Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Sampling
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. PUFA Levels and GNRI in the Survivor and Nonsurvivor Groups
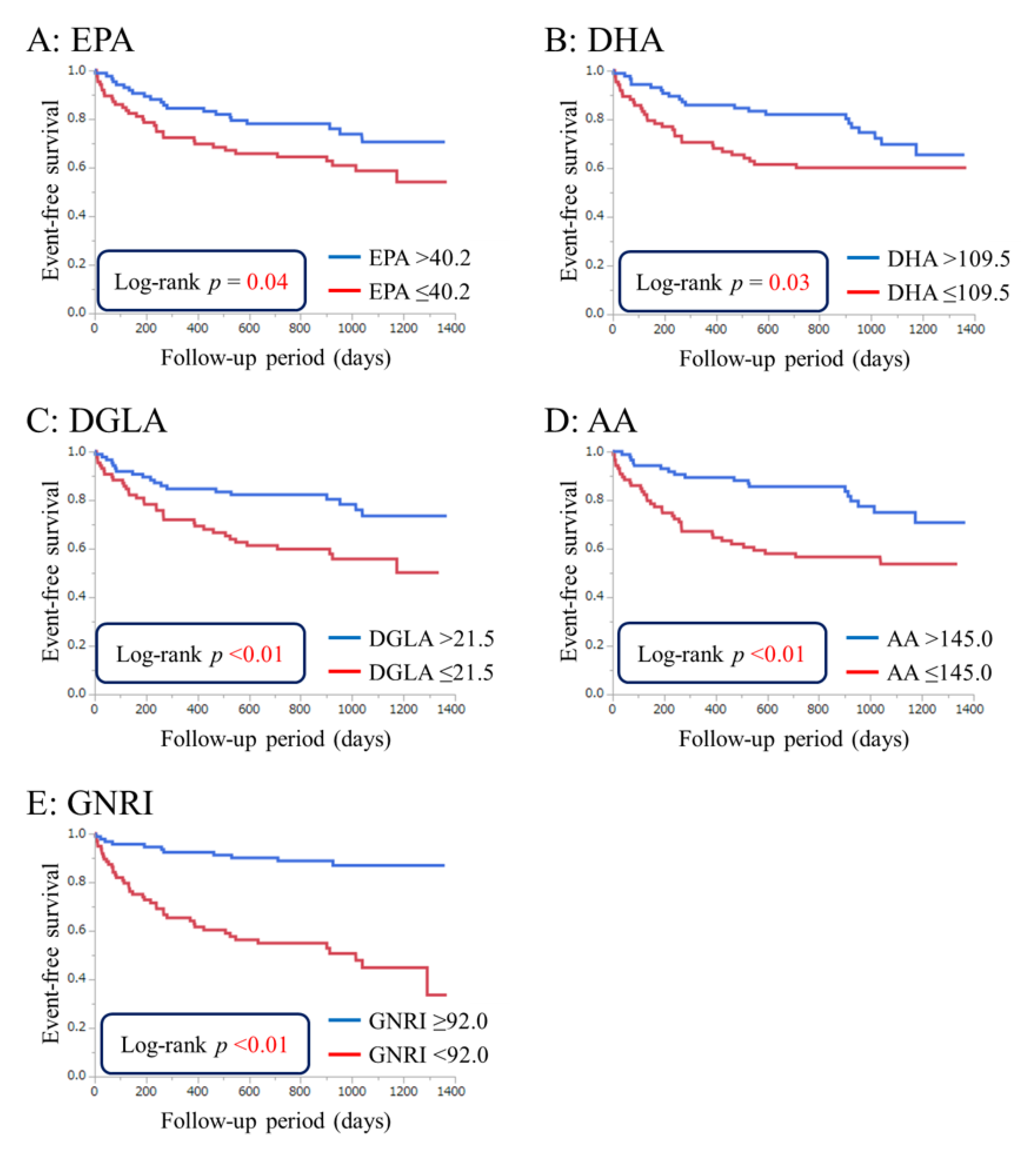
3.3. Cumulative Event-Free Survival Rates Based on PUFA Levels and GNRI
3.4. Effects of PUFA Levels on Event-Free Survival Independent of GNRI
3.5. Univariate and Multivariate Analyses of Parameters Contributing to Long-Term Mortality
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, H.; Zhang, H.; Lin, Z.; Li, X.; Kong, X.; Sun, G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail. Rev. 2016, 21, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Miyazaki, T.; Takagi, A.; Sugita, Y.; Yatsu, S.; Murata, A.; Kato, T.; Suda, S.; Ouchi, S.; Aikawa, T.; et al. Low circulating coenzyme Q10 during acute phase is associated with inflammation, malnutrition, and in-hospital mortality in patients admitted to the coronary care unit. Heart Vessels 2016, 32, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Agra Bermejo, R.M.; Gonzalez Ferreiro, R.; Varela Roman, A.; Gomez Otero, I.; Kreidieh, O.; Conde Sabaris, P.; Rodriguez-Manero, M.; Moure Gonzalez, M.; Seoane Blanco, A.; Virgos Lamela, A.; et al. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int. J. Cardiol. 2017, 230, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, N.; Nagai, T.; Furukawa, T.A.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Prognostic value of malnutrition assessed by controlling nutritional status score for long-term mortality in patients with acute heart failure. Int. J. Cardiol. 2017, 230, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin Nutr. 2005, 82, 777–783. [Google Scholar] [PubMed]
- Wada, H.; Dohi, T.; Miyauchi, K.; Doi, S.; Naito, R.; Konishi, H.; Tsuboi, S.; Ogita, M.; Kasai, T.; Hassan, A.; et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am. J. Cardiol. 2017, 119, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrolli, C.; Zagami, A.; Vanotti, A.; Piffer, S.; Faliva, M.; Rondanelli, M.; Caccialanza, R. Nutritional risk, functional status and mortality in newly institutionalised elderly. Br. J. Nutr. 2013, 110, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Klersy, C.; Pedrolli, C.; Cameletti, B.; Bonardi, C.; Quarleri, L.; Cappello, S.; Bonoldi, A.; Bonadeo, E.; Caccialanza, R. The geriatric nutritional risk index predicts hospital length of stay and in-hospital weight loss in elderly patients. Clin. Nutr. 2015, 34, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Vanotti, A. The new geriatric nutritional risk index is a good predictor of muscle dysfunction in institutionalized older patients. Clin. Nutr. 2007, 26, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pusani, C.; Limonta, D.; Vanotti, A. The association of geriatric nutritional risk index and total lymphocyte count with short-term nutrition-related complications in institutionalised elderly. J. Am. Coll. Nutr. 2008, 27, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Mauthner, O.; Claes, V.; Deschodt, M.; Jha, S.R.; Engberg, S.; Macdonald, P.S.; Newton, P.J.; De Geest, S. Handle with care: A systematic review on frailty in cardiac care and its usefulness in heart transplantation. Transplant. Rev. 2017, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R.; Group, N.S. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Hébuterne, X.; Elia, M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res. Rev. 2013, 12, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the american heart association nutrition subcommittee of the council on nutrition, physical activity, and metabolism; council on cardiovascular nursing; and council on epidemiology and prevention. Circulation 2009, 119, 902–907. [Google Scholar] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs lps dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Cialdella-Kam, L.; Nieman, D.C.; Knab, A.M.; Shanely, R.A.; Meaney, M.P.; Jin, F.; Sha, W.; Ghosh, S. A mixed flavonoid-fish oil supplement induces immune-enhancing and anti-inflammatory transcriptomic changes in adult obese and overweight women-a randomized controlled trial. Nutrients 2016, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, Y.; Tsugane, S.; Group, J.S. Intake of fish and n-3 fatty acids and risk of coronary heart disease among Japanese: The Japan public health center-based (JPHC) study cohort I. Circulation 2006, 113, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin e after myocardial infarction: Results of the gissi-prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Marchioli, R. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the gruppo italiano per lo studio della sopravvivenza nell’infarto miocardico (GISSI)-prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, R.; Levantesi, G.; Macchia, A.; Maggioni, A.P.; Marfisi, R.M.; Silletta, M.G.; Tavazzi, L.; Tognoni, G.; Valagussa, F.; Investigators, G.I.-P. Antiarrhythmic mechanisms of n-3 pufa and the results of the gissi-prevenzione trial. J. Membr. Biol. 2005, 206, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, Y.; Shimada, K.; Tani, S.; Ogawa, T.; Ando, J.; Takahashi, M.; Yamamoto, M.; Shinozaki, T.; Miyazaki, T.; Miyauchi, K.; et al. Association between the docosahexaenoic acid to arachidonic acid ratio and acute coronary syndrome: A multicenter observational study. BMC Cardiovasc. Disord. 2016, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Honda, Y.; Sugano, Y.; Nishimura, K.; Nakai, M.; Honda, S.; Iwakami, N.; Okada, A.; Asaumi, Y.; Aiba, T.; et al. Circulating omega-6, but not omega-3 polyunsaturated fatty acids, are associated with clinical outcomes in patients with acute decompensated heart failure. PLoS ONE 2016, 11, e0165841. [Google Scholar] [CrossRef] [PubMed]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar]
- Shimamoto, K.; Ando, K.; Fujita, T.; Hasebe, N.; Higaki, J.; Horiuchi, M. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens. Res. 2014, 37, 253–390. [Google Scholar] [PubMed]
