Effects of Simulated Human Gastrointestinal Digestion of Two Purple-Fleshed Potato Cultivars on Anthocyanin Composition and Cytotoxicity in Colonic Cancer and Non-Tumorigenic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Computer Controlled Dynamic Human Gastrointestinal Model
2.3. LC-ESI-TOF-MS Analysis
2.4. Ferric Reducing Antioxidant Power Assay
2.5. Cytotoxic Effects of FW with and without Purple Potato Digests on Caco-2 and CCD112-CoN Cells
3. Results
3.1. Anthocyanins in Digested Amachi and Leona Cultivars
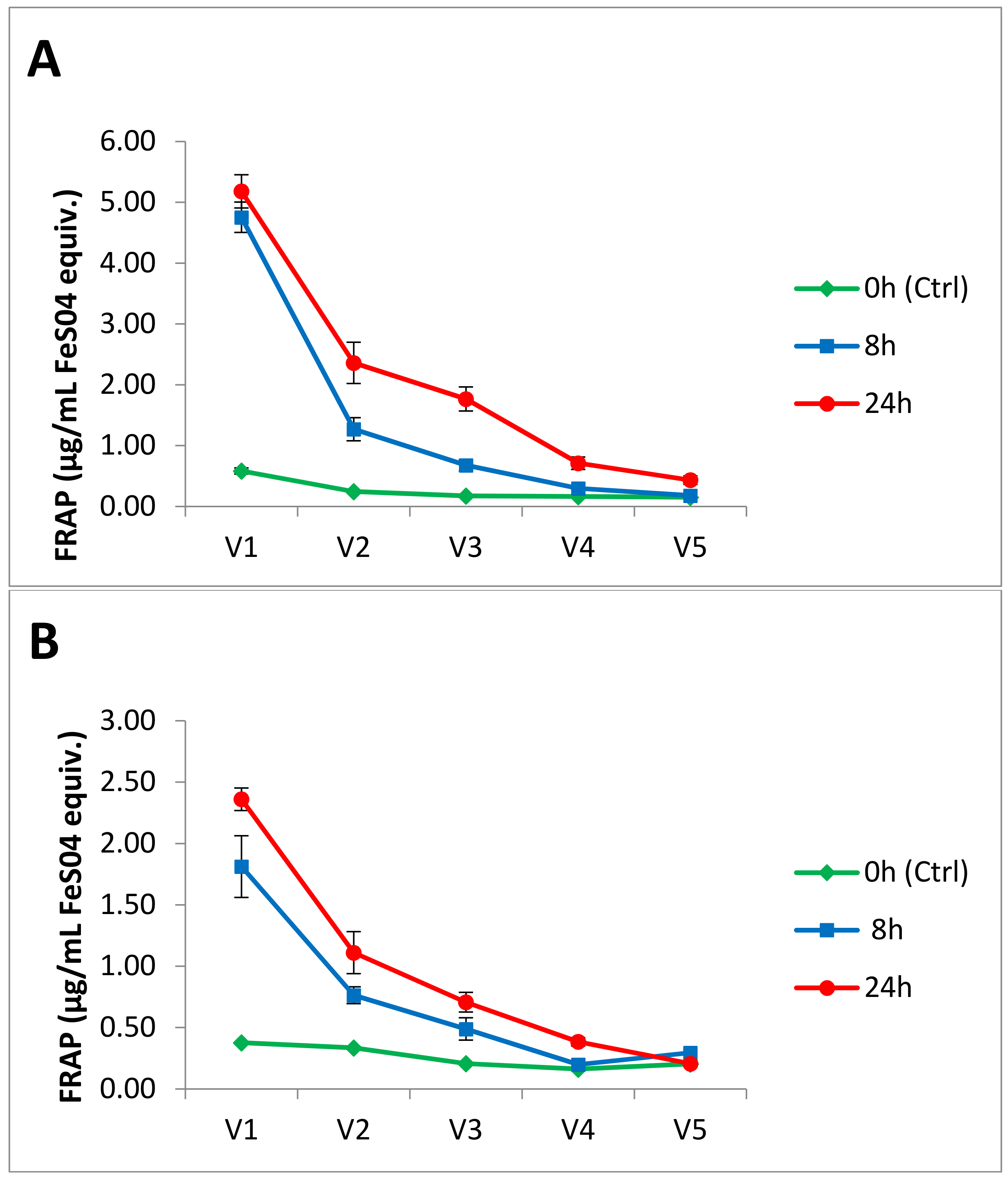
3.2. Antioxidant Activity
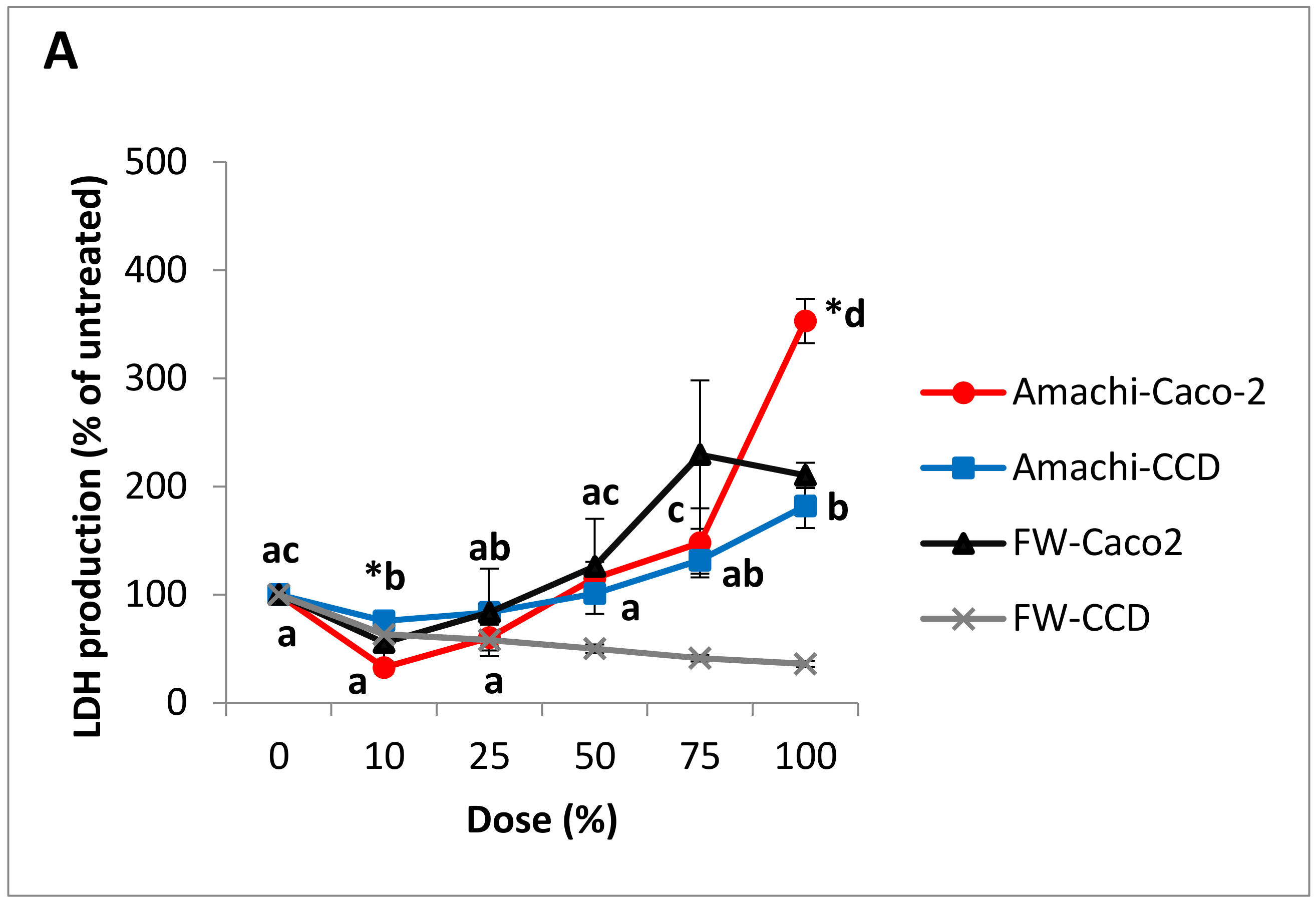
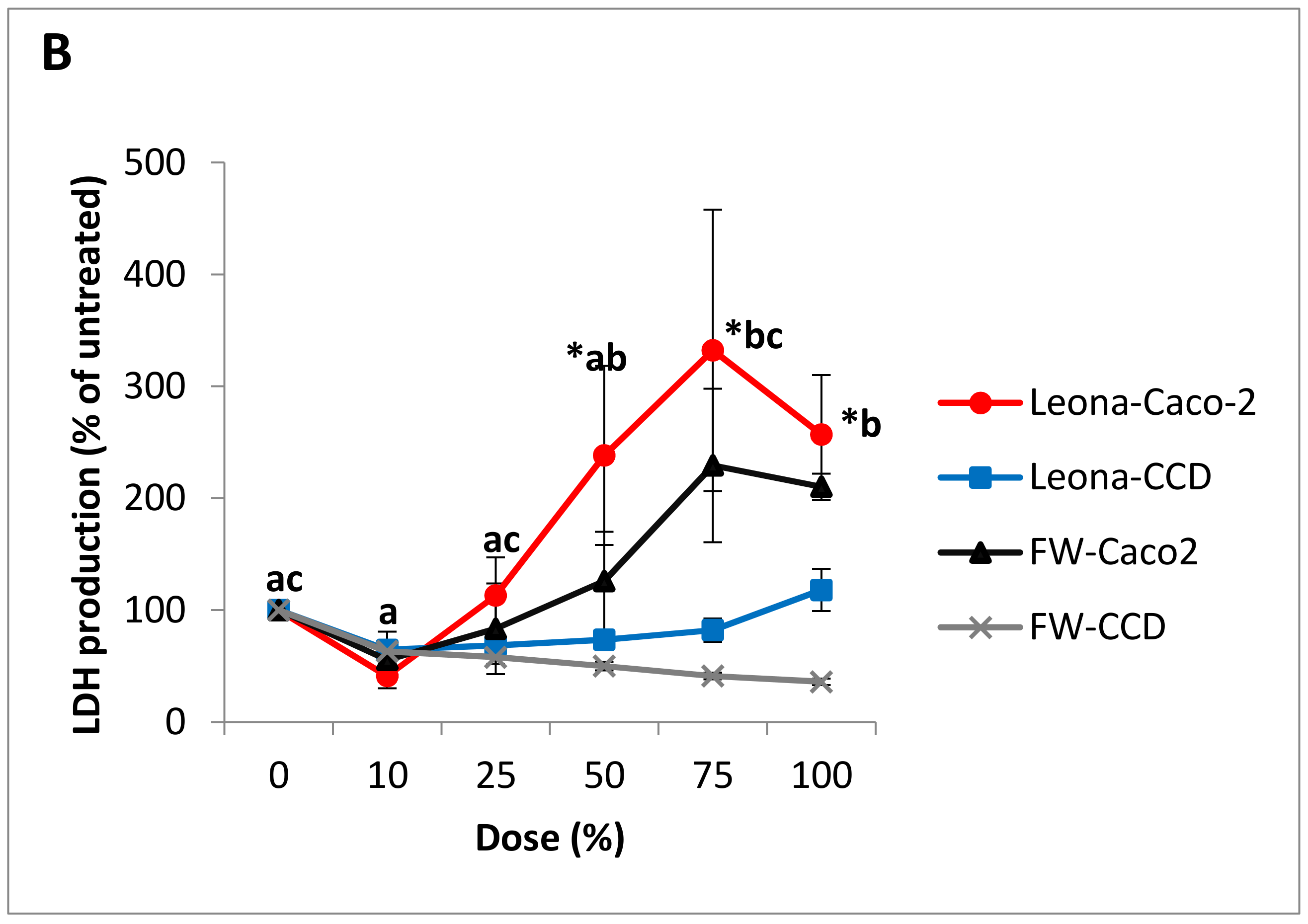
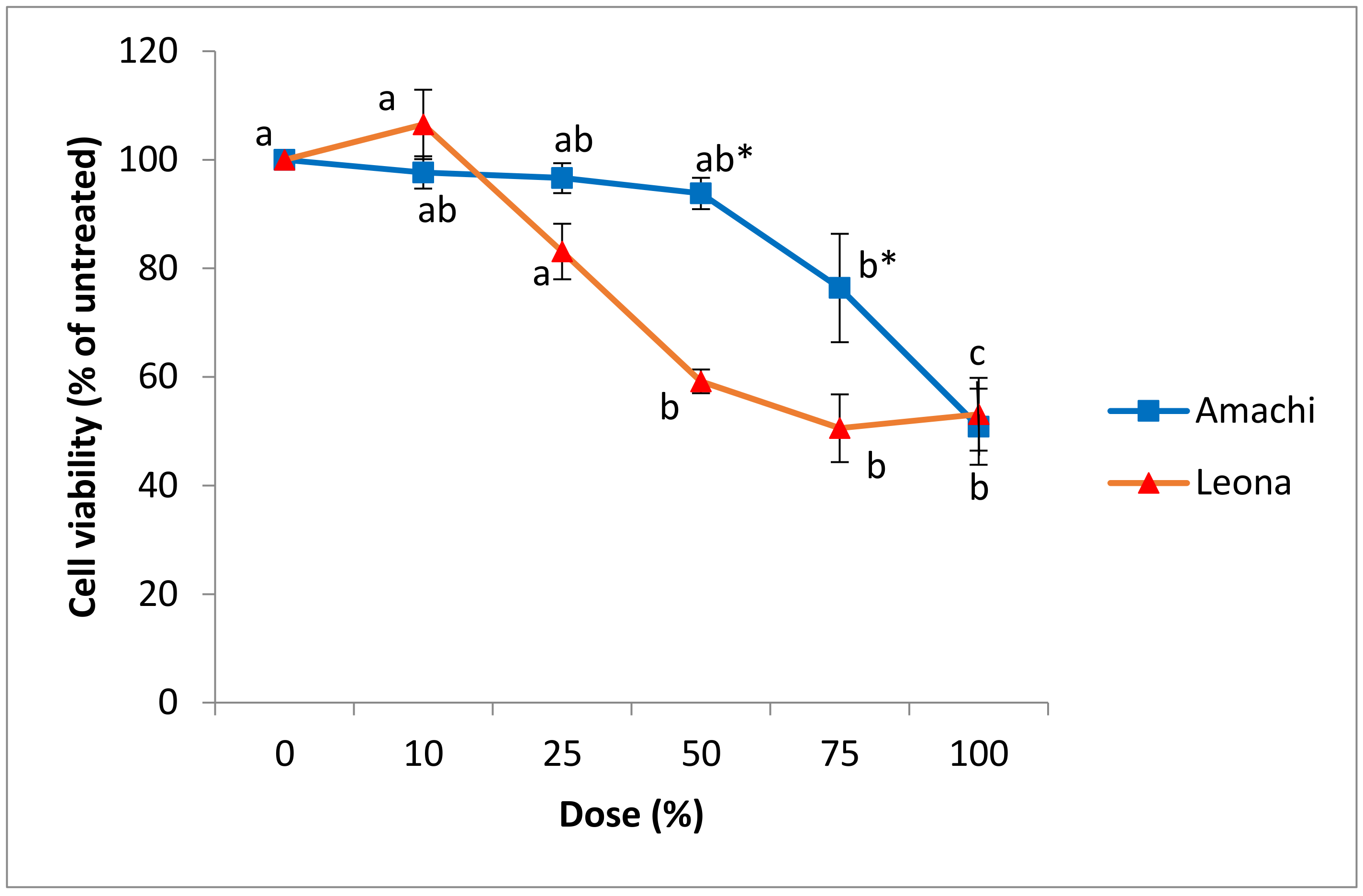
3.3. Cytotoxicity and Cell Viability in Caco-2 and CCD-112CoN Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.; Dao, L. Methods of Analysis for Functional Foods and Nutraceuticals, 1st ed.; Hurst, W.J., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Brown, C.R.; Wrolstad, R.; Durst, C.-P.; Yang, B.A. Clevidence, Breeding studies in potatoes containing high concentrations of anthocyanins. Am. J. Potato Res. 2003, 80, 241–250. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Andrenelli, L.; Vecchio, V.; Mulinacci, N. Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chem. 2011, 125, 750–759. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Michelozzi, M.; Cerratini, L.; Mulinacci, N. Coloured-fleshed potatoes after boiling: Promising sources of known antioxidant compounds. J. Food Compos. Anal. 2017, 59, 1–7. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, P.K.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an In Vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Podsȩdek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Blanquet-Diot, S.; Deat, E.; Jarrige, J.F.; Denis, S.; Beyssac, E.; Alric, M. Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: Application to the assessment of lycopene and alpha-tocopherol bioavailability from a whole food. J. Agric. Food Chem. 2009, 57, 11314–11320. [Google Scholar]
