Dietary Inflammatory Index and Colorectal Cancer Risk—A Meta-Analysis
Abstract
:1. Introduction
2. Methods
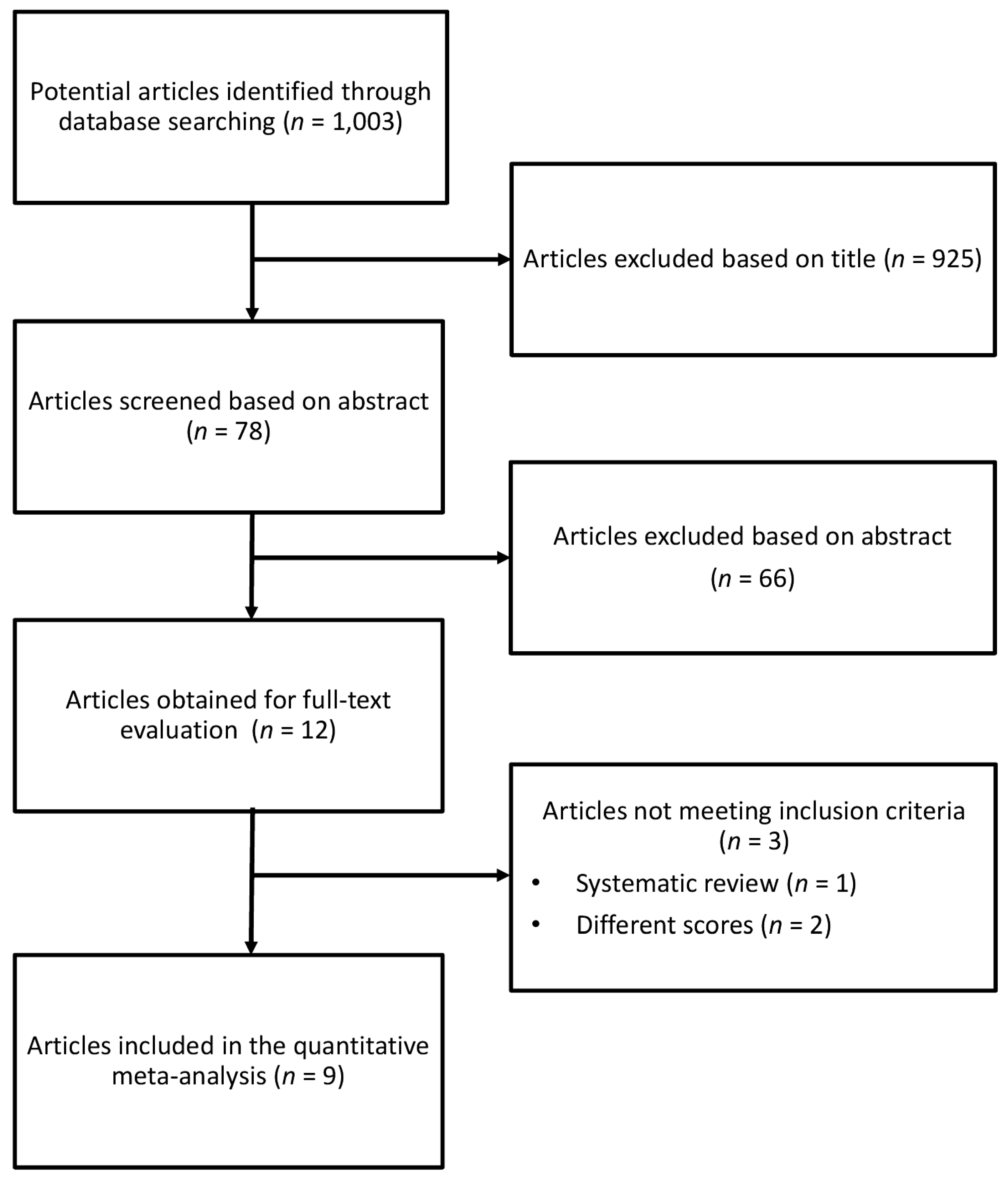
