Could Pomegranate Juice Help in the Control of Inflammatory Diseases?
Abstract
:1. Introduction
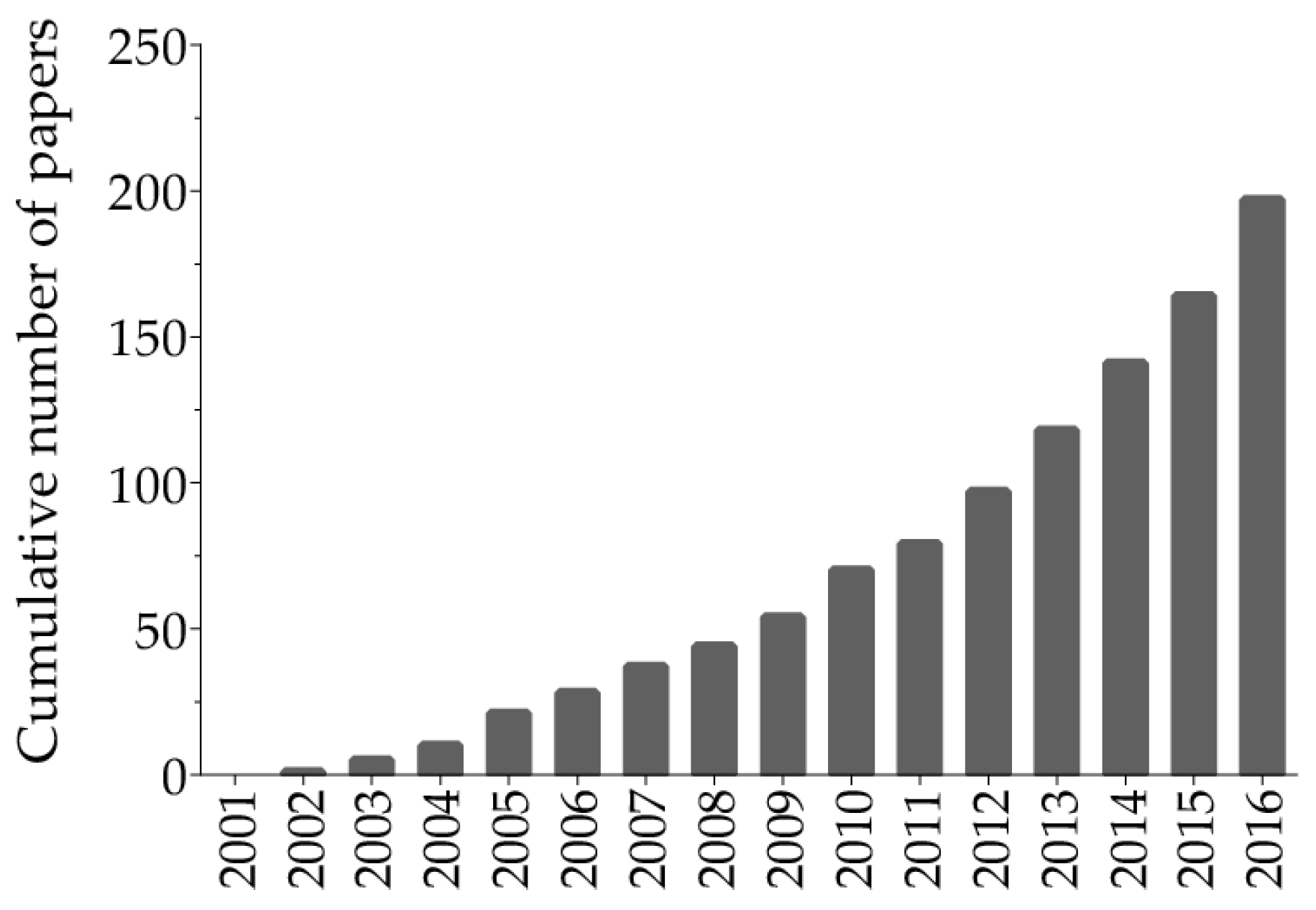
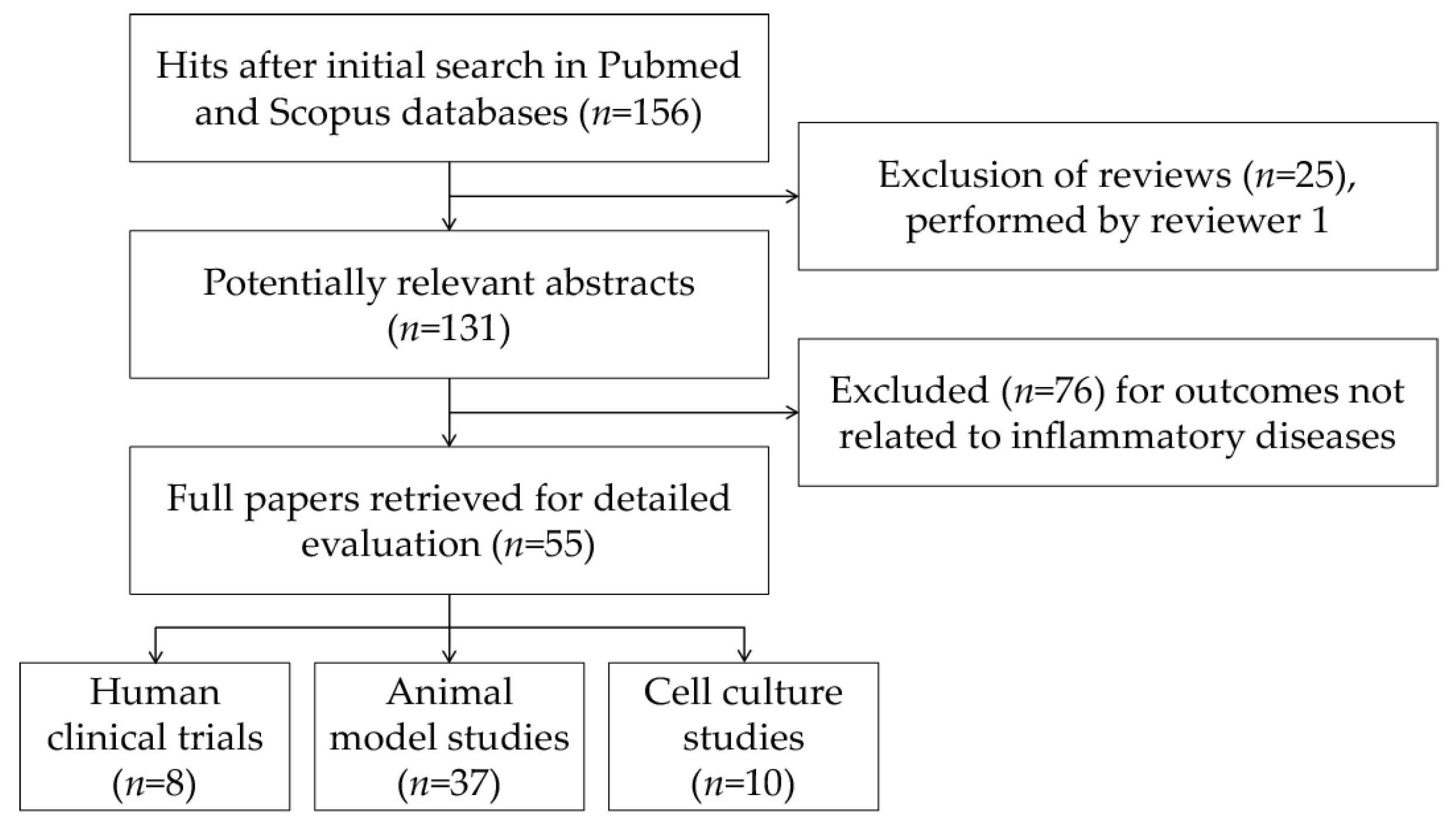
2. Search Strategy
3. Results and Discussion
3.1. Findings Related to Pomegranate Products Consumption and CID in Humans
3.2. Evidence of Anti-Inflammatory Effects of Pomegranate or Pomegranate-Derived Products in Different Animal Models of CID
3.3. In Vitro Anti-Inflammatory Activity of Pomegranate Extracts or Pomegranate-Derived Bioactive Compounds
4. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nasef, N.A.; Mehta, S.; Ferguson, L.R. Susceptibility to chronic inflammation: An update. Arch. Toxicol. 2017, 91, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Inflammation: An introduction. In Nutrition and Physical Activity in Inflammatory Diseases; Garg, M., Wood, L.G., Eds.; CABI: Wallingford, UK, 2013; pp. 1–22. [Google Scholar]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Ferri, C.; Giorgini, P.; Bo, S.; Nachtigal, P.; Grassi, D. Effects of pomegranate juice on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 115, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I.; Alavi-Majd, H.; Azadbakht, L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int. J. Vitam. Nutr. Res. 2006, 76, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Espinel-Bermudez, M.C.; Perez-Rubio, K.G. Effect of pomegranate juice on insulin secretion and sensitivity in patients with obesity. Ann. Nutr. Metab. 2011, 58, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Al-Muammar, M.N.; Khan, F. Obesity: The preventive role of the pomegranate (Punica granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendía, L.E.; Giorgini, P.; Ferri, C.; Grassi, D. Lipid profile changes after pomegranate consumption: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2016, 23, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Gurban, C.; Serban, A.; Andrica, F.; Serban, M.C. Effects of supplementation with pomegranate juice on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2016, 23, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- Bachoual, R.; Talmoudi, W.; Boussetta, T.; Braut, F.; El-Benna, J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 2011, 49, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.F.; Garreto, D.V.; da Silva, M.C.; Fortes, T.S.; de Oliveira, R.B.; Nascimento, F.R.; Da Costa, F.B.; Grisotto, M.A.; Nicolete, R. Therapeutic potential of biodegradable microparticles containing Punica granatum L. (pomegranate) in murine model of asthma. Inflamm. Res. 2013, 62, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Husari, A.; Hashem, Y.; Bitar, H.; Dbaibo, G.; Zaatari, G.; El Sabban, M. Antioxidant activity of pomegranate juice reduces emphysematous changes and injury secondary to cigarette smoke in an animal model and human alveolar cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, T.; Raad, H.; Letteron, P.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Driss, F.; El-Benna, J. Punicic acid a conjugated linolenic acid inhibits TNFa-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS ONE 2009, 4, e6458. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016, 60, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Maria Giner, R.; Ríos, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; de la Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.A.; Parikh, M.; Patel, K.V.; Patel, K.G.; Joshi, C.G.; Gandhi, T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol. Cell. Biochem. 2016, 419, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Jaggi, A.S.; Singh, N. Exploring the ameliorative potential of Punica granatum in dextran sulfate sodium induced ulcerative colitis in mice. Phytother. Res. 2009, 23, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.T. Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J. Agric. Food Chem. 2012, 60, 8866–8876. [Google Scholar] [CrossRef] [PubMed]