- Martoni, C.; Bhathena, J.; Jones, M.L.; Urbanska, A.M.; Chen, H.; Prakash, S. Investigation of microencapsulated BSH active Lactobacillus in the simulated human GI tract. J. Biomed. Biotechnol. 2007, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Molly, K.; Vande Woestyne, M.; De Smet, I.; Verstraete, W. Validation of the simulator of the human intestinal microbial ecosystem (SHIME) reactor using microorganism-associated activities. Microb. Ecol. Health Dis. 1994, 7, 191–200. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In Vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar]
- Noguer, M.; Cerezo, A.B.; Rentzsch, M.; Winterhalter, P.; Troncoso, A.M.; García-Parrilla, M.C. Simulated digestion and antioxidant activity of red wine fractions separated by high speed countercurrent chromatography. J. Agric. Food Chem. 2008, 56, 8879–8884. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Akoh, C.C.; Fischer, J.; Krewer, G. Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. J. Agric. Food Chem. 2007, 55, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrich, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Gupte, A.; Gates, L.; Mumper, R.J. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food Chem. Toxicol. 2009, 47, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Rugina, D.; Sconta, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Charepalli, V.; Reddivari, L.; Vadde, R.; Walia, S.; Radhakrishnan, S.; Vanamala, J.K.P. Eugenia jambolana (Java plum) fruit extract exhibits anti-cancer activity against early stage human HCT-116 colon cancer cells and colon cancer stem cells. Cancers 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.-Y.; Su, X.; Standard, J.; Edward, C.; Jason, G.; Betty, H.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- Bobe, G.; Want, B.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC(Min) mice fed suboptimal levels of sulindac. J. Agric. Food Chem. 2006, 54, 9322–9328. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.K.; Gupta, A.; Nines, R.G.; Kresty, L.A.; Habib, S.G.; Frankel, W.L.; LaPerle, K.; Gallaher, D.D.; Schwartz, S.J.; Stoner, G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer 2001, 40, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Miyashita, K.; Nakanishi, T.; Sano, M.; Tamano, S.; Kadota, T.; Koda, T.; Nakamura, M.; Imaida, K.; Ito, N.; et al. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-l-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1,2-dimethylhydrazine. Cancer Lett. 2001, 171, 17–25. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; He, M.; Mir, P.; Su, J.; Yang, Q. Inhibitory effect of antioxidant extracts from various potatoes on the proliferation of human colon and liver cancer cells. Nutr. Cancer 2011, 53, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Madiwale, G.P.; Reddivari, L.; Stone, M.; Holm, D.G.; Vanamala, J. Combined effects of storage and processing on the bioactive compounds and pro-apoptotic properties of color-fleshed potatoes in human colon cancer cells. J. Agric. Food Chem. 2012, 60, 11088–11096. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; Fratianni, F.; Granese, T.; Cardinale, F.; Cozzolino, A.; Nazzaro, F. In Vitro antioxidant, antimicrobial and anti-proliferative activities of purple potato extracts (Solanum tuberosum cv. Vitelotte noire) following simulated gastro-intestinal digestion. Nat. Prod. Res. 2014, 29, 1087–1091. [Google Scholar] [PubMed]
- Fonseca, C.; Burgos, G.; Rodríguez, F.; Muñoa, L.; Ordinola, M. Catálogo De Variedades De Papa Nativa Con Potencial Para La Seguridad Alimentaria Y Nutricional De Apurímac Y Huancavelica; Centro Internacional De La Papa: Lima, Peru, 2014. [Google Scholar]
- Porras, E.; Burgos, G.; Sosa, P.; Zum Felde, T. Procedures for Sampling and Sample Preparation of Sweetpotato Roots and Potato Tubers for Mineral Analysis; International Potato Center (CIP), Global Program Genetics and Crop Improvement: Lima, Peru, 2014; p. 13. ISBN 978-92-9060-445-7. [Google Scholar]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Konczak, I.; Schwartz, S. Probing anthocyanin profiles in purple sweet potato cell line (Ipomea batatas L. cv. Ayamurasaki) by high performance liquid chromatography and electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.P.; Mya, K.Y.; Win, K.Y.; Yeo, C.C.; Low, M.; He, C.; Han, M.