- Yanagisawa, N.; Shimada, K.; Miyazaki, T.; Kume, A.; Kitamura, Y.; Ichikawa, R.; Ohmura, H.; Kiyanagi, T.; Hiki, M.; Fukao, K.; et al. Polyunsaturated fatty acid levels of serum and red blood cells in apparently healthy Japanese subjects living in an urban area. J. Atheroscler. Thromb. 2010, 17, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated gfr from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cereda, E.; Pedrolli, C. The geriatric nutritional risk index. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Mitmesser, S.H. Potential impact of nutrition on immune system recovery from heavy exertion: A metabolomics perspective. Nutrients 2017, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids—A review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Shi, J.J.; Li, Y.M.; Zhang, X.Y.; Chen, Y.; Calder, P.C.; Tang, L.J. What is the impact of n-3 pufas on inflammation markers in type 2 diabetic mellitus populations?: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chaudhary, A.; Sethi, S. Oxidized omega-3 fatty acids inhibit nf-kappab activation via a pparalpha-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J. Pufas: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Toyoda-Ono, Y.; Suwa, Y.; Kiso, Y. Subchronic (13-week) oral toxicity study of dihomo-gamma-linolenic acid (DGLA) oil in rats. Food Chem. Toxicol. 2009, 47, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Lemaitre, R.N.; King, I.B.; Song, X.; Psaty, B.M.; Siscovick, D.S.; Mozaffarian, D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: The cardiovascular health study. Circulation 2014, 130, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.M.; Rich, M.W. Targeting frailty in heart failure. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The effectiveness of exercise interventions for the management of frailty: A systematic review. J. Aging Res. 2011, 2011, 569194. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.C.; Potter, J.; Vivanti, A.; Avenell, A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst. Rev. 2009, 15, CD003288. [Google Scholar]
- Davies, E.J.; Moxham, T.; Rees, K.; Singh, S.; Coats, A.J.; Ebrahim, S.; Lough, F.; Taylor, R.S. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur. J. Heart Fail. 2010, 12, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Kikukawa, H.; Sakuradani, E.; Ando, A.; Okuda, T.; Shimizu, S.; Ogawa, J. Microbial production of dihomo-γ-linolenic acid by δ5-desaturase gene-disruptants of mortierella alpina 1s-4. J. Biosci. Bioeng. 2016, 122, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Gay, J.P.; Smith, M.; Wilganowski, N.; Harris, A.; Holland, A.; Reyes, M.; Kirkham, L.; Kirkpatrick, L.L.; Zambrowicz, B.; et al. Fatty acid desaturase 1 knockout mice are lean with improved glycemic control and decreased development of atheromatous plaque. Diabetes Metab. Syndr. Obes. 2016, 9, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ueno, M.; Kubo, K.; Yamaguchi, M. Dose-response effect of dietary docosahexaenoic acid on fatty acid profiles of serum and tissue lipids in rats. J. Agric. Food Chem. 1998, 46, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Eto, M.; Shindou, H.; Kita, Y.; Otsubo, E.; Hishikawa, D.; Ishii, S.; Sakimura, K.; Mishina, M.; Shimizu, T. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metab. 2014, 20, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]