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Statistical Analysis
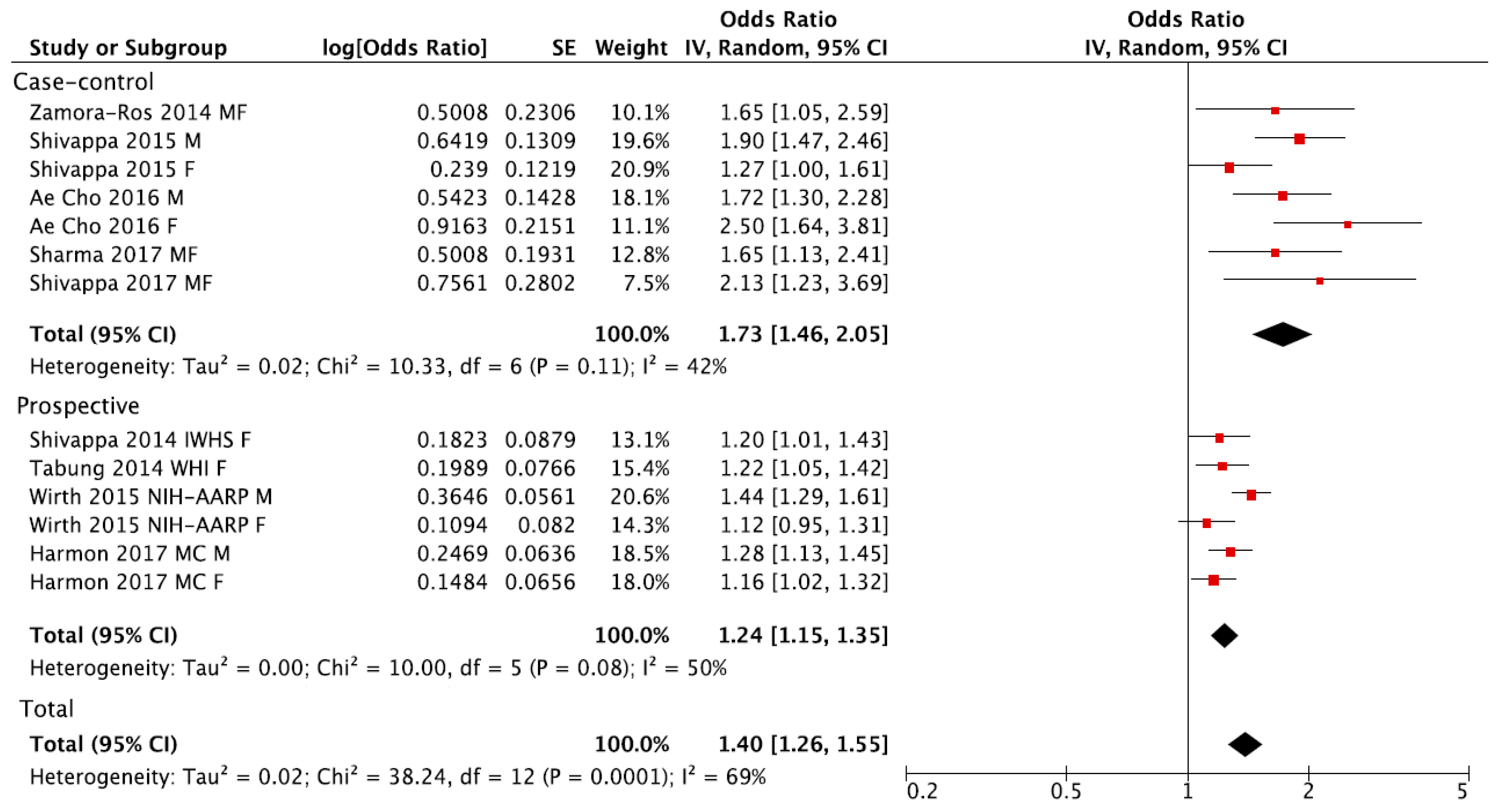
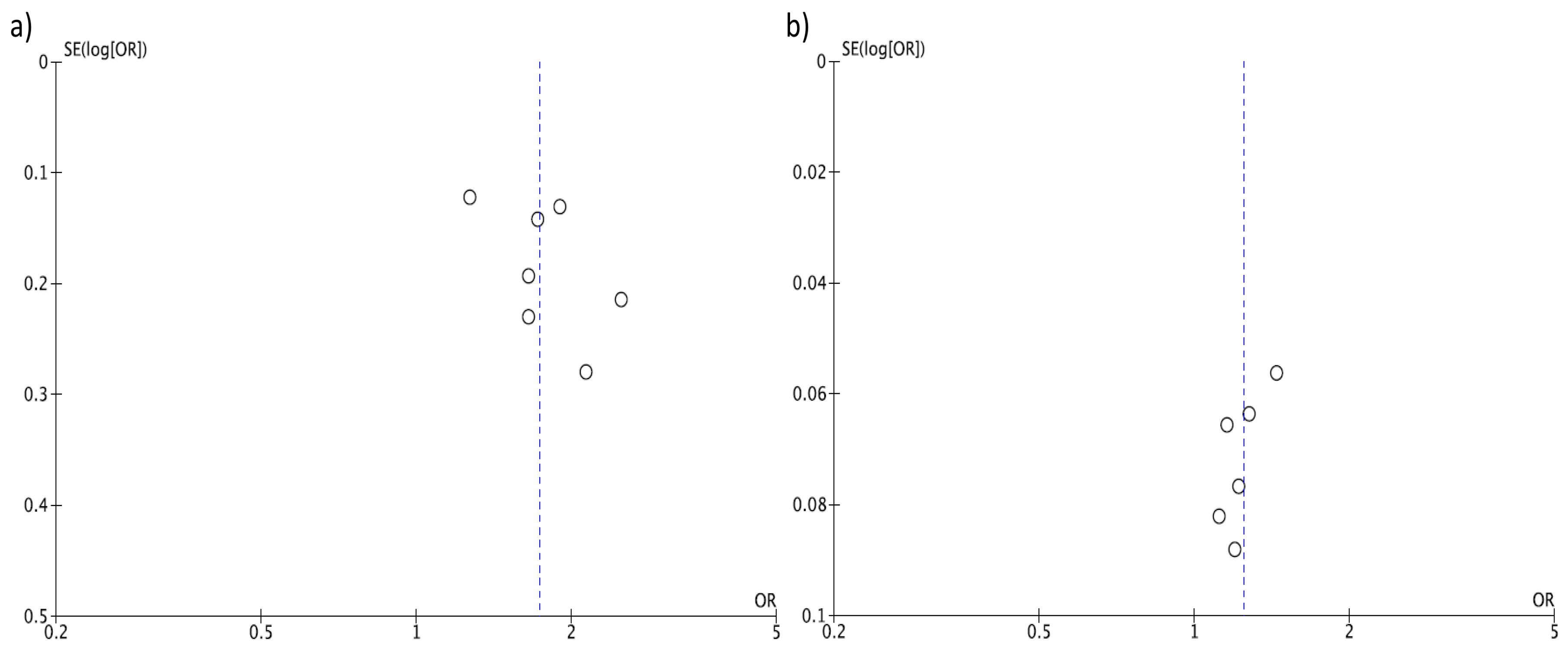
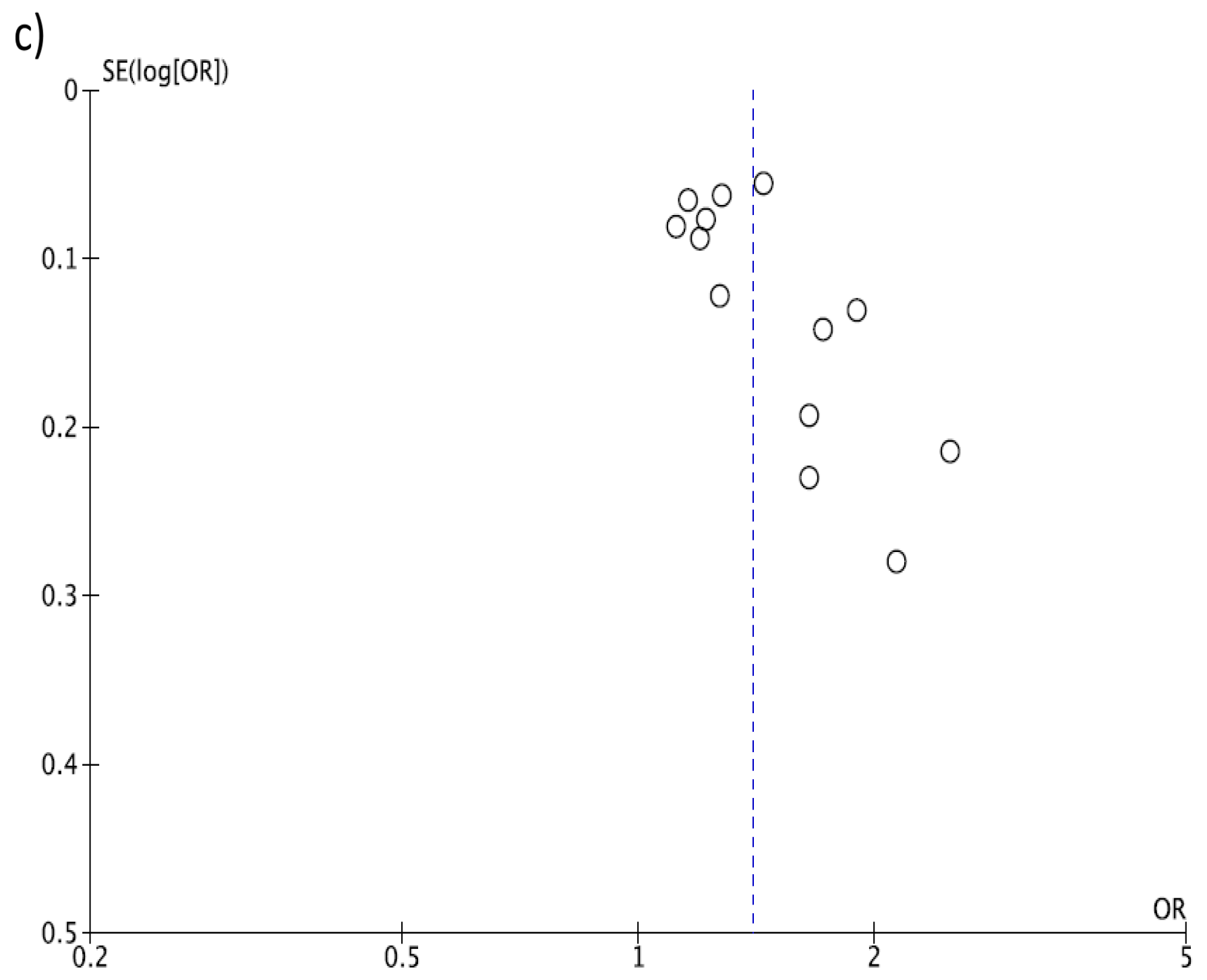