-Y. Star-shaped polyhedral oligomeric silsesquioxane-polycaprolactone-polyurethane as biomaterials for tissue engineering application. NPG Asia Mater. 2014, 6, e142. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Grootjans, S.; Goossens, V.; Dondelinger, Y.; Krysko, D.V.; Takahashi, N.; Vandenabeele, P. Determination of apoptotic and necrotic cell death In Vitro and In Vivo. Methods 2013, 61, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Andre, C.M.; Oufir, M.; Guignard, C.; Hoffman, L.; Hausman, J.-F.; Evers, D.; Larondelle, Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of β-carotene, α-tocopherol, chlorogenic acid, petanin. J. Agric. Food Chem. 2007, 55, 10839–10849. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Polit, M.F.; Ayvaz, H.; Tay, D.; Manrique, I. Characterization and quantitation of anthocyanins and other phenolics in native Andean potatoes. J. Agric. Food Chem. 2014, 62, 4408–4416. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.; Winterhalter, P. Anthocyanins from pigmented potato (Solanum tuberosum L.) varieties. Food Res. Int. 2005, 38, 943–948. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.M.; Lee, K.G.; Shin, J.S.; Ahn, H.J.; Jeong, J.C.; Kwon, O.K.; Nam, J.H.; Lee, K.T.; Jang, D.S. p-Coumaroyl Anthocyanins from the Tuber Epidermis of a Colored Potato Solanum tuberosum L. cv Jayoung. Bull. Korean Chem. Soc. 2014, 35, 8. [Google Scholar] [CrossRef]
- Lewis, C.; Walker, J.; Lancaster, J.; Sutton, K. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Colored Cultivars of Solanum tuberosum L. J. Sci. Food Agric. 1998, 77, 45–57. [Google Scholar]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In Vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kubow, S.; Iskandar, M.M.; Sabally, K.; Azadi, B.; Sadeghi Ekbatan, S.; Kumarathasan, P.; Dhar Das, D.; Prakash, S.; Burgos, G.; Zum Felde, T. Biotransformation of anthocyanins from two purple-fleshed sweet potatoo accessions in a dynamic gastrointestinal system. Food Chem. 2016, 192, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.; Kroon, P.; Cassidy, A.; Colin, K. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vicente, A.; Gil-Izquierdo, A.; Garcia-Viguera, C. In Vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E. Investigating the In Vitro bioaccessibility of polyphenols in fresh and sun-dried figs (Ficus carica L.). Int. J. Food Sci. 2013, 48, 2621–2629. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In Vitro release properties of encapsulated blueberry (Vaccinium ashei) extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, J.; Coelho, G.; Crespo, M.E.; Cruz, T.; Rodríguez-Cabezas, M.E.; Concha, A.; Gonzalez, M.; Zarzuelo, A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001, 15, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Garsetti, M.; Pellegrini, N.; Baggio, C.; Brighenti, F. Antioxidant activity in human faeces. Br. J. Nutr. 2000, 84, 705–710. [Google Scholar] [PubMed]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent In Vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy–6H–dibenzopyran–6–one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Chengbin, X.; Wei, L.; Jianhong, W.; Xiangliang, Y.; Huibi, X. Chemoprotective effect of N-acetylcysteine (NAC) on cellular oxidative damages and apoptosis induced by nano titanium dioxide under UVA irradiation. Toxicol. In Vitro 2011, 25, 110–216. [Google Scholar]
- Lin, W.S.; Huang, Y.W.; Zhou, X.D.; Ma, Y.F. In Vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Briviba, K.S.; Abrahamse, L.; Pool-Zobel, B.L.; Rechkemmer, G. Neurotensin- and EGF-induced metabolic activation of colon carcinoma cells is diminished by dietary flavonoid cyanidin but not by its glycosides. Nutr. Cancer 2001, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Vareed, S.D.; Nair, M.G. Human tumor cell growth inhibition by nontoxic anthocyanidins in fruits and vegetables. Life Sci. 2005, 76, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Ohtani, K.; Ma, Y.; Kato, S.; Semba, S.; Katoh, T.; Wakamiya, N.; Taniguchi, T. Differential effects of cyanidin and cyanidin-3-glucoside on human cell lines. Food Sci. Technol. Res. 2011, 17, 515–521. [Google Scholar] [CrossRef]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.W.; Kawakishi, S.; Osawa, T. Antioxidative