Ginseng Extract G115 Attenuates Ethanol-Induced Depression in Mice by Increasing Brain BDNF Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals and Treatment
2.3. Behavioral Testing
2.3.1. Open Field Test
2.3.2. Forced Swimming Test
2.4. Western Immunoblotting
2.5. Statistical Analysis
3. Results
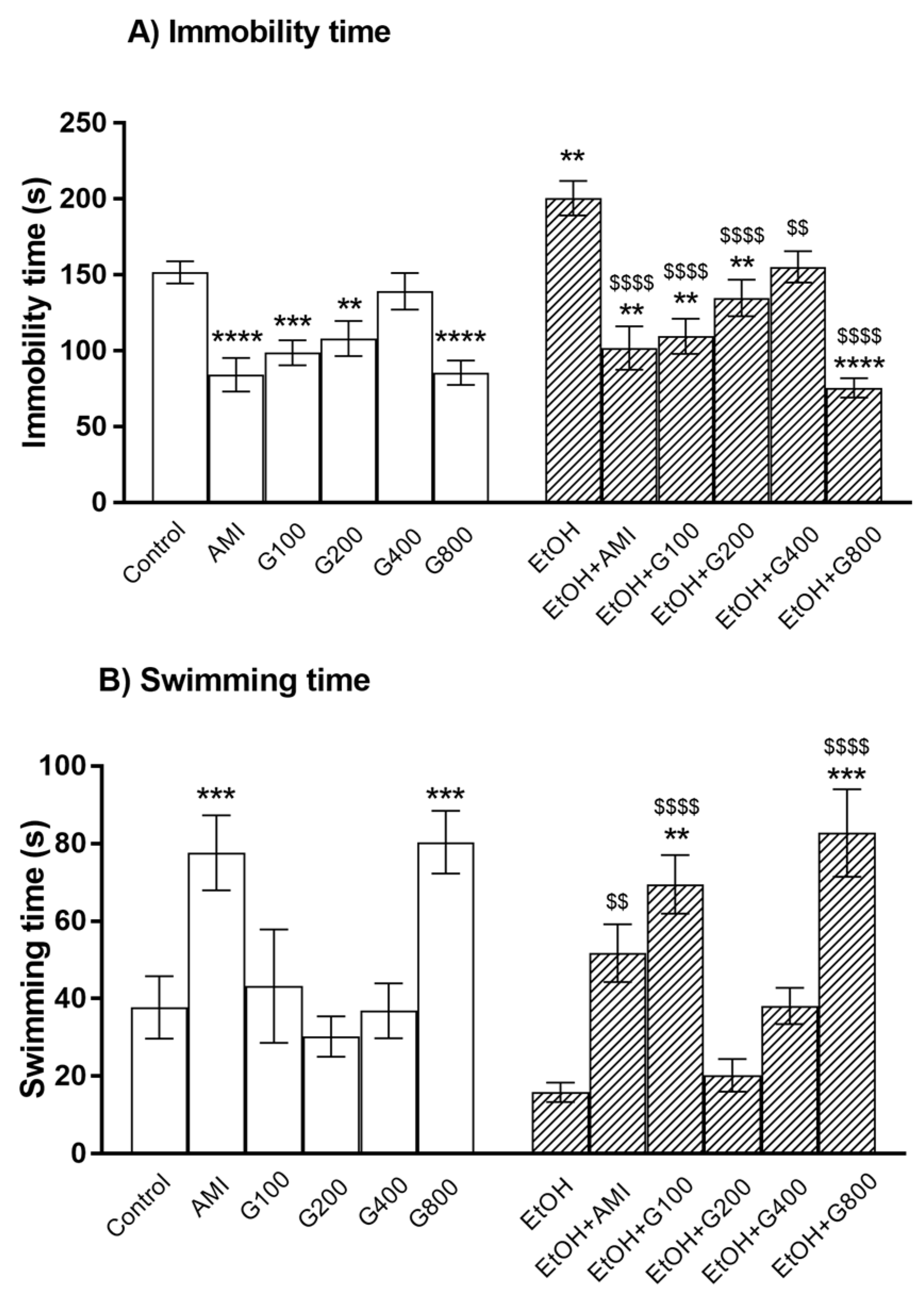
3.1. Forced Swimming Test
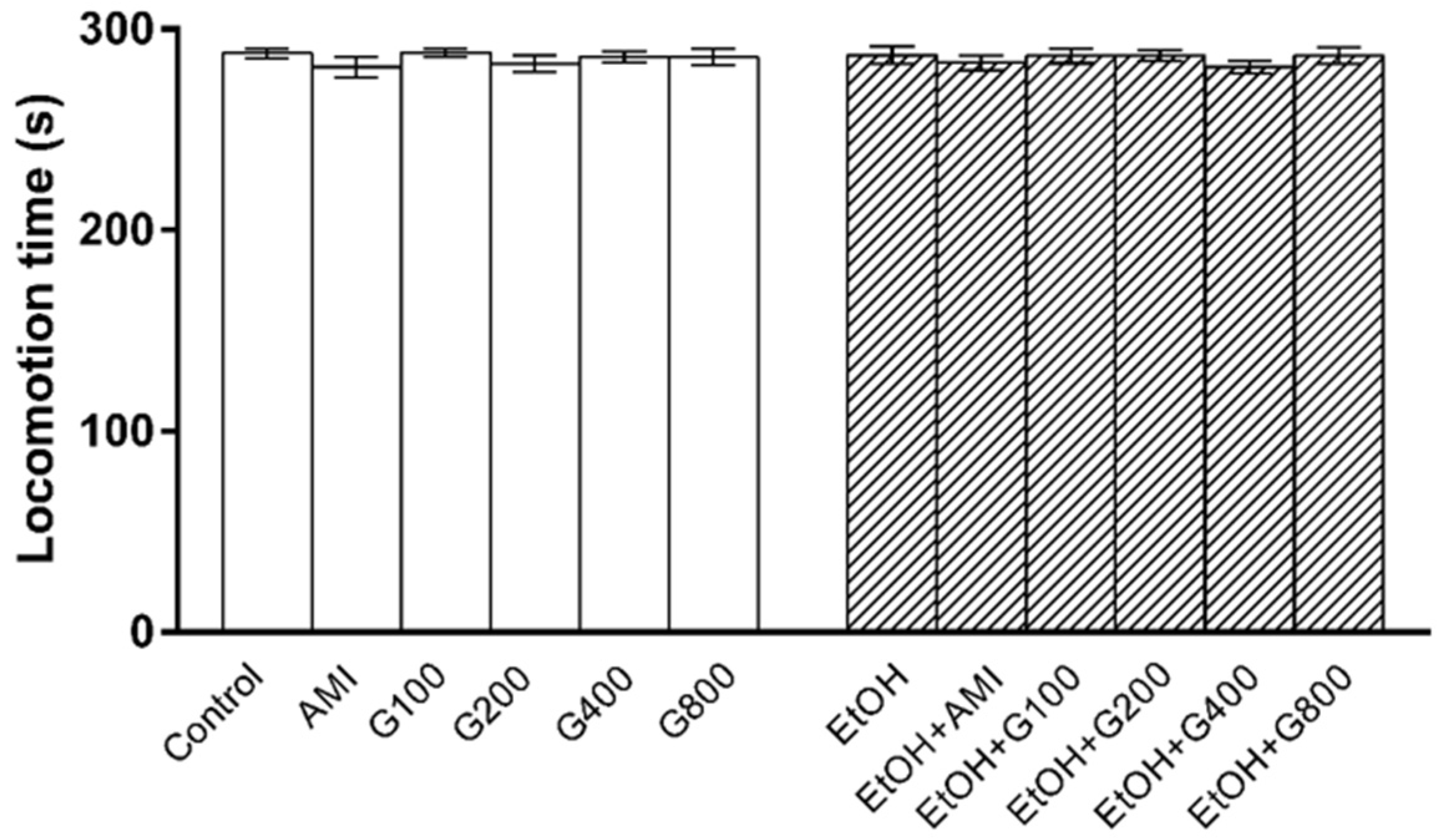
3.2. Locomotor Activity
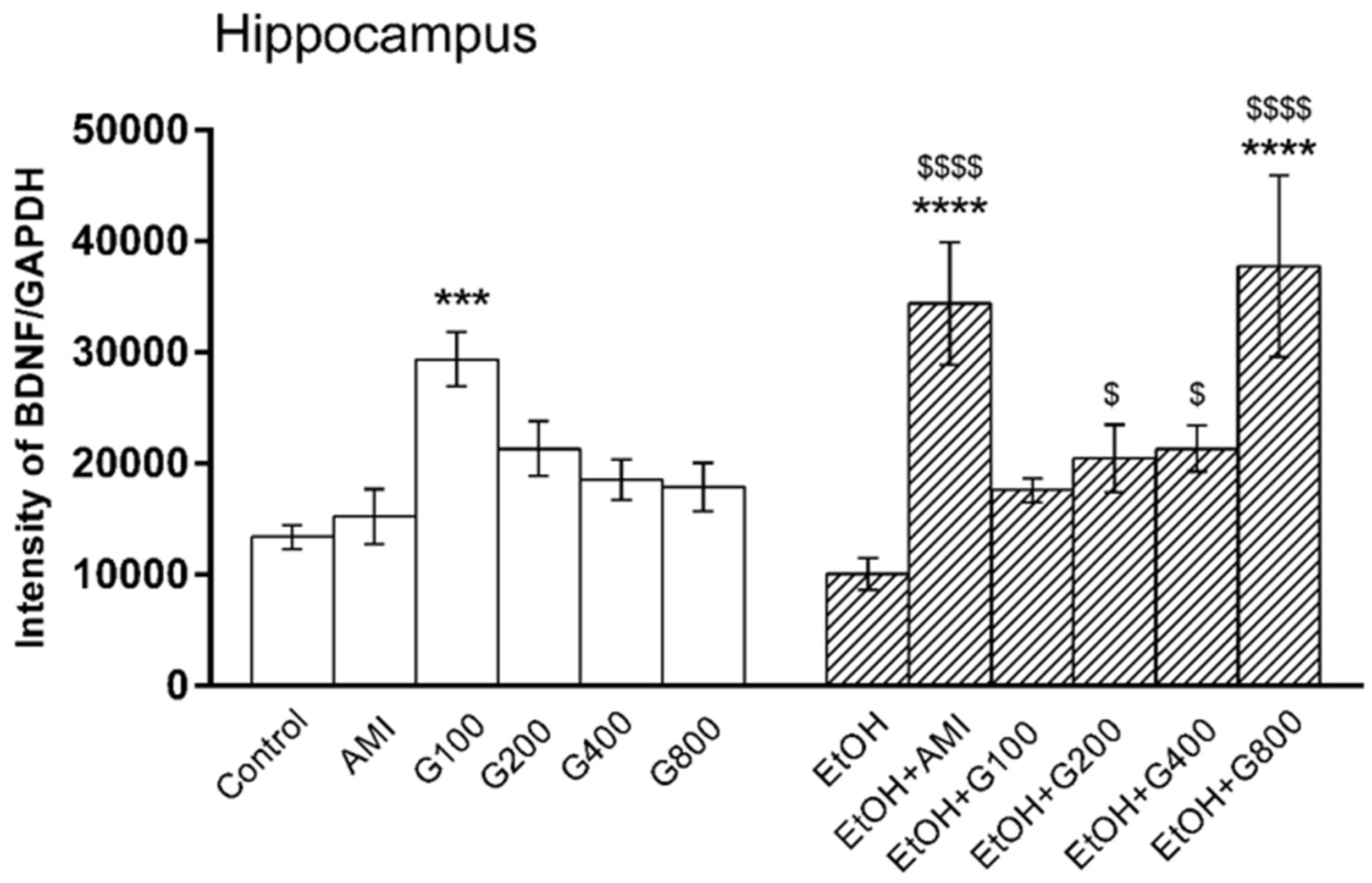
3.3. BDNF Levels in the Hippocampus
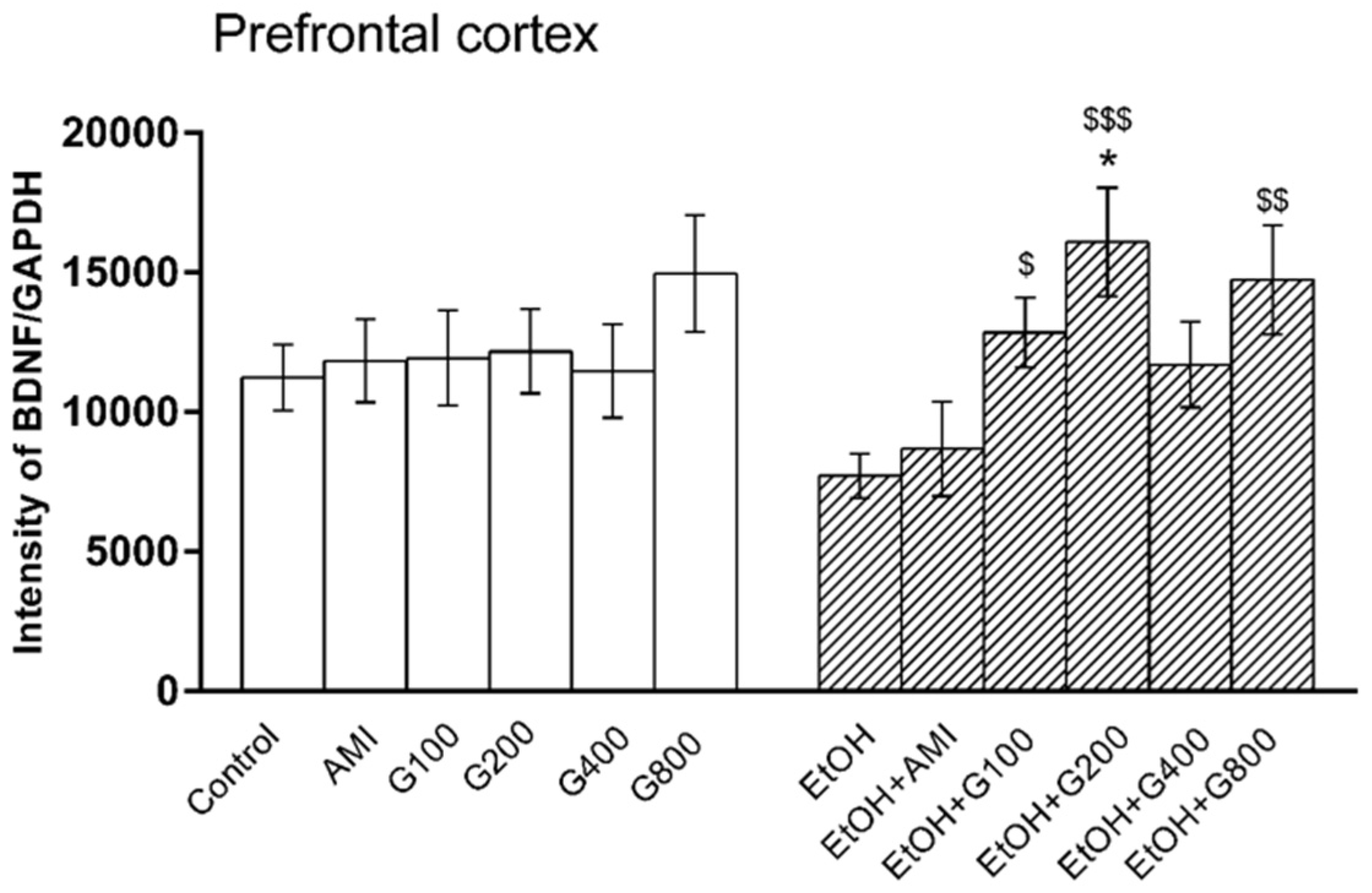
3.4. BDNF Levels in the Prefrontal Cortex
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixit, A.R.; Crum, R.M. Prospective study of depression and the risk of heavy alcohol use in women. Am. J. Psychiatry 2000, 157, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Massak, A.; Demers, A.; Rehm, J. Does the association between alcohol consumption and depression depend on how they are measured? Alcohol. Clin. Exp. Res. 2007, 31, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Preuss, U.W.; Schuckit, M.A.; Smith, T.L.; Danko, G.R.; Dasher, A.C.; Hesselbrock, M.N.; Hesselbrock, V.M.; Nurnberger, J.I., Jr. A comparison of alcohol-induced and independent depression in alcoholics with histories of suicide attempts. J. Stud. Alcohol. 