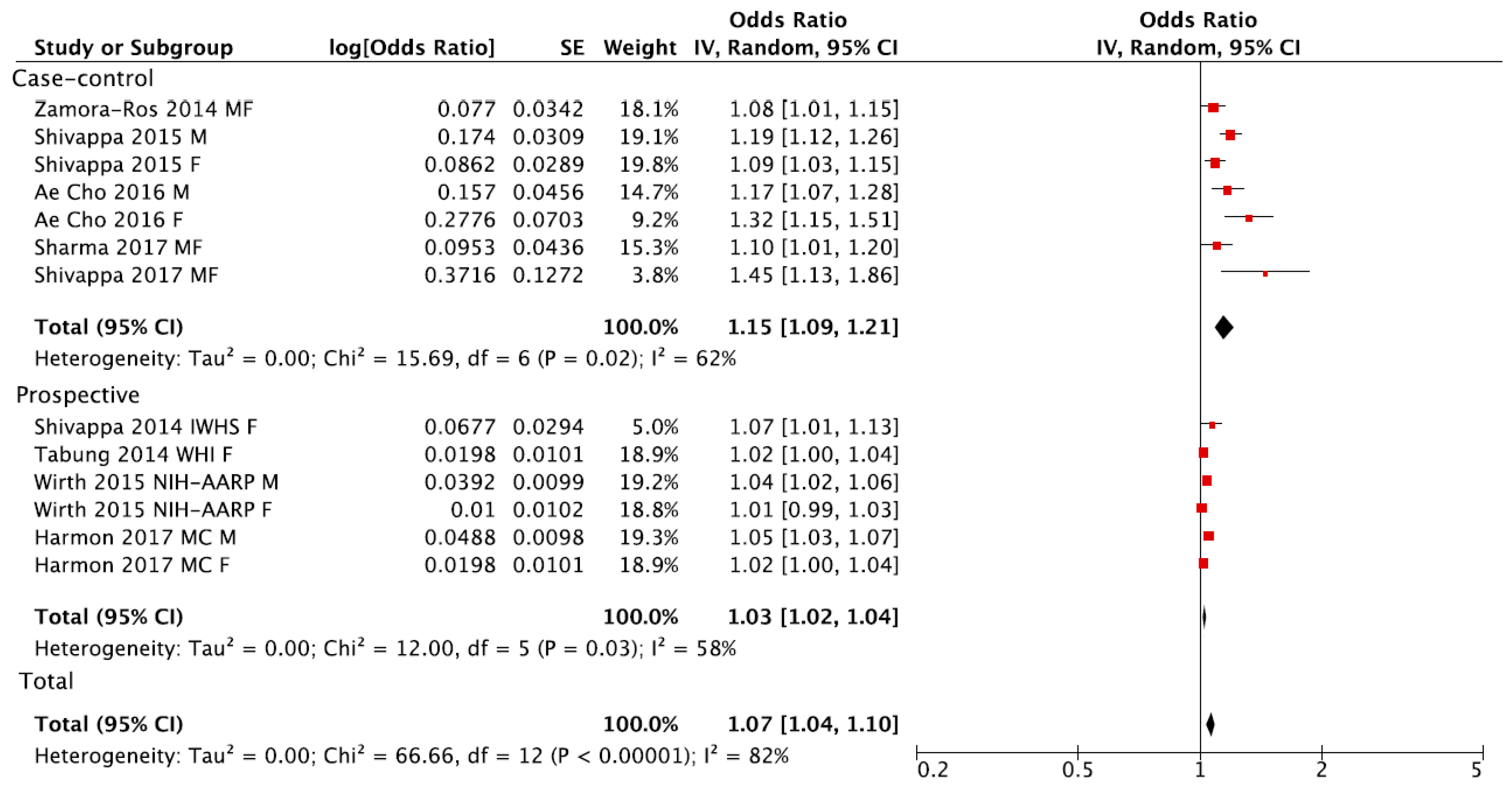
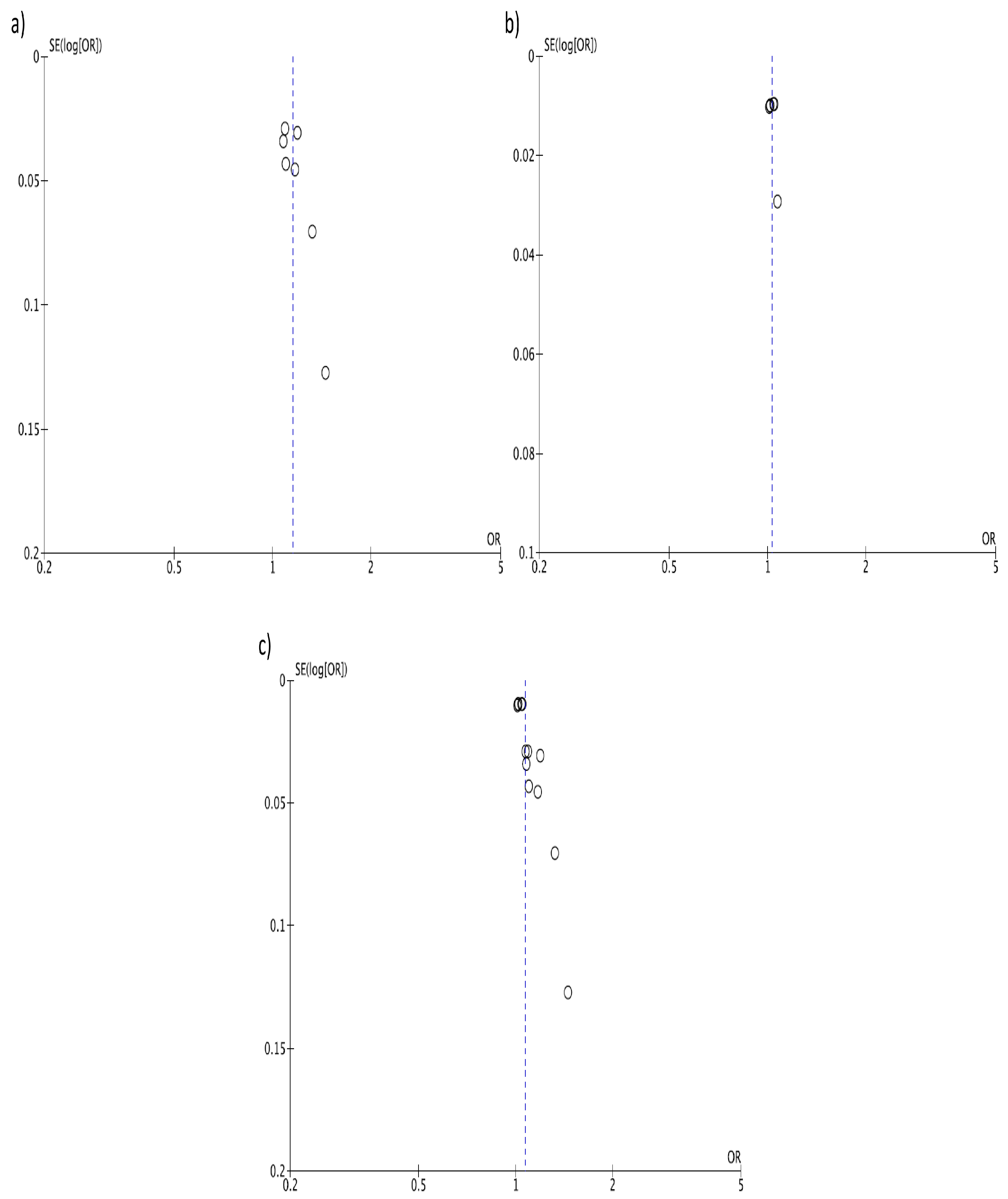
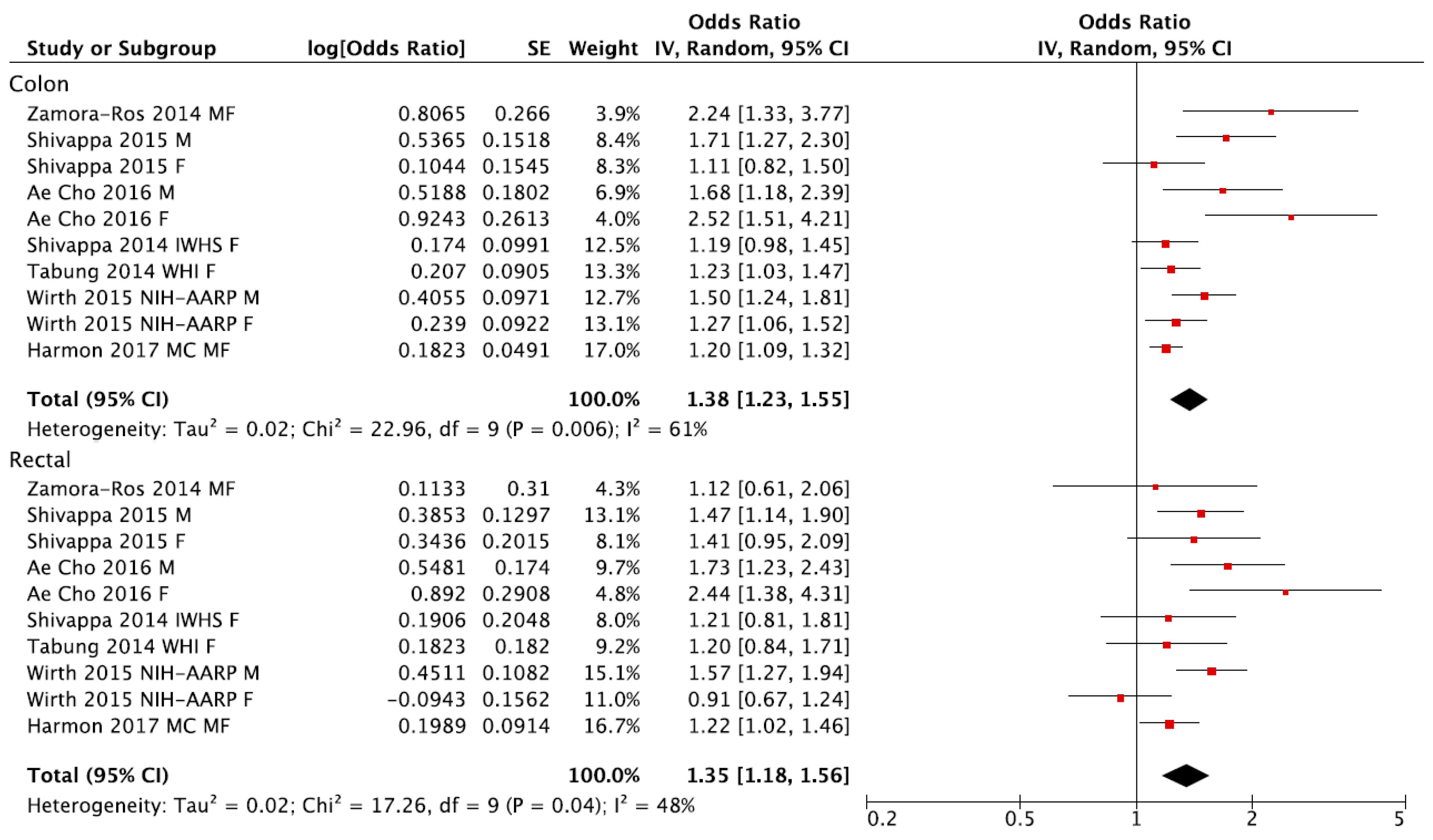
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IARC Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalance Worldwide 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 18 September 2017).
