Capsaicin Supplementation Improved Risk Factors of Coronary Heart Disease in Individuals with Low HDL-C Levels
Abstract
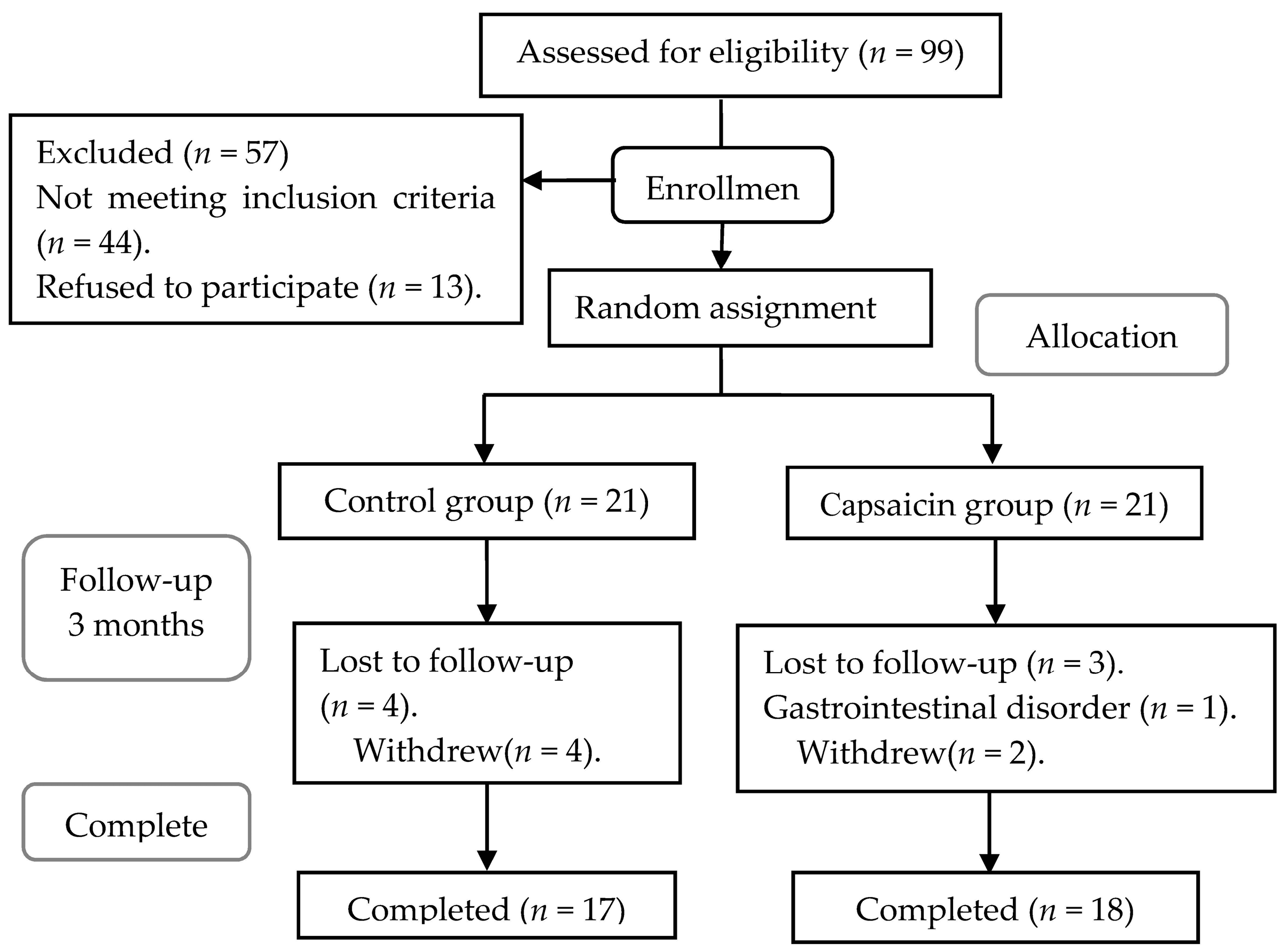
:1. Introduction
2. Methods
2.1. Populations
2.2. Study Design
2.3. Interventions
2.4. Experimental Assays
2.4.1. Anthropometric Measurements
2.4.2. Serum Lipids, Lipoproteins, Apolipoproteins, and Glucose Assays
2.4.3. Serum Liver Enzymes, Kidney Parameters, and Blood Routine Assays
2.4.4. CETP, LCAT, and PLTP Assays
2.4.5. Inflammatory Cytokine Assays
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Participants
3.2. Compliance
3.3. Effects of Capsaicin on the Serum Lipids, Lipoprotein, Apolipoproteins, and Glucose of the Participants
3.4. Effects of Capsaicin on the Blood CETP, LCAT, and PLTP of the Participants
3.5. Effects of Capsaicin on the Serum Liver, Kidney Parameters, and Blood Routine of the Participants
3.6. Effects of Capsaicin on the Inflammatory Cytokines of the Participants
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huxley, R.R.; Barzi, F.; Lam, T.H.; Czernichow, S.; Fang, X.; Welborn, T.; Shaw, J.; Ueshima, H.; Zimmet, P.; Jee, S.H.; et al. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: An individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation 2011, 124, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Ding, X.; Tang, W.; Li, Q.; Mao, D.; Wang, Y. Prevalence and risk factors associated with dyslipidemia in Chongqing, China. Int. J. Environ. Res. Public Health 2015, 12, 13455–13465. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, H.; Srinivasan, K. Hypolipidemic and antioxidant effects of dietary curcumin and capsaicin in induced hypercholesterolemic rats. Lipids 2007, 42, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Xiong, S.; Zhu, Z. Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cheang, W.S.; Wang, X.; Lei, L.; Liu, Y.; Ma, K.Y.; Zheng, F.; Huang, Y.; Chen, Z.Y. Capsaicinoids but not their analogue capsinoids lower plasma cholesterol and possess beneficial vascular activity. J. Agric. Food Chem. 2014, 62, 8415–8420. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; Tian, X.Y.; Chen, J.N.; Peng, C.; Ma, K.Y.; Zuo, Y.; Jiao, R.; Lu, Y.; Huang, Y.; Chen, Z.Y. Capsaicinoids lower plasma cholesterol and improve endothelial function in hamsters. Eur. J. Nutr. 2013, 52, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, G.; Zheng, L.; Chen, Z.; Liu, X. Hypocholesterolemic effect of capsaicinoids in rats fed diets with or without cholesterol. J. Agric. Food Chem. 2013, 61, 4287–4293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; Zhang, Q.Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Quintao, E.C.; Cazita, P.M. Lipid transfer proteins: Past, present and perspectives. Atherosclerosis 2010, 209, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zannis, V.I.; Chroni, A.; Krieger, M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. (Berl.) 2006, 84, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.; Baker, W.S.; Khan, M.I.; Thukuntla, S.; McKinney, K.H.; Abate, N.; Tuvdendorj, D. Current and future therapies for addressing the effects of inflammation on HDL cholesterol metabolism. Br. J. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am. J. Clin. Nutr. 2006, 84, 63–69. [Google Scholar] [PubMed]
- Ahuja, K.D.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur. J. Clin. Nutr. 2007, 61, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Shu, F.; Zeng, Y.; Meng, X.; Wang, B.; Diao, L.; Wang, L.; Wan, J.; Zhu, J.; Wang, J.; et al. Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-beta RsaI. J. Nutr. 2014, 144, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.J.; Lyass, A.; Brocia, R.W.; Massaro, J.M.; Vasan, R.S. Plasma lipid transfer proteins and cardiovascular disease. The Framingham Heart Study. Atherosclerosis 2013, 228, 230–236. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; McKavanagh, P.; York, E.; Nadeem, N.; Harbinson, M.; Stevenson, M.; Ball, P.; Lusk, L.; Trinick, T.; Young, I.S.; et al. Serum- and HDL3-serum amyloid A and HDL3-LCAT activity are influenced by increased CVD-burden. Atherosclerosis 2016, 244, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Investigators, A.-H.; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Opizzi, A.; Perna, S.; Faliva, M.; Solerte, S.B.; Fioravanti, M.; Klersy, C.; Cava, E.; Paolini, M.; Scavone, L.; et al. Improvement in insulin resistance and favourable changes in plasma inflammatory adipokines after weight loss associated with two months’ consumption of a combination of bioactive food ingredients in overweight subjects. Endocrine 2013, 44, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; Saleheen, D.; et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br. J. Nutr. 2003, 90, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Karalis, I.K.; Bergheanu, S.C.; Wolterbeek, R.; Dallinga-Thie, G.M.; Hattori, H.; van Tol, A.; Liem, A.H.; Wouter Jukema, J. Effect of increasing doses of Rosuvastatin and Atorvastatin on apolipoproteins, enzymes and lipid transfer proteins involved in lipoprotein metabolism and inflammatory parameters. Curr. Med. Res. Opin. 2010, 26, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.J.; Smelt, A.H.; Hattori, H.; Scheek, L.M.; van Gent, T.; de Man, F.H.; van der Laarse, A.; van Tol, A. Decreased PLTP mass but elevated PLTP activity linked to insulin resistance in HTG: Effects of bezafibrate therapy. J. Lipid Res. 2003, 44, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Vergeer, M.; Boekholdt, S.M.; Sandhu, M.S.; Ricketts, S.L.; Wareham, N.J.; Brown, M.J.; de Faire, U.; Leander, K.; Gigante, B.; Kavousi, M.; et al. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation 2010, 122, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Marmur, J.D.; Chhabra, S.; Chopra, V.; Eng, C.; Jiang, X.C. Relation of baseline plasma phospholipid transfer protein (PLTP) activity to left ventricular systolic dysfunction in patients referred for coronary angiography. Atherosclerosis 2009, 207, 261–265. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.; Dallinga-Thie, G.M.; Smit, A.J.; Wolffenbuttel, B.H.; van Tol, A.; Dullaart, R.P. Elevated plasma phospholipid transfer protein activity is a determinant of carotid intima-media thickness in type 2 diabetes mellitus. Diabetologia 2006, 49, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, S.J.; Carr, M.C.; Hokanson, J.E.; Brunzell, J.D.; Albers, J.J. PLTP activity in premenopausal women: Relationship with lipoprotein lipase, HDL, LDL, body fat, and insulin resistance. J. Lipid Res. 2000, 41, 237–244. [Google Scholar] [PubMed]
- Chen, X.; Sun, A.; Mansoor, A.; Zou, Y.; Ge, J.; Lazar, J.M.; Jiang, X.C. Plasma PLTP activity is inversely associated with HDL-C levels. Nutr. Metab. (Lond.) 2009, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; Gruppen, E.G.; Dallinga-Thie, G.M. Paraoxonase-1 activity is positively related to phospholipid transfer protein activity in type 2 diabetes mellitus: Role of large HDL particles. Clin. Biochem. 2016, 49, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.C.; Wolfbauer, G.; Kennedy, H.; Brown, B.G.; Albers, J.J. Plasma phospholipid transfer protein activity in patients with low HDL and cardiovascular disease treated with simvastatin and niacin. Biochim. Biophys. Acta 2001, 1537, 117–124. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Murtaza, N.; Jagtap, S.; Singh, D.P.; Karmase, A.; Kaur, J.; Bhutani, K.K.; Boparai, R.K.; Premkumar, L.S.; Kondepudi, K.K.; et al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J. Nutr. Biochem. 2014, 25, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef] [PubMed]
| Variables | Control (n = 17) | Capsaicin (n = 18) | p |
|---|---|---|---|
| Age (years) | 45.2 ± 4.8 | 41.9 ± 5.7 | 0.07 |
| Male/Female (n) | 14/3 | 16/2 | 0.66 |
| Height (cm) | 164.5 ± 7.4 | 166.6 ± 6.6 | 0.40 |
| Weight (kg) | 70.4 ± 7.9 | 73.9 ± 11.7 | 0.30 |
| BMI (kg/m2) | 26.03 ± 2.95 | 26.46 ± 2.76 | 0.65 |
| SBP (mmHg) | 128.8 ± 17.0 | 123.1 ± 13.7 | 0.29 |
| DBP (mmHg) | 84.9 ± 11.6 | 83.7 ± 10.9 | 0.76 |
| HDL-C (mmol/L) | 0.88 ± 0.13 | 0.92 ± 0.13 | 0.38 |