2002, 63, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Litten, R.Z.; Fertig, J.; Mattson, M.; Egli, M. Development of medications for alcohol use disorders: Recent advances and ongoing challenges. Expert Opin. Emerg. Drugs 2005, 10, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, I.; Ralevski, E.; Nich, C.; Levinson, C.; Carroll, K.; Poling, J.; Rounsaville, B.; VA VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and current depression. J. Clin. Psychopharmacol. 2007, 27, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Pettinati, H.M. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol. Psychiatry 2004, 56, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Getachew, B.; Hauser, S.R.; Taylor, R.E.; Tizabi, Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol. Biochem. Behav. 2010, 96, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Briones, T.L.; Woods, J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience 2013, 254, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Mizui, T.; Ishikawa, Y.; Kumanogoha, H.; Kojima, M. Neurobiological actions by three distinct subtypes of brain-derived neurotrophic factor: Multi-ligand model of growth factor signaling. Pharmacol. Res. 2016, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Umene-Nakano, W.; Yoshimura, R.; Ikenouchi-Sugita, A.; Hori, H.; Hayashi, K.; Ueda, N.; Nakamura, J. Serum levels of brain-derived neurotrophic factor in comorbidity of depression and alcohol dependence. Hum. Psychopharmacol. 2009, 24, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.R.; Getachew, B.; Taylor, R.E.; Tizabi, Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol. Biochem. Behav. 2011, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.; Hu, C. Efficacy and safety of ginseng. Public Health Nutr. 2000, 3, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-F. Identification and quantification of ginsenosides in various commercial ginseng preparations. Eur. J. Pharm. Sci. 1995, 3, 77–85. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, 3638. [Google Scholar] [CrossRef] [PubMed]
- Umka, J.; Mustafa, S.; ElBeltagy, M.; Thorpe, A.; Latif, L.; Bennett, G.; Wigmore, P.M. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 2010, 166, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Chen, Y.; Liu, X.; Wang, Q.; Wang, L.; Jia, W.; Wang, Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xiong, Z.; Yang, J.; Wang, W.; Wang, Y.; Hu, Z.L.; Wang, F.; Chen, J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 2012, 166, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dai, J.; Wang, Z.; Zhang, H.; Huang, Y.; Zhao, Y. The antidepressant effects of