activity of the anthocyanin pigments, cyanidin-3-O-h-d-glucoside and cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Stushnoff, C.; Holm, D.; Thompson, M.D.; Jiang, W.; Thompson, H.J.; Joyce, N.I.; Wilson, P. Antioxidant properties of cultivars and selections from the Colorado potato breeding program. Am. J. Potato Res. 2008, 85, 267–276. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L.; Papucci, L.; Tani, A.; Schiavone, N.; Tempestini, A.; Orlandini, G.E.; Capaccioli, S.; Orlandini, S.Z. Aponecrosis: Morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J. Cell. Physiol. 2000, 182, 41–49. [Google Scholar] [CrossRef]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Demkosky, C.A.; Navarre, D.A.; Smyda, M.A. High-antioxidant potatoes: Acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects. J. Agric. Food Chem. 2012, 60, 6749–6754. [Google Scholar] [CrossRef] [PubMed]

| Measured Accurate Mass | Amachi | Leona | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m/z) 2 | Proposed Compound 3 | RT | V1 | V2 | V3 | V4 | V5 | V1 | V2 | V3 | V4 | V5 |
| 287.06 | Cyanidin 4 | 3.1 | 0.38 | - | - | - | - | 0.40 | - | - | 0.31 | 0.48 |
| 301.03 | Peonidin 5 | 4.8 | 0.47 | - | 0.35 | - | - | - | - | - | - | - |
| 433.11 | Pelargonidin 3-glucoside | 18 | - | - | - | 0.06 | 0.17 | - | - | 0.05 | 0.13 | 0.25 |
| 463.12 | Petunidin 3-rhamnoside | 18 | - | - | - | - | - | - | - | 0.03 | 0.10 | 0.16 |
| 583.14 | Peonidin 3-p-hydroxybenzoyl-glucoside | 5 | 0.16 | - | - | - | - | 0.07 | 0.14 | 0.14 | 0.22 | - |
| 610.16 | Pelargonidin 3-feruloyl-glucoside | 15 | 0.12 | - | - | - | - | 0.31 | 0.20 | 0.13 | 0.10 | 0.04 |
| 611.14 | Cyanidin 3-(6-caffeoyl-glucoside) | 15 | - | - | 0.17 | - | - | - | 0.09 | 0.02 | 0.03 | 0.04 |
| 611.16 | Cyanidin 3-sophoroside | 15 | - | 0.10 | 0.15 | - | - | - | 0.12 | 0.02 | 0.04 | 0.07 |
| 612.14 | Peonidin 3-(6-p-coumaroyl-glucoside) | 3.6 | - | - | 0.09 | - | - | 0.09 | 0.07 | - | - | - |
| 625.15 | Peonidin 3-(6-caffeoyl-glucoside) | 13 | 2.3 | - | - | - | - | - | - | - | - | - |
| 625.17 | Petunidin 3-rutinoside | 13 | 2.6 | - | - | - | - | - | - | - | - | - |