- Vogel, V.G.; McPherson, R.S. Dietary epidemiology of colon cancer. Hematol. Oncol. Clin. N. Am. 1989, 3, 35–63. [Google Scholar]
- Keibel, A.; Singh, V.; Sharma, M.C. Inflammation, microenvironment, and the immune system in cancer progression. Curr. Pharm. Des. 2009, 15, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Dushenkov, S.; Ho, C.T. Modulation of inflammatory genes by natural dietary bioactive compounds. J. Agric. Food Chem. 2009, 57, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Chang, Y.-F. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J. Surg. Oncol. 2003, 83, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Cheng, T.Y.; Neuhouser, M.L.; Wener, M.H.; Zheng, Y.; Brown, E.; Miller, J.W.; Song, X.; Beresford, S.A.A.; Gunter, M.J.; et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int. J. Cancer 2013, 132, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Oliveira, A.; Lopes, C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr. Res. 2013, 33, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; dos Santos, C.N.; Pinto, P.; Re, R.; Rémond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2015, 57, 2497–2525. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. Available online: http://www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf (accessed on 18 September 2017).
- Vuong, Q.V. Epidemiological evidence linking tea consumption to human health: a review. Crit. Rev. food Sci. Nutr. 2014, 54, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, Y.Y.; Wei, X.; Yu, F.F.; Zhou, Y.H.; He, J. Tea consumption and risk of cardiovascular outcomes and total mortality: A systematic review and meta-analysis of prospective observational studies. Eur. J. Epidemiol. 2015, 30, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Sciacca, S.; Pajak, A.; Martinez-Gonzalez, M.A.; Giovannucci, E.L.; Galvano, F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: A dose-response meta-analysis. Eur. J. Epidemiol. 2016, 31, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, in press. [Google Scholar] [CrossRef]
- Godos, J.; Bella, F.; Torrisi, A.; Sciacca, S.; Galvano, F.; Grosso, G. Dietary patterns and risk of colorectal adenoma: A systematic review and meta-analysis of observational studies. J. Hum. Nutr. Diet. 2016, 29, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in high-sensitivity c-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hebert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; Gonzalez-Gross, M.; Castillo, M.J.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Wirth, M.D.; Hurley, T.G.; Hebert, J.R. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999-2002. Mol. Nutr. Food Res. 2017, 61, 4. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Shivappa, N.; Hekmatdoost, A.; Hebert, J.R.; Davoodi, S.H.; Sadeghi, M. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: Case-control study. Appl. Physiol. Nutr. Metab. 2017, 42, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Shivappa, N.; Berthon, B.S.; Gibson, P.G.; Hebert, J.R. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin. Exp. Allergy 2015, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Shivappa, N.; Davis, L.; Hurley, T.G.; Ortaglia, A.; Drayton, R.; Blair, S.N.; Hébert, J.R. Construct Validation of the Dietary Inflammatory Index among African Americans. J. Nutr. Health Aging 2017, 21, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Burch, J.; Shivappa, N.; Violanti, J.M.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Hartley, T.A.; Miller, D.B.; Mnatsakanova, A.; et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J. Occup. Environ. Med./Am. Coll. Occup. Environ. Med. 2014, 56, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Polesel, J.; Zucchetto, A.; Crispo, A.; Montella, M.; Franceschi, S.; Rossi, M.; La Vecchia, C.; Serraino, D. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. Br. J. Nutr. 2016, 115, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Rosato, V.; Rossi, M.; Montella, M.; Serraino, D.; La Vecchia, C. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer Causes Control 2016, 27, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Rosato, V.; Serraino, D.; La Vecchia, C. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer Causes Control 2016, 27, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Graffouillere, L.; Deschasaux, M.; Mariotti, F.; Neufcourt, L.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Latino-Martel, P.; Hercberg, S.; Galan, P.; et al. Prospective association between the Dietary Inflammatory Index and mortality: Modulation by antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Blair, C.K.; Prizment, A.E.; Jacobs, D.R., Jr.; Steck, S.E.; Hebert, J.R. Association between inflammatory potential of diet and mortality in the Iowa Women’s Health study. Eur. J. Nutr. 2016, 55, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hussey, J.R.; Ma, Y.; Hebert, J.R. Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in national health and nutrition examination survey iii study. Eur. J. Nutr. 2015, 56, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Shivappa, N.; Hebert, J.R.; Bellomi, M.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Palli, D.; Veronesi, G.; Gnagnarella, P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the cosmos screening study. Eur. J. Nutr. 2016, 55, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Touvier, M.; Neufcourt, L.