| All (n = 214) | Survivor Group (n = 152) | Nonsurvivor Group (n = 62) | p-Value * | |
|---|---|---|---|---|
| Age (years) | 73 (64–82) | 72 (61–81) | 74 (69–82) | 0.03 |
| Male (n, %) | 115 (53.7) | 77 (50.7) | 38 (61.3) | NS |
| Body mass index (kg/m2) | 22.9 (20.4–25.7) | 23.4 (21.1–26.7) | 21.1 (19.5–23.4) | <0.01 |
| Systolic blood pressure (mmHg) | 130 (110–149) | 135 (115–155) | 115 (94–135) | <0.01 |
| Diastolic blood pressure (mmHg) | 77 (62–90) | 80 (68–96) | 65 (56–77) | <0.01 |
| Heart rate (per min) | 93 (74–112) | 97 (75–118) | 87 (71–101) | 0.01 |
| Left ventricular ejection fraction (%) | 35 (25–49) | 38 (26–51) | 32 (20–45) | NS |
| Diabetes mellitus (n, %) | 89 (41.6) | 61 (40.1) | 28 (45.2) | NS |
| Dyslipidemia (n, %) | 143 (66.8) | 111 (73.0) | 32 (51.6) | <0.01 |
| Hypertension (n, %) | 142 (66.4) | 100 (65.8) | 42 (67.7) | NS |
| Smoking (current smoker) (n, %) | 25 (11.7) | 22 (14.6) | 3 (4.8) | NS |
| Atrial fibrillation (n, %) | 91 (42.5) | 67 (44.1) | 24 (38.7) | NS |
| Ischemic heart disease (n, %) | 85 (39.7) | 56 (36.8) | 29 (46.8) | NS |
| Laboratory data | ||||
| Total cholesterol (mg/dL) | 153 (128–178) | 156 (132–186) | 140 (116–161) | <0.01 |
| Triglycerides (mg/dL) | 77 (56–108) | 80 (58–109) | 72 (49–105) | NS |
| HDL-C (mg/dL) | 38 (33–49) | 38 (33–50) | 38 (32–46) | NS |
| LDL-C (mg/dL) | 92 (75–113) | 100 (79–117) | 82 (69–105) | <0.01 |
| Creatinine (mg/dL) | 0.94 (0.72–1.31) | 0.87 (0.67–1.22) | 1.25 (0.94–1.80) | <0.01 |
| HbA1c (%) | 6.0 (5.5–6.7) | 6.0 (5.5–6.8) | 6.0 (5.6–6.6) | NS |
| Total protein (g/dL) | 6.4 (6.0–6.7) | 6.4 (6.1–6.7) | 6.3 (5.9–6.9) | NS |
| Albumin (g/dL) | 3.4 (3.0–3.6) | 3.5 (3.1–3.8) | 3.2 (2.9–3.4) | <0.01 |
| Cholinesterase (U/L) | 183 (138–244) | 210 (154–264) | 158 (105–183) | <0.01 |
| Brain natriuretic peptide (pg/mL) | 800 (445–1610) | 627 (372–1206) | 1495 (660–2246) | <0.01 |
| EPA (μg/mL) | 40.6 (28.7–61.6) | 42.6 (31.2–62.6) | 34.4 (26.1–50.9) | 0.04 |
| DHA (μg/mL) | 109.6 (88.8–138.2) | 112.4 (93.9–140.6) | 104.3 (81.1–128.2) | 0.04 |
| DGLA (μg/mL) | 21.8 (17.4–29.5) | 23.0 (18.5–32.0) | 19.1 (14.7–22.9) | <0.01 |
| AA (μg/mL) | 145.7 (117.0–182.4) | 160.2 (122.6–190.2) | 137.2 (107.2–158.1) | <0.01 |
| GNRI | 90.8 (84.5–94.8) | 92.3 (85.6–96.6) | 87.9 (81.4–90.2) | <0.01 |
| Medication | ||||
| Diuretics (n, %) | 115 (54.5) | 66 (44.0) | 49 (80.3) | <0.01 |
| Antiplatelets (n, %) | 77 (36.5) | 44 (29.3) | 33 (54.1) | <0.01 |
| Anticoagulants (n, %) | 75 (35.5) | 47 (31.3) | 28 (45.9) | NS |
| ACE-I/ARBs (n, %) | 107 (50.7) | 72 (48.0) | 35 (57.4) | NS |
| β-blockers (n, %) | 96 (45.5) | 62 (41.3) | 34 (55.7) | NS |