| 731.16 | Cyanidin 3-(6-caffeoyl-6-p-hydroxybenzoyl-glucoside) | 10 | 3.53 | - | 0.62 | - | - | 0.96 | - | - | - | - |
| 731.18 | Cyanidin 3-p-hydroxybenzoyl-sophoroside | 10 | 3.20 | - | 1.14 | - | - | 0.96 | - | - | - | - |
| 757.19 | Cyanidin 3-p-coumaroyl-sophoroside | 7 | 0.26 | - | - | - | - | - | - | - | - | - |
| 757.21 | Pelargonidin 3-sophoroside-5-glucoside | 7 | 0.28 | - | - | - | - | 0.07 | - | - | - | - |
| 771.21 | Peonidin 3-(6”-p-coumaryl-sophoroside) | 9 | 1.14 | - | - | - | - | 0.16 | - | 0.08 | - | - |
| 773.19 | Cyanidin 3-(6”-caffeoyl-sophoroside) | 15 | - | - | 0.53 | - | - | - | - | - | - | - |
| 773.21 | Cyanidin 3-sophoroside-5-glucoside | 7 | - | - | - | 1.11 | - | - | - | - | - | - |
| 787.20 | Peonidin 3-(6″-caffeoyl-sophoroside) | 7.7 | 4.55 | - | - | 0.23 | - | 0.26 | - | - | - | - |
| 787.22 | Petunidin 3-rutinoside-5-glucoside | 7.7 | 6.12 | - | - | 0.34 | - | 0.26 | - | - | - | - |
| 801.20 | Peonidin 3-caffeoyl-feruloyl-glucoside | 10 | 0.22 | - | - | - | - | - | - | - | - | - |
| 801.22 | Peonidin 3-(6”-feruloyl-sophoroside) | 10 | 0.30 | 0.07 | - | - | - | - | - | - | - | - |
| 893.21 | Cyanidin 3-(6”-caffeoyl-6”-p-hydroxybenzoyl-sophoroside) | 11 | - | - | 0.39 | 0.08 | - | - | - | - | - | - |
| 893.23 | Cyanidin 3-p-hydroxybenzoyl-sophoroside-5-glucoside | 11 | - | - | 0.39 | 0.07 | - | - | - | - | - | - |
| 903.23 | Cyanidin 3-(6’,6”-dicoumaroyl-sophoroside) | 20 | 2.20 | - | - | - | - | - | - | - | - | - |
| 907.22 | Peonidin 3-caffeoyl-p-hydroxybenzoyl-sophoroside | 13 | - | - | 0.04 | 0.02 | - | - | - | - | - | - |
| 907.25 | Peonidin 3-p-hydroxybenzoyl-sophoroside-5-glucoside | 20 | - | - | 0.02 | - | - | - | - | - | - | - |
| 917.27 | Peonidin 3-p-coumaroyl-rutinoside-5-glucoside | 22 | 16.42 | - | - | - | - | 0.06 | - | - | - | - |
| 919.25 | Cyanidin 3-p-coumaroyl-sophoroside-5-glucoside | 18 | 2.53 | - | - | - | - | - | - | - | - | - |
| 920.23 | Cyanidin 3-caffeoyl-p-coumaroyl-sophoroside | 18 | 1.11 | - | - | - | - | - | - | - | - | - |
| 931.25 | Cyanidin 3-feruloyl-sophoroside-5-glucoside | 20 | 0.09 | - | - | - | - | - | - | - | - | - |