- Hollebeeck, S.; Winand, J.; Hérent, M.F.; During, A.; Leclercq, J.; Larondelle, Y.; Schneider, Y.J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012, 3, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Promsong, A.; Chung, W.O.; Satthakarn, S.; Nittayananta, W. Ellagic acid modulates the expression of oral innate immune mediators: Potential role in mucosal protection. J. Oral Pathol. Med. 2015, 44, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Akhtar, N.; Anbazhagan, A.N.; Ramamurthy, S.; Shukla, M.; Haqqi, T.M. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. J. Inflamm. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Sheikholeslami, S.; Mirmiran, P.; Chary, A.; Hedayati, M.; Shafiee, A.; Azizi, F. Effect of pomegranate seed oil on serum TNF-a level in dyslipidemic patients. Int. J. Food Sci. Nutr. 2012, 63, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Hashemi, M.; Rafieian-Kopaei, M. Clinical investigation of the acute effects of pomegranate juice on blood pressure and endothelial function in hypertensive individuals. ARYA Atheroscler. 2013, 9, 326–331. [Google Scholar] [PubMed]
- Shishehbor, F.; Mohammad Shahi, M.; Zarei, M.; Saki, A.; Zakerkish, M.; Shirani, F.; Zare, M. Effects of concentrated pomegranate juice on subclinical inflammation and cardiometabolic risk factors for type 2 diabetes: A quasi-experimental study. Int. J. Endocrinol. Metab. 2016, 14, e33835. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, H.; Alizadeh, M. Effects of pomegranate juice on cardiovascular risk factors in patients with metabolic syndrome: A double-blinded, randomized crossover controlled trial. Plant Foods Hum. Nutr. 2017, 72, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shema-Didi, L.; Sela, S.; Ore, L.; Shapiro, G.; Geron, R.; Moshe, G.; Kristal, B. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: A randomized placebo-controlled trial. Free Radic. Biol. Med. 2012, 53, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Rivara, M.B.; Mehrotra, R.; Linke, L.; Ruzinski, J.; Ikizler, T.A.; Himmelfarb, J. A pilot randomized crossover trial assessing the safety and short-term effects of pomegranate supplementation in hemodialysis patients. J. Ren. Nutr. 2015, 25, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Al Hariri, M.; Zibara, K.; Farhat, W.; Hashem, Y.; Soudani, N.; Al Ibrahim, F.; Hamade, E.; Zeidan, A.; Husari, A.; Kobeissy, F. Cigarette smoking-induced cardiac hypertrophy, vascular inflammation and injury are attenuated by antioxidant supplementation in an animal model. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarallah, A.; Igdoura, F.; Zhang, Y.; Tenedero, C.B.; White, E.J.; MacDonald, M.E.; Igdoura, S.A.; Trigatti, B.L. The effect of pomegranate extract on coronary artery atherosclerosis in SR-BI/APOE double knockout mice. Atherosclerosis 2013, 228, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martínez-Pérez, M.M.; Calderón-Ramos, Z.G.; Belefant-Miller, H.; Cancino-Diaz, J.C. Pomegranate juice increases levels of paraoxonase1 (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011, 44, 1381–1385. [Google Scholar] [CrossRef]
- Çukurova, Z.; Hergünsel, O.; Eren, G.; Gedikbaşi, A.; Uhri, M.; Demir, G.; Tekdöş, Y. The effect of pomegranate juice on diabetes-related oxidative stress in rat lung. Türkiye Klinikeri J. Med. Sci. 2012, 32, 444–452. [Google Scholar] [CrossRef]
- de Nigris, F.; Balestrieri, M.L.; Williams-Ignarro, S.; D’Armiento, F.P.; Fiorito, C.; Ignarro, L.J.; Napoli, C. The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric Oxide 2007, 17, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Dushkin, M.; Khrapova, M.; Kovshik, G.; Chasovskikh, M.; Menshchikova, E.; Trufakin, V.; Shurlygina, A.; Vereschagin, E. Effects of Rhaponticum carthamoides versus Glycyrrhiza glabra and Punica granatum extracts on metabolic syndrome signs in rats. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Harzallah, A.; Hammami, M.; Kępczyńska, M.A.; Hislop, D.C.; Arch, J.R.; Cawthorne, M.A.; Zaibi, M.S. Comparison of potential preventive effects of pomegranate flower, peel and seed oil on insulin resistance and inflammation in high-fat and high-sucrose diet-induced obesity mice model. Arch. Physiol. Biochem. 2016, 122, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR g and a by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Jafari, B.; Hekmatdoost, A. Pomegranate juice prevents development of non-alcoholic fatty liver disease in rats by attenuating oxidative stress and inflammation. J. Sci. Food Agric. 2017, 97, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Taheri Rouhi, S.Z.; Sarker, M.M.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague-Dawley rats. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef]
