Effect of Oral Pre-Meal Administration of Betaglucans on Glycaemic Control and Variability in Subjects with Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
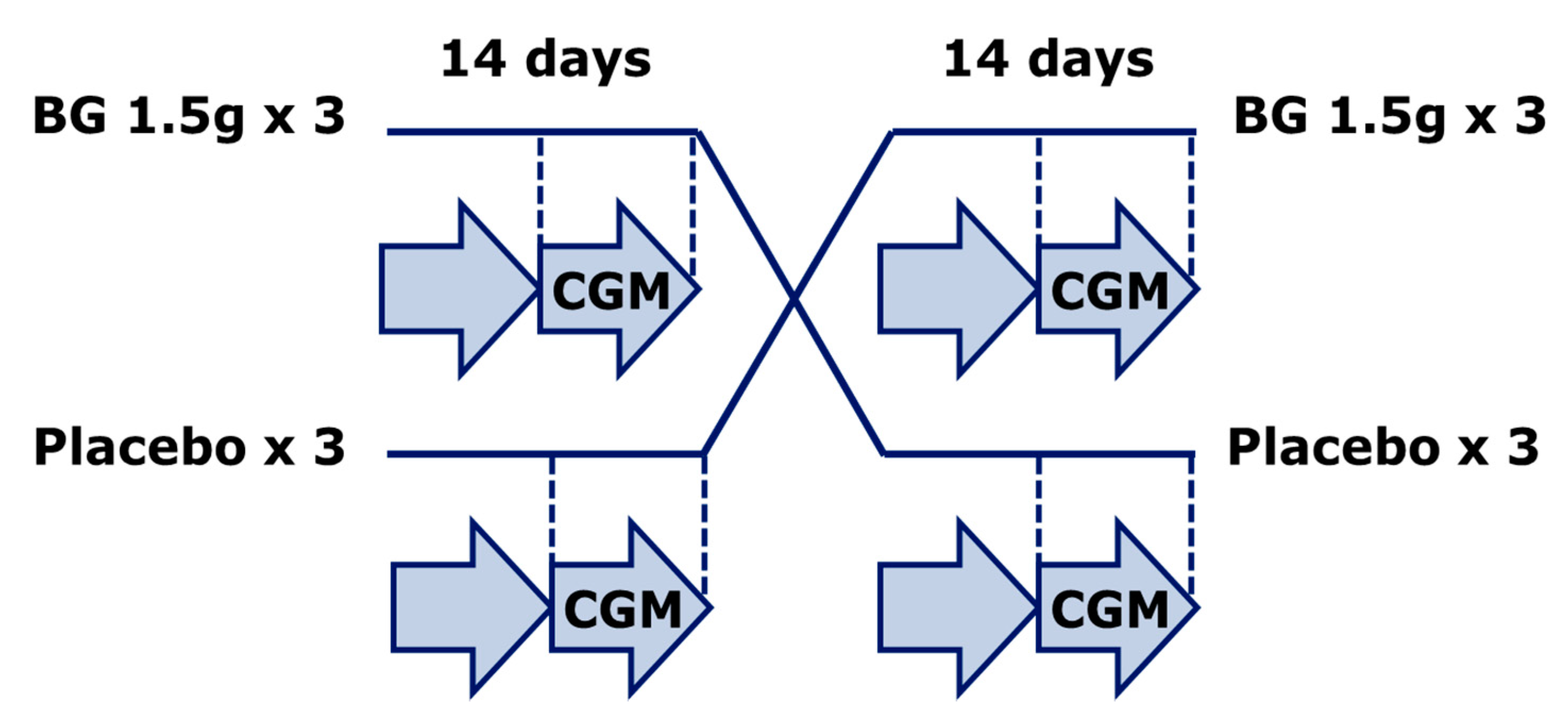
2.1. Study Set-Up and Subjects
2.2. Calculation of Indices for Continuous Glucose Monitoring (CGM) Data Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jenkins, D.J.; Kendall, C.W.; Vuksan, V.; Vidgen, E.; Parker, T.; Faulkner, D.; Mehling, C.C.; Garsetti, M.; Testolin, G.; Cunnane, S.C.; et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: Serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr. 2002, 75, 834–839. [Google Scholar] [PubMed]
- Othman, R.A.; Moghadasian, M.H.; Jones, P.J. Cholesterol-lowering effects of oat β-glucan. Nutr. Rev. 2011, 69, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Michiels, J.; Vanhaecke, L.; de Smet, S.; Kravchuk, O.; van de Meene, A.; Gidley, M.J. Reduction in circulating bile acid and restricted diffusion across the intestinal epithelium are associated with a decrease in blood cholesterol in the presence of oat β-glucan. FASEB J. 2016, 30, 4227–4238. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.L.; Zhao, T.; Zhou, Y.; Shi, X.; Zou, Y.; Zhao, G. Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9. [Google Scholar] [CrossRef] [Green Version]
- Cloetens, L.; Ulmius, M.; Johansson-Persson, A.; Åkesson, B.; Önning, G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr. Rev. 2012, 70, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Škrha, J.; Šoupal, J.; Škrha, J., Jr.; Prázný, M. Glucose variability, HbA1c and microvascular complications. Rev. Endocr. Metab. Disord. 2016, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, C.S.; Dejgaard, T.F.; Madsbad, S. Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol. 2016, 4, 766–780. [Google Scholar] [CrossRef]
- Rodbard, D. Interpretation of continuous glucose monitoring data: Glycemic variability and quality of glycemic control. Diabetes Technol. Ther. 2009, 11, S55–S67. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol. Ther. 2009, 11, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Werzowa, J.; Pacini, G.; Hecking, M.; Fidler, C.; Haidinger, M.; Brath, H.; Thomas, A.; Säemann, M.D.; Tura, A. Comparison of glycemic control and variability in patients with type 2 and posttransplantation diabetes mellitus. J. Diabetes Complicat. 2015, 29, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Farngren, J.; Schweizer, A.; Foley, J.E.; Pacini, G.; Ahrén, B. Four points pre-prandial self-monitoring of blood glucose for the assessment of glycemic control and variability in patients with type 2 diabetes treated with insulin and vildagliptin. Int. J. Endocrinol. 2015, 2015, 484231. [Google Scholar] [CrossRef] [PubMed]