| 933.26 | Petunidin 3-p-coumaroyl-rutinoside-5-glucoside | 20 | 132.79 | 0.40 | 0.07 | 0.01 | 0.02 | 0.15 | 0.13 | - | 0.04 | 0.26 |
| 949.23 | Peonidin 3-dicaffeoyl-sophoroside | 18 | 3.64 | - | - | - | - | - | - | - | - | - |
| 949.26 | Peonidin 3-(6″′-caffeoyl-sophoroside)-5-glucoside | 18 | 3.47 | - | - | - | - | - | - | - | - | - |
| 963.25 | Peonidin 3-caffeoyl-feruloyl-sophoroside | 20 | 4.39 | - | - | - | - | - | - | - | - | - |
| 963.27 | Peonidin 3-(6”-feruloyl-sophoroside)-5-glucoside | 20 | 4.45 | - | - | - | - | - | - | - | - | - |
| Total Anthocyanins Measured | 196.72 | 0.57 | 3.96 | 1.92 | 0.19 | 3.75 | 0.75 | 0.47 | 0.97 | 1.3 | ||
| Dose | Anthocyanin Concentration (mg/L Cyanidin 3-Rutinoside Equivalents) | |
|---|---|---|
| % FW in Cell Culture Media | Cv. Amachi | Cv. Leona |
| 0% | 0 | 0 |
| 10% | 0.203 | 0.091 |
| 25% | 0.507 | 0.228 |
| 50% | 1.013 | 0.457 |
| 75% | 1.520 | 0.685 |
| 100% | 2.027 | 0.913 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubow, S.; Iskandar, M.M.; Melgar-Bermudez, E.; Sleno, L.; Sabally, K.; Azadi, B.; How, E.; Prakash, S.; Burgos, G.; Felde, T.z. Effects of Simulated Human Gastrointestinal Digestion of Two Purple-Fleshed Potato Cultivars on Anthocyanin Composition and Cytotoxicity in Colonic Cancer and Non-Tumorigenic Cells. Nutrients 2017, 9, 953. https://doi.org/10.3390/nu9090953
Kubow S, Iskandar MM, Melgar-Bermudez E, Sleno L, Sabally K, Azadi B, How E, Prakash S, Burgos G, Felde Tz. Effects of Simulated Human Gastrointestinal Digestion of Two Purple-Fleshed Potato Cultivars on Anthocyanin Composition and Cytotoxicity in Colonic Cancer and Non-Tumorigenic Cells. Nutrients. 2017; 9(9):953. https://doi.org/10.3390/nu9090953
Chicago/Turabian StyleKubow, Stan, Michèle M. Iskandar, Emiliano Melgar-Bermudez, Lekha Sleno, Kebba Sabally, Behnam Azadi, Emily How, Satya Prakash, Gabriela Burgos, and Thomas zum Felde. 2017. "Effects of Simulated Human Gastrointestinal Digestion of Two Purple-Fleshed Potato Cultivars on Anthocyanin Composition and Cytotoxicity in Colonic Cancer and Non-Tumorigenic Cells" Nutrients 9, no. 9: 953. https://doi.org/10.3390/nu9090953