- Vilahur, G.; Padró, T.; Casani, L.; Mendieta, G.; López, J.A.; Streitenberger, S.; Badimon, L. Polyphenol-enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Rev. Esp. Cardiol. 2015, 68, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yan, C.; Shi, Y.; Cao, K.; Xu, J.; Wang, X.; Chen, C.; Luo, C.; Li, Y.; Gao, J.; et al. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: The protective effects of pomegranate with its active component punicalagin. Antioxid. Redox Signal. 2014, 21, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-κB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, O.; Larush, L.; Frid, K.; Keller, G.; Friedman-Levi, Y.; Ovadia, H.; Abramsky, O.; Magdassi, S.; Gabizon, R. Treatment of a multiple sclerosis animal model by a novel nanodrop formulation of a natural antioxidant. Int. J. Nanomed. 2015, 10, 7165–7174. [Google Scholar]
- Braidy, N.; Essa, M.M.; Poljak, A.; Selvaraju, S.; Al-Adawi, S.; Manivasagm, T.; Thenmozhi, A.J.; Ooi, L.; Sachdev, P.; Guillemin, G.J. Consumption of pomegranates improves synaptic function in a transgenic mice model of Alzheimer’s disease. Oncotarget 2016, 7, 64589–64604. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Subash, S.; Akbar, M.; Al-Adawi, S.; Guillemin, G.J. Long-term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 2015, 10, e0120964. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Farbood, Y.; Naghizadeh, B.; Shabani, S.; Mirshekar, M.A.; Sarkaki, A. Beneficial effects of ellagic acid against animal models of scopolamine- and diazepam-induced cognitive impairments. Pharm. Biol. 2016, 54, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Morzelle, M.C.; Salgado, J.M.; Telles, M.; Mourelle, D.; Bachiega, P.; Buck, H.S.; Viel, T.A. Neuroprotective effects of pomegranate peel extract after chronic infusion with amyloid-b peptide in mice. PLoS ONE 2016, 11, e0166123. [Google Scholar] [CrossRef] [PubMed]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Cannon, J.R.; Greenamyre, J.T. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol. Aging 2014, 35, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Afkhami Goli, A.; Asadpour, E.; Ghorbani, A.; Sadeghnia, H.R. Protective effect of Punica granatum L. against serum/glucose deprivation-induced PC12 cells injury. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Ergin, V.; Burcu Bali, E.; Kuniaková, M.; Karasu, Ç. Pomegranate seed oil modulates functions and survival of BV-2 microglial cells in vitro. Int. J. Vitam. Nutr. Res. 2014, 84, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; Baco, G.; Khela, S.; Okorji, U.; Olajide, O. Pomegranate inhibits neuroinflammation and amyloidogenesis in IL-1b-stimulated SK-N-SH cells. Eur. J. Nutr. 2016, 55, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017, 71, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition 2008, 24, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Seong, A.R.; Yoo, J.Y.; Choi, K.; Lee, M.H.; Lee, Y.H.; Lee, J.; Jun, W.; Kim, S.; Yoon, H.G. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem. Biophys. Res. Commun. 2011, 410, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Thoppil, R.J.; Mandal, A.; Samtani, K.A.; Darvesh, A.S.; Bishayee, A. Pomegranate bioactive constituents suppress cell proliferation and induce apoptosis in an experimental model of hepatocellular carcinoma: Role of Wnt/b-catenin signaling pathway. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.E.; Esmat, A.; Hassona, M.D.; Tadros, M.G.; Abdel-Naim, A.B.; Guns, E.S. The effect of pomegranate fruit extract on testosterone-induced BPH in rats. Prostate 2015, 75, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mukhtar, H. Prostate cancer prevention through pomegranate fruit. Cell Cycle 2006, 5, 371–373. [Google Scholar] [PubMed]
- Wang, L.; Alcon, A.; Yuan, H.; Ho, J.; Li, Q.J.; Martins-Green, M. Cellular and molecular mechanisms of pomegranate juice-induced anti-metastatic effect on prostate cancer cells. Integr. Biol. 2011, 3, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Kojadinovic, M.I.; Arsic, A.C.; Debeljak-Martacic, J.D.; Konic-Ristic, A.I.; Kardum, N.D.; Popovic, T.B.; Glibetic, M.D. Consumption of pomegranate juice decreases blood lipid peroxidation and levels of arachidonic acid in women with metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Clinical Tricals.gov. Available online: http://www.clinicaltrials.gov (accessed on 20 February 2017).
