * (1.62 ± 6.7) p = (0.002) | R: 4.34* (0.88 ± 7.8) (p = 0.015) | |||||
| C: 90.0 ± 130 | C: 87.5 ± 13.2 | C: −0.17 (−2.53, 2.19) (p = 0.886) | ||||||
| Truong, et al. [62] Canada | ||||||||
| Winters-Stone, et al. [57] United States | ||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. https://doi.org/10.3390/nu9091003
Baguley BJ, Bolam KA, Wright ORL, Skinner TL. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients. 2017; 9(9):1003. https://doi.org/10.3390/nu9091003
Chicago/Turabian StyleBaguley, Brenton J., Kate A. Bolam, Olivia R. L. Wright, and Tina L. Skinner. 2017. "The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review" Nutrients 9, no. 9: 1003. https://doi.org/10.3390/nu9091003