| Calcium channel blockers (n, %) | 59 (28.0) | 40 (26.7) | 19 (31.2) | NS |
| Inotropic agents (n, %) | 34 (16.1) | 16 (10.7) | 18 (29.5) | <0.01 |
| Statins (n, %) | 52 (24.6) | 37 (24.7) | 15 (24.6) | NS |
| Oral hypoglycemic agents (n, %) | 43 (20.3) | 32 (21.2) | 11 (18.0) | NS |
| Insulin (n, %) | 23 (10.8) | 18 (11.9) | 5 (8.2) | NS |
| Univariate | Multivariate (EPA) | Multivariate (DHA) | Multivariate (DGLA) | Multivariate (AA) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | P | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age, 1 year increase | 1.03 | 1.01–1.06 | <0.01 | 1.04 | 1.01–1.07 | 0.01 | 1.05 | 1.02–1.09 | <0.01 | 1.04 | 1.00–1.07 | 0.03 | 1.04 | 1.01–1.08 | <0.01 |
| Male | 1.42 | 0.86–2.39 | NS | 0.88 | 0.44–1.74 | NS | 0.74 | 0.37–1.44 | NS | 0.85 | 0.44–1.66 | NS | 0.83 | 0.42–1.64 | NS |
| Body mass index, 1 kg/m2 increase | 0.87 | 0.81–0.93 | <0.01 | 0.95 | 0.87–1.03 | NS | 0.96 | 0.87–1.04 | NS | 0.95 | 0.86–1.03 | NS | 0.94 | 0.86–1.03 | NS |
| Diabetes mellitus | 1.13 | 0.68–1.86 | NS | 0.87 | 0.43–1.71 | NS | 0.87 | 0.44–1.72 | NS | 0.72 | 0.35–1.46 | NS | 0.85 | 0.42–1.68 | NS |
| Dyslipidemia | 0.43 | 0.26–0.71 | <0.01 | 0.39 | 0.20–0.76 | <0.01 | 0.43 | 0.22–0.82 | 0.01 | 0.51 | 0.26–0.98 | 0.04 | 0.46 | 0.24–0.89 | 0.02 |
| Hypertension | 1.08 | 0.64–1.88 | NS | 0.78 | 0.41–1.54 | NS | 0.84 | 0.44–1.70 | NS | 0.76 | 0.39–1.53 | NS | 0.68 | 0.35–1.36 | NS |
| Smoking (current smoker) | 0.31 | 0.08–0.84 | 0.02 | 0.49 | 0.09–1.82 | NS | 0.44 | 0.07–1.78 | NS | 0.40 | 0.07–1.64 | NS | 0.38 | 0.07–1.47 | NS |
| Creatinine, 0.1 mg/dL increase | 1.09 | 1.06–1.13 | <0.01 | 1.07 | 1.02–1.12 | <0.01 | 1.09 | 1.04–1.14 | <0.01 | 1.10 | 1.05–1.16 | <0.01 | 1.11 | 1.05–1.17 | <0.01 |
| LVEF, 1% increase | 0.99 | 0.98–1.01 | NS | 1.00 | 0.98–1.02 | NS | 0.99 | 0.97–1.01 | NS | 0.99 | 0.98–1.01 | NS | 0.99 | 0.97–1.01 | NS |
| GNRI, increase by 1 | 0.94 | 0.92–0.97 | <0.01 | 0.95 | 0.91–0.99 | 0.02 | 0.94 | 0.91–0.98 | <0.01 | 0.94 | 0.90–0.98 | <0.01 | 0.94 | 0.91–0.98 | <0.01 |
| EPA, 10 μg/mL increase | 0.90 | 0.79–1.01 | NS | 0.88 | 0.73–1.03 | NS | |||||||||
| DHA, 10 μg/mL increase | 0.91 | 0.84–0.99 | 0.02 | 0.87 | 0.78–0.96 | <0.01 | |||||||||
| DGLA, 10 μg/mL increase | 0.55 | 0.37–0.76 | <0.01 | 0.54 | 0.34–0.80 | <0.01 | |||||||||
| AA, 10 μg/mL increase | 0.91 | 0.85–0.96 | <0.01 | 0.92 | 0.86–0.98 | <0.01 | |||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses. Nutrients 2017, 9, 956. https://doi.org/10.3390/nu9090956
Ouchi S, Miyazaki T, Shimada K, Sugita Y, Shimizu M, Murata A, Kato T, Aikawa T, Suda S, Shiozawa T, et al. Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses. Nutrients. 2017; 9(9):956. https://doi.org/10.3390/nu9090956
Chicago/Turabian StyleOuchi, Shohei, Tetsuro Miyazaki, Kazunori Shimada, Yurina Sugita, Megumi Shimizu, Azusa Murata, Takao Kato, Tatsuro Aikawa, Shoko Suda, Tomoyuki Shiozawa, and et al. 2017. "Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses" Nutrients 9, no. 9: 956. https://doi.org/10.3390/nu9090956