- Kaulmann, A.; Bohn, T. Bioactivity of polyphenols: Preventive and adjuvant strategies toward reducing inflammatory bowel diseases-promises, perspectives, and pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [PubMed]
- Labsi, M.; Khelifi, L.; Mezioug, D.; Soufli, I.; Touil-Boukoffa, C. Antihydatic and immunomodulatory effects of Punica granatum peel aqueous extract in a murine model of echinococcosis. Asian Pac. J. Trop. Med. 2016, 9, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Pantuck, A.J.; Leppert, J.T.; Zomorodian, N.; Aronson, W.; Hong, J.; Barnard, R.J.; Seeram, N.; Liker, H.; Wang, H.; Elashoff, R.; et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006, 12, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Kroon, P.A.; Saha, S.; de Biase, D.; D’Antuono, L.F.; Bordoni, A. Mixed pro- and anti-oxidative effects of pomegranate polyphenols in cultured cells. Int. J. Mol. Sci. 2014, 15, 19458–19471. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, G.; Danesi, F.; Del Rio, D.; Mena, P. The importance of studying cell metabolism when testing the bioactivity of phenolic compounds. Trends Food Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Danesi, F.; Philpott, M.; Huebner, C.; Bordoni, A.; Ferguson, L.R. Food-derived bioactives as potential regulators of the IL-12/IL-23 pathway implicated in inflammatory bowel diseases. Mutat. Res. 2010, 690, 139–144. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomised clinical trial. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
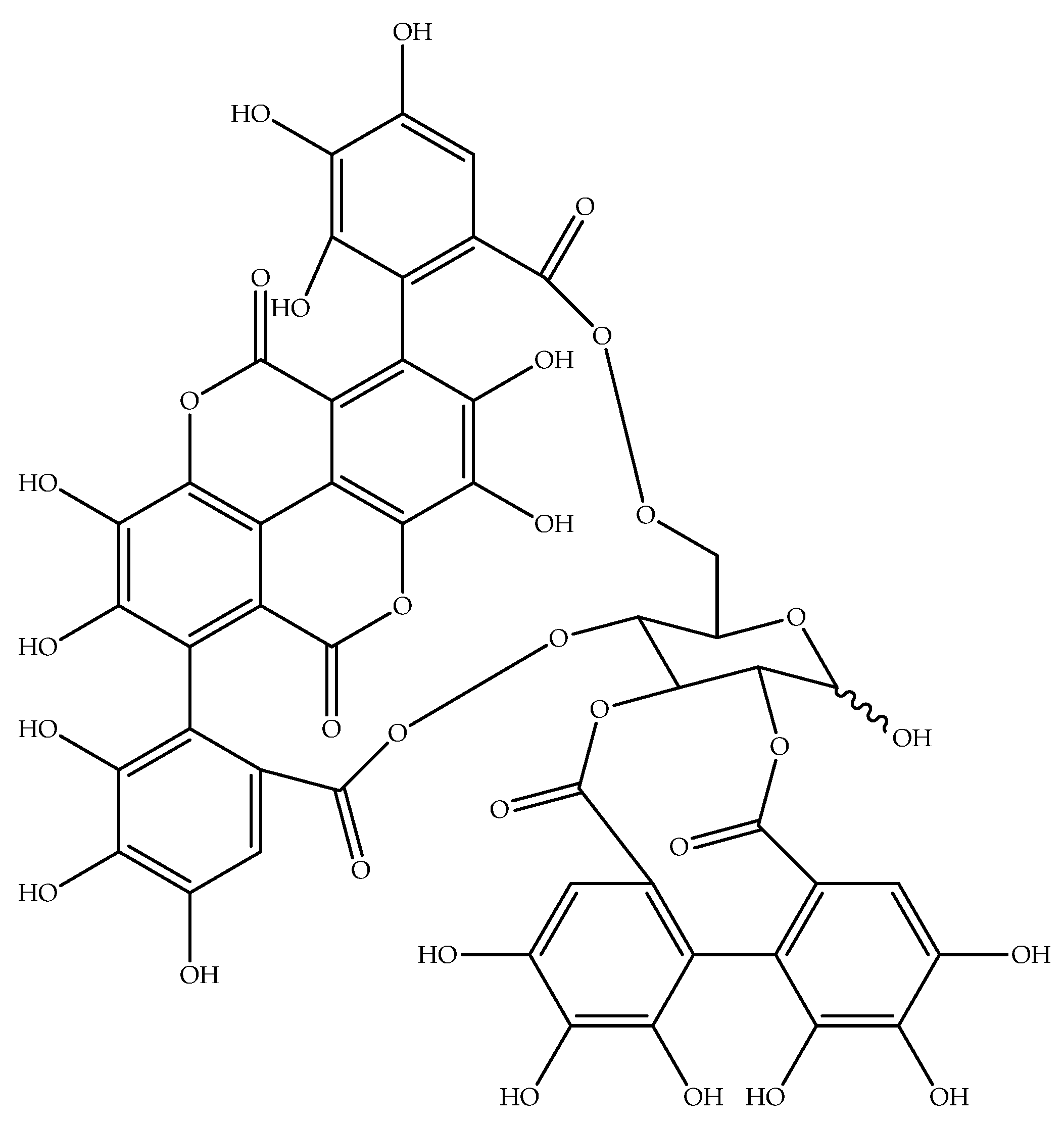
| CID | Human Clinical Trials (No. of Subjects) | Animal Model Studies | Cell Culture Studies |
|---|---|---|---|
| Asthma and COPD | - | 3 [17,18,19] | - |
| IBD | - | 8 [20,21,22,23,24,25,26,27] | 2 [28,29] |
| Immune system | - | - | 2 [30,31] |
| Metabolic and cardiovascular disorders § | 7 (51 [32], 13 [33], 31 [34], 30 [35], 101 [36], 24 [37], 27 [38]) | 14 [39,40,41,42,43,44,45,46,47,48,49,50,51,52] | - |
| Neurodegenerative diseases | - | 8 [53,54,55,56,57,58,59,60] | 4 [53,61,62,63] |
| Psoriasis | - | - | - |
| RA | 1 (55 [64]) | 1 [65] | 1 [66] |
| Other disorders §§ | - | 3 [67,68,69] | 1 [70] |
| Study Design | Population | Subjects (Gender, No., Age) | Intervention | Control/Comparator | Duration | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Double-blind, placebo-controlled, randomised | Dyslipidaemic patients | F & M: 51, 42–64 years | Pomegranate seed oil 400 mg/day × 2 | Paraffin 400 mg/day × 2 | 4 weeks | ↓ TG, ↓ HDL-C, ↓ TG/HDL-C ratio, ↔ TNF-α | Asghari et al., 2012 [32] |
| Pre- and post-test | Hypertensive patients | M: 13, 39–68 years | Pomegranate juice 150 mL/day | - | 1 day | ↓ SBP, ↓ DBP, ↔ CRP, ↔ ICAM-1, ↔ VCAM-1, ↔ IL-6, ↔ E-selectin | Asgary et al., 2013 [33] |
| Pre- and post-test | Type 2 diabetic patients | F: 16, M: 15, 38–54 years | Concentrated pomegranate juice 50 g/day | - | 4 weeks | ↔ SBP, ↔ DBP, ↑ TC, ↑ HDL-C, ↔ TG, ↔ LDL-C, ↔ glycaemia, ↓ IL-6, ↔ TNF-α, ↔ CRP, ↓ adiponectin, ↑ TAC | Shishehbor et al., 2016 [34] |
| Double-blind, placebo-controlled, randomised crossover | Patients with metabolic syndrome | F: 16, M: 14, 42–62 years | Pomegranate juice 500 mL/day | Placebo 500 mL/day | 1 week | ↓ SBP, ↓ DBP, ↓ CRP, ↑ TG, ↑ VLDL-C | Moazzen & Alizadeh 2017 [35] |
| Double-blind, placebo-controlled, randomised | Haemodialysis patients | F: 46, M: 55, 55–81 years | Pomegranate juice 100 mL/day | Placebo 100 mL/day × 1 | 12 months | ↓ IL-6, ↓ TNF-α, ↓ MPO, ↓ AOPP, ↓ oxidised fibrinogen, ↓ MDA | Shema-Didi et al., 2012 [36] |
| Pilot, open, randomised crossover | Haemodialysis patients | F: 13, M: 11, 47–75 years | Pomegranate juice 100 mL/day; Pomegranate extract POMxTM 1050 mg/day (both containing 650 mg GAE) | - | 4 weeks | ↔ SBP, ↔ DBP, ↔ CRP, ↔ IL-6, ↔ F2-isoprostanes, ↔ isofurans, TG, ↔ TC, ↔ HDL-C, ↔ LDL-C | Rivara et al., 2015 [37] |
| Double-blind, placebo-controlled, randomised, parallel-arm | Haemodialysis patients | F: 10, M: 17, 49–59 years | Pomegranate extract POMxTM 1 g/day (containing 600–755 mg GAE) | Placebo 1 pill/day | 6 months | ↓ SBP, ↓ DBP, ↔ CRP, ↔ IL-6, ↔ TC, ↔ HDL-C, ↔ LDL-C, ↔ TG, ↔ ORAC, ↔ AOPP, ↔ 8-OHdG, ↔ ox-LDL, ↔ arylesterase activity, ↔ lactonase activity, ↔ PON activity | Wu et al., 2015 [38] |
| Double-blind, placebo-controlled, randomised | RA patients | F & M: 55, 37–61 years | Pomegranate extract POMxTM 250 mg/day × 2 | Placebo (cellulose) 250 mg/day × 2 | 8 weeks | ↔ CRP, ↔ MMP3, ↔ MDA, ↑ GPx, ↓ ESR, ↓ DAS28, ↓ HAQ, ↓ swollen joints, ↓ tender joints, ↓ pain intensity, ↓ morning stiffness | Ghavipour et al., 2017 [64] |
| clintrials.gov Identifier | Study Focus | Study Design, Duration | Sponsor | Estimated Enrolment | Study Start Date | Estimated Completion Date |
|---|---|---|---|---|---|---|