ginseng total saponins in male C57BL/6N mice by enhancing hippocampal inhibitory phosphorylation of GSK-3b. Phytother. Res. 2014, 28, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Teng, J.; Chen, W.; Ge, Q.; Yang, Z.; Yu, C.; Yang, Z.; Jia, W. 20(S)-protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sang, W.; Yang, X.-S.; Zhai, M.-J.; Wang, L.-L.; Qin, P.-Y.; Wu, L.; Zhou, X.-R.; Wang, L.-J.; Li, J.-Y.; et al. Antidepressant effects of ginsenosides from Panax notoginseng. J. Integr. Agric. 2012, 11, 483–488. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Calabrese, F.; Bedogni, F.; Tongiorgi, E.; Fumagalli, F.; Racagni, G.; Riva, M.A. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int. J. Neuro-Psychopharmacol. 2006, 9, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Reus, G.Z.; Dos Santos, M.A.; Abelaira, H.M.; Ribeiro, K.F.; Petronilho, F.; Vuolo, F.; Colpo, G.D.; Pfaffenseller, B.; Kapczinski, F.; Dal-Pizzol, F.; et al. Imipramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav. Brain Res. 2013, 242, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, Y.; Chen, A.C.H.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [PubMed]
- Murakami, S.; Imbe, H.; Morikawa, Y.; Kubo, C.; Senba, E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005, 53, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Rantamäki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Z.-Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lyon, L.; ElBeltagy, M.; Umka, J.; Markwick, R.; Startin, C.; Bennett, G.; Wigmore, P. Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology 2011, 215, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Sairanen, M.; O’Leary, O.F.; Knuuttila, J.E.; Castren, E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience 2007, 144, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kozisek, M.E.; Middlemas, D.; Bylund, D.B. The differential regulation of BDNF and TrkB levels in juvenle rats four days of escitalopram and desipramine treatment. Neuropharmacology 2008, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, G.; Tang, C.; Wang, L.; Cheng, H.; Zhang, Y.; Ma, J.; Shi, M.; Zhao, G. Ginsenoside Rd maintains adult neural stem cell proliferation during lead-impaired neurogenesis. Neurol. Sci. 2013, 34, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Zhou, X.-Y.; Hou, J.-C.; Zhu, H.; Wang, Z.; Liu, J.-X.; Zheng, Y.-Q. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol. Sin. 2015, 36, 421–428. [Google Scholar] [CrossRef] [PubMed]
| Groups | Treatment | Groups | Treatment |
|---|---|---|---|
| Normal Mice | Ethanol-Treated Mice | ||