| Variables | Control (n = 17) | Capsaicin (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 3 Months | Change | 0 Month | 3 Months | Change | Pt | PM12 | PM23 | |
| TC (mmol/L) | 4.28 ± 0.87 | 4.73 ± 1.27 | 0.45 ± 1.04 | 4.56 ± 0.67 | 4.61 ± 1.16 | 0.05 ± 0.93 | 0.30 | 0.28 | 0.12 |
| LDL-C (mmol/L) | 2.42 ± 0.55 | 2.33 ± 0.42 | −0.09 ± 0.46 | 2.46 ± 0.44 | 2.50 ± 0.54 | 0.04 ± 0.37 | 0.83 | 0.27 | 0.48 |
| HDL-C (mmol/L) | 0.88 ± 0.13 | 0.86 ± 0.13 | −0.02 ± 0.14 | 0.92 ± 0.13 | 1.00 ± 0.13 | 0.08 ± 0.14 | 0.38 | 0.005 | 0.030 |
| non-HDL-C (mmol/L) | 3.40 ± 0.80 | 3.88 ± 1.3 | 0.47 ± 1.03 | 3.64 ± 0.68 | 3.61 ± 1.13 | −0.03 ± 0.91 | 0.35 | 0.15 | 0.08 |
| TC/HDL-C | 4.88 ± 0.79 | 5.72 ± 2.36 | 0.84 ± 1.92 | 5.06 ± 1.13 | 4.64 ± 1.12 | −0.42 ± 1.13 | 0.59 | 0.027 | 0.043 |
| TG (mmol/L) | 2.91 (1.69, 3.88) | 2.38 (1.65, 4.45) | 0.29 (−0.39, 1.03) | 2.62 (1.91, 5.57) | 2.05 (1.43, 3.57) | −0.65 (−1.71, 0.04) | 0.66 | 0.019 | 0.027 |
| Lp(a) (mg/L) | 61.0 (26.5, 200.5) | 60.0 (32.5, 134.5) | 0.0 (−33.0, 14.5) | 33.0 (21.25, 74.75) | 26.0 (14.25, 56.25) | −8.5 (−19.25, 5.25) | 0.23 | 0.34 | 0.15 |
| ApoAI (g/L) | 1.09 ± 0.14 | 1.11 ± 0.11 | 0.02 ± 0.11 | 1.11 ± 0.17 | 1.12 ± 0.10 | 0.01 ± 0.19 | 0.70 | 0.86 | 0.82 |
| ApoB (g/L) | 0.96 ± 0.19 | 0.90 ± 0.19 | −0.06 ± 0.19 | 0.90 ± 0.18 | 0.90 ± 0.17 | −0.01 ± 0.17 | 0.34 | 0.63 | 0.76 |
| ApoE (mg/dL) | 5.03 ± 1.55 | 6.55 ± 4.25 | 1.52 ± 3.21 | 5.74 ± 2.85 | 5.43 ± 3.14 | −0.32 ± 2.97 | 0.37 | 0.11 | 0.16 |
| Glucose (mmol/L) | 5.27 ± 0.41 | 5.30 ± 0.38 | 0.03 ± 0.46 | 5.49 ± 2.24 | 5.47 ± 1.71 | −0.02 ± 0.68 | 0.70 | 0.97 | 0.64 |
| Variables | Control (n = 17) | Capsaicin (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 3 Months | Change | 0 Month | 3 Months | Change | Pt | PM12 | PM23 | |
| CETP concentration (mg/L) | 2.02 ± 0.89 | 2.37 ± 1.15 | 0.35 ± 0.88 | 2.07 ± 1.06 | 2.30 ± 1.07 | 0.22 ± 0.94 | 0.87 | 0.71 | 0.92 |
| CETP activity (mmol/h) | 73.2 ± 8.34 | 73.14 ± 7.9 | −0.06 ± 8.97 | 72.91 ± 9.72 | 71.61 ± 10.94 | −1.3 ± 11.75 | 0.93 | 0.67 | 0.99 |
| LCAT concentration (mg/L) | 6.27 ± 3.73 | 7.38 ± 4.12 | 1.10 ± 2.55 | 7.25 ± 4.89 | 7.67 ± 4.46 | 0.42 ± 3.28 | 0.51 | 0.65 | 0.67 |
| LCAT activity (390/470 nm) | 0.94 ± 0.07 | 0.97 ± 0.11 | 0.03 ± 0.12 | 0.95 ± 0.18 | 0.95 ± 0.09 | 0.01 ± 0.15 | 0.80 | 0.66 | 0.73 |
| PLTP activity (mmol/h) | 1.52 ± 0.38 | 1.64 ± 0.42 | 0.12 ± 0.39 | 1.67 ± 0.39 | 1.44 ± 0.32 | −0.22 ± 0.4 | 0.27 | 0.030 | 0.043 |
| Variables | Control (n = 17) | Capsaicin (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 3 Months | Change | 0 Month | 3 Months | Change | Pt | PM12 | PM23 | |
| Albumin(g/L) | 45.7 ± 1.6 | 45.9 ± 2.4 | 0.2 ± 2.5 | 46.7 ± 3.0 | 46.0 ± 2.7 | −0.7 ± 2.7 | 0.23 | 0.64 | 0.43 |