| NCT02093130 | Memory in older adults | Double-blind, placebo-controlled, parallel arm, randomised, 12 months | University of California (Los Angeles, CA, USA) | 212 | January 2014 | December 2017 |
| NCT02258776 | Ageing and inflammation of the skin | Single-blind, placebo-controlled, parallel arm, randomised, 12 weeks | University of California (Los Angeles, CA, USA) | 15 | October 2015 | January 2018 |
| NCT03000101 | Inflammation in IBD | Double-blind, placebo-controlled, parallel arm, randomised, 12 weeks | St. Orsola-Malpighi Hospital (Bologna, Italy) | 36 | December 2016 | June 2018 |
| Disease Model | Animal Model | Tested Product(s), Vehicle, Duration | Disease Induction | Effects | Reference |
|---|---|---|---|---|---|
| Respiratory diseases | BalbC mice | Pomegranate peel aqueous extract (200 mg/kg b.w.) via intraperitoneal injection for 2 days | LPS-induced lung inflammation | ↓ total cells in BAL, ↓ neutrophils in BAL | Bachoual et al., 2011 [17] |
| BalbC mice | Encapsulated pomegranate leave extract (10 mg/mL) or non-encapsulated pomegranate leave extract (20 mg/kg b.w.) via nostril for 4 days | Ovalbumin-induced asthma | ↓ leukocytes, neutrophils, and eosinophils in BAL, ↓ macrophages in BAL (non-encapsulated extract only), ↔ lymphocytes in BAL, ↓ IL-1β and IL-5 in BAL | de Oliveira et al., 2013 [18] | |
| C57BL/6J mice | Pomegranate juice (80 μmol/kg b.w.) via bottle for 1 week or 1 month or 3 months | Cigarette smoke-induced lung stress | ↓ IL-1β and IL-6 expression in lung (1 week only), ↓ TNF-α expression in lung | Husari et al., 2016 [19] | |
| IBD | Wistar rats | Punicic acid (400 mg/0.5 mL PBS) or pomegranate seed oil (0.5 mL) via oral administration for 10 days | TNBS-induced colitis | Punicic acid: ↓ Wallace and Ameho scores, ↓ MPO activity in colon, ↓ F2-isoprostane in colon; Pomegranate seed oil: ↓ Wallace and Ameho scores | Boussetta et al., 2009 [20] |
| Swiss albino mice | Pomegranate flower hydro-alcoholic extract (100 or 200 mg/kg b.w.) or EA-rich fraction of pomegranate flower (100 or 200 mg/kg b.w.) via oral administration for 7 days | DSS-induced colitis | ↓ macroscopic and histopathological changes in colon, ↓ colon MPO activity, ↓ histamine content in colon, ↓ MDA level in colon, ↓ superoxide anion production in colon | Singh et al., 2009 [27] | |
| Fischer rats | Pomegranate extract (250 mg/kg b.w.) or urolithin A (15 mg/kg b.w.) via chow for 10 days | DSS-induced colitis | ↓ colon tissue damage (urolithin A only), ↑ FRAP (pomegranate extract only), ↓ MDA in colon (pomegranate extract only), ↓ COX-2 gene and protein expression in colon, ↓ iNOS expression in colon, ↓ PGE2 and NO levels in colon (pomegranate extract only), ↓ PTGES protein expression in colon | Larrosa et al., 2010 [22] | |
| Wistar rats | EA (10 and 20 mg/kg b.w.) via oral gavage for 48, 24 and 1 h prior to the induction of colitis and 24 h later | TNBS-induced colitis | ↓ colon macroscopic damage, ↓ b.w. loss, ↓ colon weight/length, ↓ histological damage in colon, ↓ colon MPO activity, ↓ iNOS and COX-2 protein expression in colon, ↓ JNK and ERK phosphorylation in colon, ↓ NF-κB activation in colon | Rosillo et al., 2011 [25] | |
| Wistar rats | Pomegranate extract (250 or 500 mg/kg feed) or EA (10 mg/kg feed) or EA-enriched pomegranate extract (pomegranate extract 250 mg/kg feed + EA 10 mg/kg feed) via chow for 30 days prior to the induction of colitis and 14 days later | TNBS-induced colitis | ↓ colon macroscopic damage, ↓ b.w. loss (all treatments, apart from extract 250 mg/kg), ↓ colon weight/length (EA and EA-enriched extract only), ↓ colon MPO activity, ↓ TNF-α level in colon, ↓ iNOS and COX-2 protein expression in colon, ↓ JNK and ERK phosphorylation in colon, ↓ NF-κB activation in colon, ↔ colon PPAR-γ protein expression | Rosillo et al., 2012 [24] | |
| C57BL/6 mice | EA (0.5% w/w, equivalent to 25 mg/mouse) via chow for 56 days | DSS-induced colitis | ↓ disease symptoms, ↓ DAI, ↓ iNOS and COX-2 protein expression in colon, ↓ JNK and ERK phosphorylation in colon, ↓ NF-κB activation in colon, ↓ IL-6 gene expression in colon, ↓ STAT3 phosphorylation in colon | Marín et al., 2013 [23] | |
| Sprague-Dawley rats | Pomegranate beverage (containing 2504.74 mg/L GAE) ad libitum for 3 weeks prior to the induction of colitis and 7 weeks later | DSS-induced colitis | ↓ colonocyte proliferative index, ↓ expression of hs-CRP, TNF-α, IL-1β, and IL-6 in intestinal mucosa, ↓ IL-1β and IL-6 levels in serum, ↑ IL-10 level in serum, ↓ p-p70-S6K/p70-S6K, ↓ p-rpS6/rpS6 | Kim et al., 2016 [21] | |
| Sprague-Dawley rats | Pomegranate juice (400 mg/kg b.w.) or pomegranate powder (4 mg/kg b.w.) via oral administration for 18 days | DNBS-induced colitis | ↔ histopathological scores, ↓ CMDI and DAI, ↓ MDA in colon (juice only), ↔ colon MPO activity, ↓ colon NO production, ↓ colon SOD activity, ↓ serum cortisol level, ↓ IL-1β, IL-18, TNF-α, and NF-κB expression in colon | Shah et al., 2016 [26] | |