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Long-term association between the dietary inflammatory index and cognitive functioning: Findings from the su.Vi.Max study. Eur. J. Nutr. 2017, 56, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Ruiz-Canela, M.; de la Fuente-Arrillaga, C.; Gea, A.; Shivappa, N.; Hebert, J.R.; Martinez-Gonzalez, M.A. Dietary inflammatory index, cardiometabolic conditions and depression in the seguimiento universidad de navarra cohort study. Br. J. Nutr. 2015, 114, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Steck, S.E.; Hofseth, L.J.; Shehadah, I.; Bani-Hani, K.E.; Al-Jaberi, T.; Al-Nusairr, M.; Heath, D.; Tayyem, R. Dietary inflammatory index and odds of colorectal cancer in a case-control study from Jordan. Appl. Physiol. Nutr. Metab. 2017, 42, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.E.; Wirth, M.D.; Boushey, C.J.; Wilkens, L.R.; Draluck, E.; Shivappa, N.; Steck, S.E.; Hofseth, L.; Haiman, C.A.; Le Marchand, L.; et al. The dietary inflammatory index is associated with colorectal cancer risk in the multiethnic cohort. J. Nutr. 2017, 147, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hebert, J.R. The dietary inflammatory index is associated with colorectal cancer in the national institutes of health-american association of retired persons diet and health study. Br. J. Nutr. 2015, 113, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Caan, B.; Hou, L.; Johnson, K.C.; Mossavar-Rahmani, Y.; Shivappa, N.; et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the women’s health initiative. Cancer Causes Control 2015, 26, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Shivappa, N.; Steck, S.E.; Canzian, F.; Landi, S.; Alonso, M.H.; Hebert, J.R.; Moreno, V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the bellvitge colorectal cancer case-control study. Genes Nutr. 2015, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Prizment, A.E.; Blair, C.K.; Jacobs, D.R., Jr.; Steck, S.E.; Hebert, J.R. Dietary inflammatory index and risk of colorectal cancer in the iowa women’s health study. Cancer Epidemiol. Prev. Biomark. 2014, 23, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Shin, A.; Kim, J. Dietary inflammatory index and risk of colorectal cancer: A case-control study in Korea. Nutrients 2016, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Wang, P.P.; Zhu, Y.; Woodrow, J.R.; Mulay, S.; Parfrey, P.S.; McLaughlin, J.R.; Hebert, J.R.; Shivappa, N.; Li, Y.; et al. Inflammatory diet and risk of colorectal cancer: A population based case-control study in newfoundland, Canada. Nutrition 2017. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Zucchetto, A.; Montella, M.; Serraino, D.; Steck, S.E.; La Vecchia, C.; Hebert, J.R. Inflammatory potential of diet and risk of colorectal cancer: A case-control study from italy. Br. J. Nutr. 2015, 114, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wells GA, S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 1999. [Google Scholar]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The healthy eating index: Design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a dash-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Boushey, C.J.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. High-quality diets associate with reduced risk of colorectal cancer: Analyses of diet quality indexes in the multiethnic cohort. Gastroenterology 2017. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Neuhouser, M.L.; George, S.M.; Thomson, C.A.; Ho, G.Y.; Rohan, T.E.; Kato, I.; Nassir, R.; Hou, L.; Manson, J.E. Diet quality and colorectal cancer risk in the women’s health initiative observational study. Am. J. Epidemiol. 2016, 184, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Hebert, J.R.; Shivappa, N.; Hand, G.A.; Hurley, T.G.; Drenowatz, C.; McMahon, D.; Shook, R.P.; Blair, S.N. Anti-inflammatory dietary inflammatory index scores are associated with healthier scores on other dietary indices. Nutr. Res. 2016, 36, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Bassett, J.K.; Shivappa, N.; Hebert, J.R.; English, D.R.; Giles, G.G.; Severi, G. Dietary inflammatory index, mediterranean diet score, and lung cancer: A prospective study. Cancer Causes Control 2016, 27, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Zazpe, I.; Shivappa, N.; Hebert, J.R.; Sanchez-Tainta, A.; Corella, D.; Salas-Salvado, J.; Fito, M.; Lamuela-Raventos, R.M.; Rekondo, J.; et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the predimed (prevencion con dieta mediterranea) trial. Br. J. Nutr. 2015, 113, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.; Assmann, K.E.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Hercberg, S.; Touvier, M.; Kesse-Guyot, E. Long-term associations between inflammatory dietary scores in relation to long-term c-reactive protein status measured 12 years later: Findings from the supplementation en vitamines et mineraux antioxydants (su.Vi.Max) cohort. Br. J. Nutr. 2017, 117, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkanen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome—The insulin resistance atherosclerosis study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Bruce, W.R.; Giacca, A.; Medline, A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidem. Biomar. 