| 1. Control | Water p.o. + Water i.p. | 7. EtOH | Water p.o. + Ethanol i.p. |
| 2. AMI | Amitriptyline p.o. + Water i.p. | 8. EtOH + AMI | Amitriptyline p.o. + Ethanol i.p. |
| 3. G100 | G115 100 mg/kg p.o. + Water i.p. | 9. EtOH + G100 | G115 100 mg/kg p.o. + Ethanol i.p. |
| 4. G200 | G115 200 mg/kg p.o. + Water i.p. | 10. EtOH + G200 | G115 200 mg/kg p.o. + Ethanol i.p. |
| 5. G400 | G115 400 mg/kg p.o. + Water i.p. | 11. EtOH + G400 | G115 400 mg/kg p.o. + Ethanol i.p. |
| 6. G800 | G115 800 mg/kg p.o. + Water i.p. | 12. EtOH + G800 | G115 800 mg/kg p.o. + Ethanol i.p. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonlert, W.; Benya-aphikul, H.; Umka Welbat, J.; Rodsiri, R. Ginseng Extract G115 Attenuates Ethanol-Induced Depression in Mice by Increasing Brain BDNF Levels. Nutrients 2017, 9, 931. https://doi.org/10.3390/nu9090931
Boonlert W, Benya-aphikul H, Umka Welbat J, Rodsiri R. Ginseng Extract G115 Attenuates Ethanol-Induced Depression in Mice by Increasing Brain BDNF Levels. Nutrients. 2017; 9(9):931. https://doi.org/10.3390/nu9090931
Chicago/Turabian StyleBoonlert, Weerawan, Hattaya Benya-aphikul, Jariya Umka Welbat, and Ratchanee Rodsiri. 2017. "Ginseng Extract G115 Attenuates Ethanol-Induced Depression in Mice by Increasing Brain BDNF Levels" Nutrients 9, no. 9: 931. https://doi.org/10.3390/nu9090931