| Metabolic and cardiovascular disorders | Zucker rats | Concentrated pomegranate juice or pomegranate fruit extract (6.25 mL/L) via drinking water or pomegranate seed oil (1 mL/L) via chow for 5 weeks | Obese metabolic syndrome model | ↔ TC, ↔ LDL-C, ↔ HDL-C, ↑ TG (seed oil only), ↔ daytime MAP, ↔ BPM, ↔ motor activity, ↓ arterial TSP-1 protein expression, ↑ eNOS protein expression (apart from oil), ↓ arterial TGF-β1 protein expression (except oil), ↓ nitrate and nitrite levels (apart from oil), ↔ insulin and glucose levels | de Nigris et al., 2007 [43] |
| db/db mice | Pomegranate seed oil (1 g/100 g feed) via chow for 30 days | Diabetes and obesity model | ↓ glycaemia, ↓ blood insulin, ↑ expression of genes PPAR-α, CD36, and FABP4 in adipose tissue, ↔ expression of genes PPAR-γ, ACAD, and SCD1 in adipose tissue, ↑ expression of genes PPAR-γ, CD36, FABP4, ACAD, and SCD1 in muscle, ↔ expression of genes PPAR-α in muscle, ↓ TNF-α expression and NF-κB activation in adipose tissue and liver | Hontecillas et al., 2009 [46] | |
| CD-1 mice | Pomegranate juice (12.5 mL/L juice diluted in water, equivalent to 0.35 mmol polyphenols) via drinking water for 4 months | Streptozotocin-induced diabetes | ↑ hepatic PON-1 expression and activity, ↓ glycaemia, ↔ blood TC and TG levels | Betanzos-Cabrera et al., 2011 [41] | |
| Sprague-Dawley rats | Pomegranate juice (100 μL) via gastric gavage for 10 weeks | Streptozotocin-induced diabetes | ↔ GSH in lung, ↑ SOD activity in lung, ↓ protein carbonyl content in lung, ↓ serum sialic acid, ↓ eNOS protein in lung | Çukurova et al., 2012 [42] | |
| SR-BI/apoE double knockout mice | Pomegranate extract (307.5 ml/L) via drinking water for 2 weeks | Coronary heart disease model | ↑ TC, ↔ serum apoA and apoB, ↓ atherosclerosis, ↔ SAA and serum MCP-1, ↓ MCP-1 in plaques, ↓ lipid accumulation, macrophage infiltration, and MCP-1 levels in heart, ↓ myocardial fibrosis, cardiac enlargement, and ECG abnormalities | Al-Jarallah et al., 2013 [40] | |
| BalbC mice | Pomegranate peel extract (0.2% w/v diluted in water, equivalent to 6 mg per mouse) via drinking water for 4 weeks | High-fat diet-induced obesity and hypercholesterolaemia | ↔ body weight gain, ↔ adiposity, ↔ glycaemia and insulin response, ↓ serum TC and LDL-C, ↔ serum HDL-C and TG, ↔ hepatic TC and TG, ↔ IL-1β, IL-6, and COX-2 expression in liver, ↔ IL-1β expression in colon, ↓ IL-6 and COX-2 expression in colon | Neyrinck et al., 2013 [47] | |
| Wistar rats | EA (0.8 g/kg feed) via chow for 8 weeks after the induction of metabolic syndrome | High-fat and high-carbohydrate diet-induced metabolic syndrome | ↑ retroperitoneal, epididymal, omental, and total abdominal fat, ↔ whole-body fat mass, ↓ whole-body lean mass, ↓ glycaemia, ↓ plasma TG, TC, NEFA, uric acid, urea, and CRP, ↓ plasma ALT, AST, ALP, and LDH activity, ↔ plasma albumin and bilirubin, ↓ SBD, ↑ coronary endothelial-dependent relaxation, ↔ Nrf2 protein expression in heart, ↑ Nrf2 protein expression in liver, ↓ NF-κB expression in heart and liver, ↑ CPT1 expression in heart and liver | Panchal et al., 2013 [49] | |
| Wistar Albino Glaxo rats | Pomegranate extract (300 mg/kg b.w.) via chow for 8 weeks | High-fat diet-induced metabolic syndrome | ↔ weight of epididymal adipose tissue, ↔ glycaemia, ↓ LDL-C, ↔ TC, HDL-C, TG, and FFA, ↔ SBP, ↓ serum corticosterone, ↔ adrenal corticosterone, ↓ serum IL-6 and TNF-α, ↓ TG in liver | Dushkin et al., 2014 [44] | |
| Sprague-Dawley rats | PUNI-enriched pomegranate extract (150 mg/kg b.w.) via oral gavage for 8 weeks | High-fat diet-induced NAFLD | ↓ body weight gain, ↓ serum TG, HDL-C, and LDL-C, ↔ serum C, ↓ serum insulin, leptin, and adiponectin, ↓ HOMA-IR, ↓ serum ALT level, ↓ liver tissue weight, ↓ hepatic TG and TC, ↓ expression of SREBP-1c precursor protein, ↔ expression of SREBP-1c mature protein, expression of FA biosynthesis-related genes (↓ SREBP-1c, ↓ FAS, ↓ ACC1, ↓ SCD1), expression of TG biosynthesis-related genes (↓ ACLY, ↔ GPAM, ↑ DGAT-1 and-2), ↓ serum CRP level, IL-1β, IL-4, IL-6, and TNFα, ↓ serum IgA, IgG, and IgM, ↓ protein carbonyl content in liver tissue and liver mitochondria, ↓ lipid peroxidation in liver, ↑ hepatic total SOD activity, ↓ hepatic GSH and GSSG levels, ↑ GSH/GSSG ratio, ↓ Nrf2, HO-1, NQO-1, and UCP2 protein expression in liver, ↑ ATP content in liver, ↑ activities of mitochondrial complexes I, II, and IV in liver, ↑ expression of genes PGC-1-α and PPAR-α in liver, ↔ expression of PGC-1β gene in liver, ↑ PGC-1α protein expression in liver, ↑ expression of genes CPT1A, CPT1B, and ACAD in liver | Zou et al., 2014 [52] | |