2000, 9, 1271–1279. [Google Scholar]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Rio, D.D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Wagner Robb, S.; Hebert, J.R.; Huang, H.; Wirth, M.D.; Shivappa, N.; Ebell, M.H. The association between dietary inflammatory index scores and the prevalence of colorectal adenoma. Public Health Nutr. 2017, 20, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Sardo Molmenti, C.L.; Steck, S.E.; Thomson, C.A.; Hibler, E.A.; Yang, J.; Shivappa, N.; Greenlee, H.; Wirth, M.D.; Neugut, A.I.; Jacobs, E.T.; et al. Dietary inflammatory index and risk of colorectal adenoma recurrence: A pooled analysis. Nutr. Cancer 2017, 69, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Yen, M.F.; Wang, W.M.; Wong, J.M.; Chen, T.H. A case-cohort study for the disease natural history of adenoma-carcinoma and de novo carcinoma and surveillance of colon and rectum after polypectomy: Implication for efficacy of colonoscopy. Br. J. Cancer 2003, 88, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Xirasagar, S.; Li, Y.-T.; Burch, J.B.; Daguise, V.; Hurley, T.G.; Hebert, J.R. Reducing colorectal cancer incidence and disparities: Performance and outcomes of a screening colonoscopy program in south carolina. Adv. Public Health 2014. [Google Scholar] [CrossRef] [PubMed]
- Xirasagar, S.; Hurley, T.G.; Sros, L.; Hebert, J.R. Quality and safety of screening colonoscopies performed by primary care physicians. Med. Care 2010, 48, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Gilsing, A.M.; Fransen, F.; de Kok, T.M.; Goldbohm, A.R.; Schouten, L.J.; de Bruine, A.P.; van Engeland, M.; van den Brandt, P.A.; de Goeij, A.F.; Weijenberg, M.P. Dietary heme iron and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis 2013, 34, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, P.P.; Zhao, J.; Green, R.; Sun, Z.; Roebothan, B.; Squires, J.; Buehler, S.; Dicks, E.; Zhao, J.; et al. Dietary n-nitroso compounds and risk of colorectal cancer: A case-control study in newfoundland and labrador and ontario, canada. Br. J. Nutr. 2014, 111, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V.; Niaz, M.S.; Ramesh, A. Polycyclic aromatic hydrocarbons and digestive tract cancers: A perspective. J. Environ. Sci. Health Part C 2011, 29, 324–357. [Google Scholar] [CrossRef] [PubMed]
- Young, G.P.; Hu, Y.; Le Leu, R.K.; Nyskohus, L. Dietary fibre and colorectal cancer: A model for environment—Gene interactions. Mol. Nutr. Food Res. 2005, 49, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Neuhouser, M.L.; Cheng, T.D.; Tinker, L.F.; Shikany, J.M.; Snetselaar, L.; Martinez, J.A.; Kato, I.; Beresford, S.A.; Chapkin, R.S.; et al. The interaction between dietary fiber and fat and risk of colorectal cancer in the women’s health initiative. Nutrients 2016, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Gittelsohn, J.; Shankar, A.V.; Pokhrel, R.P.; West, K.P., Jr. Accuracy of estimating food intake by observation. J. Am. Diet. Assoc. 1994, 94, 1273–1277. [Google Scholar] [CrossRef]
- Hebert, J.R.; Ebbeling, C.B.; Matthews, C.E.; Hurley, T.G.; Ma, Y.; Druker, S.; Clemow, L. Systematic errors in middle-aged women’s estimates of energy intake: Comparing three self-report measures to total energy expenditure from doubly labeled water. Anna. Epidemiol. 2002, 12, 577–586. [Google Scholar] [CrossRef]
- Hebert, J.R.; Ma, Y.; Clemow, L.; Ockene, I.S.; Saperia, G.; Stanek, E.J., III; Merriam, P.A.; Ockene, J.K. Gender differences in social desirability and social approval bias in dietary self-report. Am. J. Epidemiol. 1997, 146, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Metzner, H.L.; Lamphiear, D.E.; Hawthorne, V.M. Characteristics of individuals and long term reproducibility of dietary reports: The tecumseh diet methodology study. J. Clin. Epidemiol. 1990, 43, 1169–1178. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Lane, D.S.; Ho, G.Y.F.; Hou, L.; Snetselaar, L.; Ockene, J.K.; et al. Changes in the inflammatory potential of diet over time and risk of colorectal cancer in postmenopausal women. Am. J. Epidemiol. 2017. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Study Design | Study Cohort, Country | Sex, Age Range/Mean (Years) | No. of Individuals/Controls | No. of Cases | Follow-Up (Years) | No. of Food Parameters to Calculate DII | Adjustments |
|---|---|---|---|---|---|---|---|---|
| Shivappa et al. 2014 [45] | Cohort | Iowa Women’s Health Study, USA | Females, 62 ± 4 | 34,703 | 1636 | 19.6 | 37 | Age, BMI, smoking status, pack-years of smoking, education, hormone replacement therapy use, total energy intake, NSAIDs and history of diabetes. |
| Wirth et al. 2015 [42] | Cohort | NIH-AARP, USA | Both males and females. Age: 62 ± 5.4 | 489,422 | 6944 | 9.1 | 35 | Age, smoking status, BMI, self-reported diabetes, energy intake, physical activity, marital status, education, race and census-based income. |
| Harmon et al. 2017 [41] | Cohort | Multiethnic Cohort | Both males and females. Age: 45–75 | 190,963 | 4388 | 20 | 28 | Age, sex, BMI, race, self-reported previous diagnosis of diabetes, asthma, and heart attack; use of supplements; smoking status; family history of colon cancer; education; hormone (i.e., estrogen or progesterone) use; aspirin use. |
| Tabung et al. 2015 [43] | Cohort | Women’s Health Initiative, USA | Females. Age: 50–79 | 152,536 | 1920 | 11.3 | 32 | Age, total energy intake, body mass index, race/ethnicity, physical activity, educational level, smoking status, family history of colorectal cancer, hypertension, diabetes, arthritis, history of colonoscopy, history of occult blood tests, NSAID use, category and duration of estrogen use, category and duration of estrogen & progesterone use, dietary modification trial arm, hormone therapy trial arm and calcium and vitamin trial arm |