| Globulin (g/L) | 29.1 ± 3.1 | 29.9 ± 2.9 | 0.8 ± 3.4 | 29.4 ± 2.7 | 29.8 ± 2.6 | 0.3 ± 3.2 | 0.73 | 0.78 | 0.83 |
| Total protein (g/L) | 74.8 ± 3.4 | 76.0 ± 3.4 | 1.2 ± 4.0 | 76.1 ± 4.1 | 75.8 ± 3.4 | −0.3 ± 4.0 | 0.31 | 0.57 | 0.63 |
| A/G ratio | 1.59 ± 0.17 | 1.55 ± 0.17 | −0.04 ± 0.21 | 1.60 ± 0.17 | 1.56 ± 0.18 | −0.04 ± 0.2 | 0.83 | 0.97 | 0.66 |
| GGT (IU/L) | 22.0 (20.5, 52.5) | 21.0 (17.0, 45.3) | 3.0 (1.0, 9.0) | 29.0 (24.5, 116.5) | 25.5 (18.8, 45.8) | 4.0 (−1.5, 7.25) | 0.16 | 0.54 | 0.30 |
| ALT (IU/L) | 26.9 ± 19.4 | 27.1 ± 9.9 | 0.1 ± 15.7 | 23.3 ± 7.8 | 27.2 ± 10.3 | 3.9 ± 7.2 | 0.46 | 0.58 | 0.59 |
| AST (IU/L) | 24.1 ± 7.6 | 24.3 ± 3.6 | 0.2 ± 7.8 | 22.3 ± 4.2 | 24.9 ± 5.9 | 2.6 ± 5.2 | 0.39 | 0.54 | 0.87 |
| ALP (IU/L) | 94.4 ± 31.8 | 95.7 ± 25.0 | 1.4 ± 17.3 | 81.8 ± 18.8 | 79.6 ± 18.4 | −2.3 ± 12.2 | 0.16 | 0.10 | 0.11 |
| Creatinine (μmol/L) | 74.46 ± 11.93 | 74.48 ± 13.20 | 0.02 ± 6.08 | 75.3 ± 13.15 | 81.46 ± 12.50 | 6.16 ± 10.22 | 0.85 | 0.029 | 0.015 |
| Urea nitrogen (mmol/L) | 5.04 ± 1.08 | 5.39 ± 1.35 | 0.35 ± 1.17 | 5.06 ± 1.65 | 5.13 ± 1.34 | 0.07 ± 1.37 | 0.97 | 0.49 | 0.20 |
| Uric acid (μmol/L) | 378.0 ± 76.4 | 406.6 ± 77.5 | 28.7 ± 50.1 | 390.7 ± 88.9 | 407.1 ± 91.9 | 16.4 ± 45.1 | 0.65 | 0.52 | 0.68 |
| Variables | Control (n = 17) | Capsaicin (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Month | 3 Months | Change | 0 Month | 3 Months | Change | Pt | PM12 | PM23 | |
| CRP (μg/mL) | 1.67 (1.10, 2.36) | 1.89 (1.39, 2.76) | 0.24 (−0.73, 1.04) | 1.38 (1.13, 2.03) | 1.09 (0.83, 1.74) | −0.39 (−1.08, 0.26) | 0.69 | 0.034 | 0.042 |
| TNF-α (pg/mL) | 31.49 ± 14.75 | 35.04 ± 16.85 | 3.55 ± 13.41 | 33.42 ± 18.11 | 34.92 ± 15.59 | 1.49 ± 15.65 | 0.73 | 0.78 | 0.95 |
| SAA (μg/mL) | 4.08 ± 1.64 | 4.73 ± 2.28 | 0.66 ± 1.89 | 4.69 ± 3.04 | 5.34 ± 3.30 | 0.65 ± 2.75 | 0.46 | 0.83 | 0.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Ran, L.; Wang, J.; Yu, L.; Lang, H.-D.; Wang, X.-L.; Mi, M.-T.; Zhu, J.-D. Capsaicin Supplementation Improved Risk Factors of Coronary Heart Disease in Individuals with Low HDL-C Levels. Nutrients 2017, 9, 1037. https://doi.org/10.3390/nu9091037
Qin Y, Ran L, Wang J, Yu L, Lang H-D, Wang X-L, Mi M-T, Zhu J-D. Capsaicin Supplementation Improved Risk Factors of Coronary Heart Disease in Individuals with Low HDL-C Levels. Nutrients. 2017; 9(9):1037. https://doi.org/10.3390/nu9091037
Chicago/Turabian StyleQin, Yu, Li Ran, Jing Wang, Li Yu, He-Dong Lang, Xiao-Lan Wang, Man-Tian Mi, and Jun-Dong Zhu. 2017. "Capsaicin Supplementation Improved Risk Factors of Coronary Heart Disease in Individuals with Low HDL-C Levels" Nutrients 9, no. 9: 1037. https://doi.org/10.3390/nu9091037