| Pigs | Pomegranate extract Pomanox® (625 mg equivalent to 200 mg punicalagins) via chow for 10 days | High-fat diet-induced coronary endothelial dysfunction | ↑ coronary endothelial-dependent relaxation, ↑ Akt and eNOS phosphorylation in coronary artery, ↔ MCP-1 gene expression in coronary artery, ↓ MCP-1 protein content in coronary artery, ↓ coronary DNA oxidative damage, ↓ LDL-C oxidation | Vilahur et al., 2015 [51] | |
| Sprague-Dawley rats | Pomegranate juice concentrate (equivalent to 80 μmol polyphenols/mL) via drinking water for 5 weeks | Cigarette smoking-induced cardiac hypertrophy | ↔ DBP and SBP, ↓ ROS in aortic tissue, ↓ heart to body weight ratio, ↓ fibrotic marker (ObR and Fn1) and kinin receptor (Bdkrb1 and Bdkrb2) expression in aorta, ↓ IL-1β expression in aorta, ↔ TNF-α expression in aorta | Al Hariri et al., 2016 [39] | |
| C57Bl/6 mice | Pomegranate peel (250 mg/kg b.w.) or Pomegranate flower extract (250 mg/kg b.w.) or Pomegranate seed oil (2 mL/kg b.w.) for 6 weeks | High-fat and high-sugar diet-induced obesity | ↔ b.w. gain, ↓ glycaemia (28 days- seed oil treatment only), ↔ plasma insulin level, ↔ plasma TC, HDL-C, and TG, ↔ hepatic ALT and AST, ↔ hepatic TG, ↑ plasma IL-2 (peel extract only), ↓ plasma IL-6 (apart from flower extract), ↑ plasma IL-10 (flower extract only), ↓ plasma TNF-α (apart from peel extract), ↑ IFN-γ (seed oil only) | Harzallah et al., 2016 [45] | |
| Sprague-Dawley rats | Pomegranate juice (60 mL) via drinking water for 7 weeks | High-fat and high-sugar diet-induced NAFLD | ↓ plasma ALT and AST, ↔ plasma GGT and ALP, ↓ glycaemia and insulin, ↓ plasma TG, ↔ plasma TC, HDL-C, and LDL-C, ↓ hepatic IL-1β, IL-6, TNF-α, and TGF-β1 expression, ↑ hepatic IL-10 expression, ↔ GSH level, TBARS level, GR activity, CAT activity, SOD activity in liver, ↑ hepatic GPx activity, ↓ hepatic steatosis and ballooning, ↓ lobular and portal inflammation in liver | Noori et al., 2017 [48] | |
| Sprague-Dawley rats | Pomegranate juice (1 mL) or pomegranate seed extract (100 mg/mL) via oral administration, by force-feeding for 21 days | Streptozotocin-nicotinamide induced type 2 diabetes | ↔ b.w. gain, ↔ glycaemia and plasma insulin level, ↓ TC and TG (juice only), ↔ LDL-C and HDL-C (juice only), ↑ TC, LDL-C, and HDL-C (seed extract only), ↔ TG (seed extract only), ↓ plasma IL-6 and NF-κB levels, ↓ plasma TNF-α level (juice only), ↑ number and size of Islets of Langerhans (juice only) | Taheri Rouhi et al., 2017 [50] | |
| Neurodegenerative diseases | APPswe/PS1dE9 mice | Pomegranate extract (6.25 mL/L) via drinking water for 3 months | Transgenic model overexpressing APP, developing amyloid plaques and progressive cognitive deficits | ↑ behavioural performance, ↓ TNF-α in spleen and brain, ↓ NFATc1 activation in spleen and brain, ↑ p-NFATc2/NFATc2 ratio in brain, ↓ p-IκB/IκB ratio in brain, ↓ plaques in brain | Rojanathammanee et al., 2013 [59] |
| Lewis rats | Pomegranate juice (juice diluted 1:40 in water, equivalent to ~0.6–0.7 mg polyphenols) via drinking water for 2 weeks | Rotenone-induced degeneration of neurones | ↓ rearing behaviour, ↔ postural instability, ↔ catecholamine levels, ↓ dopamine fibres in striatum, ↓ nigral dopaminergic neurones, ↑ nitrotyrosine in substantia nigra, ↑ iNOS induction, ↑ NF-κB activation, ↑ caspase activation, ↔ IL-1β, TNF-α, and COX-2 protein expression | Tapias et al., 2014 [60] | |
| C57BL/6 mice | Pomegranate seed oil as emulsified nanodroplets (10 μL) via gavage for 10 days | MOG-induced experimental autoimmune encephalomyelitis | ↓ demyelination and oxidation of brain lipids, ↓ MDA in brain | Binyamin et al., 2015 [54] | |
| APPsw/Tg2576 mice | Pomegranate fruit (4% w/w) via chow for 15 months | Transgenic model overexpressing APP, developing amyloid plaques and progressive cognitive deficits | ↓ IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, and eotaxin levels in serum, ↓ Aβ-1 40 and 42 levels in brain, ↑ ATP levels the cortex and hippocampus, ↓ IL-1β, IL-6, TNF-α levels in cortex and hippocampus | Essa et al., 2015 [56] | |
| APPsw/Tg2576 mice | Pomegranate fruit (4% w/w) via chow for 15 months | Transgenic model overexpressing APP, developing amyloid plaques and progressive cognitive deficits | ↓ expression of genes IL-1β, IL-10, TNF-α, IGF-1, iNOS, and CCL2, ↑ BDNF gene expression, ↑ PSD-95, Munc18-1, SNAP25, and synaptophysin protein expression, ↑ p-CaMKIIα/CaMKIIα protein expression, ↑ p-CREB/CREB protein expression, ↑ BECN1 protein expression, ↑ LC3-I and LC3-II protein expression, ↑ Akt and mTOR protein expression, ↑ p70-S6K protein expression, ↔ APP and CTF-α protein expression, ↓ BACE-1, CTF-β, and sAPP-β protein expression, ↔ ADAM-10 and ADAM-17 protein expression | Braidy et al., 2016 [55] | |