- Panahi, S.; Ezatagha, A.; Temelli, F.; Vasanthan, T.; Vuksan, V. Beta-glucan from two sources of oat concentrates affect postprandial glycemia in relation to the level of viscosity. J. Am. Coll. Nutr. 2007, 26, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; O’Neil, C.E.; Greenway, F.L. Dietary fiber and satiety: The effects of oats on satiety. Nutr. Rev. 2016, 74, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; van den Borne, J.J.G.C.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristense, M.; Mars, M.; Schols, H.A.; Feskens, E.J.M. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Raederstorff, D.; Wolever, T.M. Effect of Consuming Oat Bran Mixed in Water before a Meal on Glycemic Responses in Healthy Humans-A Pilot Study. Nutrients 2016, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Nineham, R.; Craddock, C.; Craig-McFeely, P.; Donaldson, K.; Leigh, T.; Snook, J. Fibre in diabetes. Lancet 1979, 1, 434–435. [Google Scholar] [CrossRef]
- Fuessl, S.; Adrian, T.E.; Bacarese-Hamilton, A.J.; Bloom, S.R. Guar in NIDD: Effect of different modes of administration on plasma glucose and insulin responses to a starch meal. Pract. Diabetes Int. 1986, 3, 258–260. [Google Scholar] [CrossRef]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jesudason, D.R.; Stevens, J.E.; Keogh, J.B.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Sustained effects of a protein “preload” on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: A randomized clinical trial. Diabetes Res. Clin. Pract. 2015, 108, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Dingari, N.C.; Spegazzini, N.; Dasari, R.R.; Horowitz, G.L.; Barman, I. Emerging trends in optical sensing of glycemic markers for diabetes monitoring. TrAC Trends Anal. Chem. 2015, 64, 100–108. [Google Scholar] [CrossRef] [PubMed]
| CGM Parameter | Placebo | Betaglucan | p-Value |
|---|---|---|---|
| Mean glucose (mg/dL) | 175 ± 10 | 176 ± 9 | 0.6 |
| Maximal glucose (mg/dL) | 341 ± 15 | 378 ± 13 | 0.004 |
| Minimal glucose (mg/dL) | 59 ± 5 | 50 ± 4 | 0.07 |
| 80–200 mg/dL range (% of values) | 59 ± 4 | 57 ± 4 | 0.4 |
| <80 mg/dL (% of values) | 8 ± 2 | 10 ± 2 | 0.5 |
| >200 mg/dL (% of values) | 33 ± 5 | 34 ± 4 | 0.7 |
| ADDR (unitless) | 62 ± 5 | 79 ± 4 | 0.003 |
| GRADE (unitless) | 11 ± 1.1 | 12 ± 1.0 | 0.5 |
| M-value (unitless) | 19 ± 2.9 | 21 ± 3.3 | 0.5 |
| Standard deviation (mg/dL) | 65 ± 5 | 72 ± 4 | <0.05 |
| Coefficient of variation (%) | 38 ± 2 | 41 ± 1 | 0.2 |
| Glucose Range (mg/dL) | 283 ± 14 | 329 ± 12 | 0.003 |
| J-index ([mg/dL] 2) | 60 ± 6 | 63 ± 6 | 0.3 |
| CONGA (mg/dL) | 5.3 ± 0.6 | 5.2 ± 0.4 | 0.9 |
| MAGE (mg/dL) | 120 ± 9 | 119 ± 10 | 0.9 |
| Lability Index ((mg2)/h) | 0.82 ± 0.09 | 0.87 ± 10 | 0.8 |
| Shape Index (10−3 (mg/dL)/min2) | 3.20 ± 0.15 | 3.06 ± 0.13 | 0.4 |
| Autocorrelation (unitless) | 0.22 ± 0.02 | 0.28 ± 0.03 | 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frid, A.; Tura, A.; Pacini, G.; Ridderstråle, M. Effect of Oral Pre-Meal Administration of Betaglucans on Glycaemic Control and Variability in Subjects with Type 1 Diabetes. Nutrients 2017, 9, 1004. https://doi.org/10.3390/nu9091004
Frid A, Tura A, Pacini G, Ridderstråle M. Effect of Oral Pre-Meal Administration of Betaglucans on Glycaemic Control and Variability in Subjects with Type 1 Diabetes. Nutrients. 2017; 9(9):1004. https://doi.org/10.3390/nu9091004
Chicago/Turabian StyleFrid, Anders, Andrea Tura, Giovanni Pacini, and Martin Ridderstråle. 2017. "Effect of Oral Pre-Meal Administration of Betaglucans on Glycaemic Control and Variability in Subjects with Type 1 Diabetes" Nutrients 9, no. 9: 1004. https://doi.org/10.3390/nu9091004