| Shivappa et al. 2015 [48] | Case-control | Italy | Both males and females. Age: Case-60 ± 10 Controls-56 ± 11 | 4154 controls | 1953 | - | 31 | Age, sex, study center, education, BMI, alcohol consumption, physical activity, history of colorectal cancer, and energy intake |
| Zamora-Ros et al. 2015 [44] | Case-control | Spain | Both males and females. Age: 65.8 ± 12 | 401 controls | 424 | - | 33 | Age, sex, total energy intake, BMI, first-degree family history of CRC, physical activity, tobacco use, and medication use (aspirin and NSAID) |
| Cho et al. 2016 [46] | Case-control | South Korea | Both males and females. Age: Cases = 56.6 Control = 56.1 | 1846 controls | 923 | - | 36 | Age, sex, BMI, education, family history of colorectal cancer, physical activity, and total energy intake. |
| Shivappa et al. 2017 [40] | Case-control | Jordan | Both males and females. Age: Cases: 52 ± 11 Controls: 54 ± 12 | 202 controls | 153 | - | 18 | Age, sex, education, physical activity, body mass index, smoking, and family history of colorectal cancer. |
| Sharma et al. 2017 [47] | Case-control | Canada | Both males and females. Age: Cases: 62 ± 9 Controls: 60 ± 9 | 685 controls | 547 | - | 29 | Age, sex, BMI, physical activity, cholesterol level, triglycerides, family history of CRC, polyps, diabetes, history of colon screening, smoking, alcohol consumption, regular use of NSAIDs, and reported HRT, females only. |
| Subgroup | No. of Datasets (No. of Studies) | RR (95% CI) | I2 (%) | Pheterogeneity |
|---|---|---|---|---|
| Colorectal | ||||
| Total | 13 (9) | 1.40 (1.26, 1.55) | 69% | 0.0001 |
| Study design | ||||
| Prospective | 6 (4) | 1.24 (1.15, 1.35) | 50% | 0.08 |
| Case-control | 7 (5) | 1.73 (1.46, 2.05) | 42% | 0.11 |
| Gender | ||||
| Men | 4 (4) | 1.51 (1.29, 1.75) | 68% | 0.02 |
| Women | 6 (6) | 1.25 (1.10, 1.41) | 61% | 0.02 |
| Geographical location | ||||
| North America | 7 (5) | 1.26 (1.16, 1.36) | 50% | 0.06 |
| Europe | 3 (2) | 1.57 (1.19, 2.07) | 61% | 0.08 |
| Asia | 3 (2) | 1.97 (1.57, 2.49) | 9% | 0.33 |
| Adjustment for smoking | ||||
| No | 4 (2) | 1.74 (1.34, 2.25) | 69% | 0.02 |
| Yes | 9 (7) | 1.28 (1.18, 1.40) | 52% | 0.03 |
| Adjustment for BMI | ||||
| No | 1 (1) | 1.65 (1.13, 2.42) | NA | NA |
| Yes | 12 (8) | 1.39 (1.25, 1.54) | 70% | 0.0001 |
| Adjustment for physical activity | ||||
| No | 3 (2) | 1.22 (1.12, 1.32) | 0% | 0.55 |
| Yes | 10 (7) | 1.51 (1.31, 1.74) | 70% | 0.0004 |
| Adjustment for NSAID | ||||
| No | 8 (5) | 1.50 (1.28, 1.75) | 76% | 0.0002 |
| Yes | 5 (4) | 1.25 (1.15, 1.37) | 21% | 0.28 |
| Colon | ||||
| Total | 10 (7) | 1.38 (1.23, 1.55) | 61% | 0.006 |
| Study design | ||||
| Prospective | 5 (4) | 1.25 (1.16, 1.35) | 11% | 0.34 |
| Case-control | 5 (3) | 1.70 (1.29, 2.24) | 62% | 0.03 |
| Gender | ||||
| Men | 3 (3) | 1.58 (1.36, 1.83) | 0% | 0.71 |
| Women | 5 (5) | 1.27 (1.10, 1.48) | 51% | 0.09 |
| Geographical location | ||||
| North America | 5 (4) | 1.25 (1.16, 1.35) | 11% | 0.34 |
| Europe | 3 (2) | 1.56 (1.06, 2.29) | 71% | 0.03 |
| Asia | 2 (1) | 1.97 (1.34, 2.90) | 39% | 0.20 |
| Adjustment for smoking | ||||
| No | 4 (2) | 1.62 (1.20, 2.19) | 66% | 0.03 |
| Yes | 6 (5) | 1.29 (1.16, 1.43) | 46% | 0.10 |
| Adjustment for BMI | ||||
| No | 0 (0) | NA | NA | NA |
| Yes | 10 (7) | 1.38 (1.23, 1.55) | 61% | 0.006 |
| Adjustment for physical activity | ||||
| No | 2 (2) | 1.20 (1.10, 1.31) | 0% | 0.94 |
| Yes | 8 (5) | 1.48 (1.27, 1.72) | 59% | 0.02 |
| Adjustment for NSAID | ||||
| No | 7 (4) | 1.43 (1.23, 1.66) | 58% | 0.03 |
| Yes | 3 (3) | 1.29 (1.07, 1.56) | 62% | 0.07 |
| Rectal | ||||
| Total | 10 (7) | 1.35 (1.18, 1.56) | 48% | 0.04 |
| Study design | ||||
| Prospective | 5 (4) | 1.23 (1.03, 1.47) | 54% | 0.07 |
| Case-control | 5 (3) | 1.55 (1.30, 1.85) | 7% | 0.36 |
| Gender | ||||
| Men | 3 (3) | 1.56 (1.35, 1.81) | 0% | 0.75 |
| Women | 5 (5) | 1.28 (0.97, 1.69) | 59% | 0.05 |
| Geographical location | ||||
| North America | 5 (4) | 1.23 (1.03, 1.47) | 54% | 0.07 |
| Europe | 3 (2) | 1.41 (1.15, 1.73) | 0% | 0.72 |
| Asia | 2 (1) | 1.90 (1.41, 2.56) | 3% | 0.31 |
| Adjustment for smoking | ||||
| No | 4 (2) | 1.60 (1.34, 1.91) | 4% | 0.37 |
| Yes | 6 (5) | 1.22 (1.04, 1.44) | 43% | 0.12 |
| Adjustment for BMI | ||||
| No | 0 (0) | NA | NA | NA |
| Yes | 10 (7) | 1.35 (1.18, 1.56) | 48% | 0.04 |
| Adjustment for physical activity | ||||
| No | 2 (2) | 1.22 (1.03, 1.43) | 0% | 0.97 |
| Yes | 8 (5) | 1.40 (1.17, 1.68) | 54% | 0.03 |
| Adjustment for NSAID | ||||
| No | 7 (4) | 1.43 (1.18, 1.73) | 58% | 0.03 |
| Yes | 3 (3) | 1.21 (1.04, 1.41) | 0% | 0.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk—A Meta-Analysis. Nutrients 2017, 9, 1043. https://doi.org/10.3390/nu9091043
Shivappa N, Godos J, Hébert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. Dietary Inflammatory Index and Colorectal Cancer Risk—A Meta-Analysis. Nutrients. 2017; 9(9):1043. https://doi.org/10.3390/nu9091043
Chicago/Turabian StyleShivappa, Nitin, Justyna Godos, James R. Hébert, Michael D. Wirth, Gabriele Piuri, Attilio F. Speciani, and Giuseppe Grosso. 2017. "Dietary Inflammatory Index and Colorectal Cancer Risk—A Meta-Analysis" Nutrients 9, no. 9: 1043. https://doi.org/10.3390/nu9091043