| Wistar rats and mice | EA (10, 30, and 100 mg/kg b.w.) via intraperitoneal injection in a single administration | Scopolamine- and diazepam-induced cognitive impairments | ↓ amnesia in EPM and PA tests in mice ([EA] ≥ 30 mg/kg), ↓ amnesia in EPM test in rats ([EA] ≥ 30 mg/kg) | Mansouri et al., 2016 [57] | |
| C57Bl/6 mice | Pomegranate peel extract as microparticles (800 mg/kg b.w.) via oral administration for 35 days | Amyloid-β peptide-induced neurodegeneration | ↔ locomotor activity in an activity cage, ↔/↑ spatial memory in the Barnes maze, ↓ senile plaques, ↑ BDNF level in cortex and hippocampus, ↓ acetylcholinesterase activity in cortex and hippocampus, ↓ MDA in liver, ↔ SOD activity in hippocampus, cortex and serum, ↓ TNF-α in cortex, ↔ TNF-α in serum | Morzelle et al., 2016 [58] | |
| ICR mice | PUNI (1.5 mg/kg b.w.) via drinking water for 4 weeks | LPS-induced cognitive impairment | ↓ Aβ and BACE-1 protein expression, ↓ GFAP and AIF-1 protein expression, ↓ IL-1β, IL-6, and TNF-α release, ↑ GSH/GSSG ratio, ↓ ROS level, ↓ MDA, ↓ IκB phosphorylation, ↓ p50 and p65 protein expression | Kim et al., 2017 [53] | |
| RA | DBA/1 Lac J mice | POMxTM extract (13.6 or 34 mg/kg b.w.) via oral gavage for 10 days | Collagen-induced arthritis with chicken CII (Chondrex) | ↓ incidence and delay of arthritis, ↓ synovitis, ↓ pannus formation, ↓ joint degradation, ↓ IL-1β expression in ankle joints (13.6 mg/kg only), IL-6 expression in ankle joints, ↓ TNF-α expression in ankle joints (34 mg/kg only) | Shukla et al., 2008 [65] |
| Hepatocellular carcinoma | Sprague-Dawley rats | Pomegranate emulsion (1 or 10 g/kg b.w.) via oral gavage for 4 weeks prior to the DENA exposure and 18 weeks later | DENA-induced hepatocarcinogenesis | ↓ cyclin D1 expression (10 g/kg only), ↑ Bax/Bcl-2 ratio (10 g/kg only), ↓ β-catenin expression (10 g/kg only), ↑ GSK-3 expression (10 g/kg only) | Bhatia et al., 2013 [67] |
| Prostatic hyperplasia | Sprague-Dawley rats | Pomegranate fruit extract (25, 50, and 100 mg/kg b.w.) via oral gavage for 4 weeks | Testosterone-induced prostatic hyperplasia | ↓ prostate weight, ↓ PAP activity, ↑ GSH, ↔ total glutathione, ↑ SOD activity (100 mg/kg only), ↔ CAT activity, ↓ MDA, ↓ iNOS and COX-2 expression, ↔ AR, NF-κB, ER-α, and p-Akt expression | Ammar et al., 2015 [68] |
| Prostate cancer | Athymic nude mice | Pomegranate fruit extract (0.1% and 0.2% w/v) via oral administration for 28–51 days (until the implanted tumour reached to a volume of 1200 mm3) | Implantation with androgen-responsive CWR22Rn1 cells | ↓ PSA secretion | Malik & Mukhtar 2006 [69] |
| Cell Model | Primary Cell/Cell Line | Tested Compound(s), Dose, Duration | Pro-Inflammatory Treatment | Biological Effects | Reference |
|---|---|---|---|---|---|
| Intestinal cells | CCD18-Co | Uro-A (40 μM) + Uro-B (5 μM) + EA (1 μM) for 12–48 h in concomitant exposure with pro-inflammatory stimulus | IL-1β (1 ng/mL) or TNF-α (50 ng/mL) | ↓ IL-8 release, ↓ PGE2 release (only upon IL-1β stimulus), ↓ PAI-1 release, ↔ ICAM-1 and VCAM-1 release, ↔ MCP-1, ↓ cell migration and adhesion | Giménez-Bastida et al., 2012 [28] |
| Caco-2 | Pomegranate husk extract (containing 8.1 μM PUNI and 7.9 μM EA) or PUNI (50 μM) for 1 h as pre-treatment and 24 h in concomitant exposure with pro-inflammatory stimulus | basolateral side: IL-1β (25 μg/L) + TNF-α (50 μg/L) + IFN-γ (50 μg/L); apical side: LPS (1 mg/L) | ↓ IL-6 and MCP-1transcription, ↔ IL-8 transcription, ↓ IL-6, IL-8, and MCP-1 secretion | Hollebeeck et al., 2012 [29] | |
| Immune cells | KU812 | POMxTM extract (20, 40, and 100 μg/mL) for 2 h prior to pro-inflammatory stimulus | PMA (40 nM) + A23187 (1 μM) | ↓ IL-6 and IL-8 transcription, ↓ IL-6 and IL-8 secretion, ↓ JNK and ERK phosphorylation, ↓ NF-κB activation | Rasheed et al., 2009 [31] |
| Primary HGE | EA (12.5, 25, 50, and 100 μM) for 18 h | - | ↓ IL-8 transcription ([EA] ≥ 25 μM), ↑ BD2 transcription ([EA] ≥ 25 μM), ↑ SLPI transcription, ↓ CCL20 transcription ([EA] ≥ 50 μM), ↓ CXCL5 transcription ([EA] ≥ 50 μM), ↔ IL-1β secretion, ↑ IL-2 secretion ([EA] = 12.5 μM), ↓ IL-2 secretion ([EA] = 50 μM), ↔ IL-4, IL-6, and TNF-α secretion, ↓ IL-8 secretion ([EA] = 50 μM), ↔ MCP-1 secretion, ↑ CCL5 secretion ([EA] ≥ 12.5 μM), ↑ BD2 secretion ([EA] = 100 μM), ↔ SLPI secretion | Promsong et al., 2015 [30] | |
| Neuronal cells | PC12 | Pulp aqueous extract (6.25–800 μg/mL), pulp hydro-alcoholic extract (6.25, 12.5, 25, 50, 100, 200, 400, and 800 μg/mL), PJ extract (6.25, 12.5, 25, 50, 100, 200, 400, and 800 μg/mL) for 2 h prior glucose deprivation | Serum glucose deprivation | ↓ DNA damage ([PJ] ≥ 400 μg/mL) | Forouzanfar et al., 2013 [61] |
| BV-2 | Pomegranate seed oil (25 μg/mL) for 24 h | LPS (1 mg/mL) | ↓ NO production, ↓ TNF-α release, ↓ iNOS induction, ↓ caspase 3 activation | Račková et al., 2014 [62] | |
| SK-N-SH | PJ extract (25, 50, 100, and 200 μg/mL) for 24 h | IL-1β (10 U/mL) | ↓ PGE2 release, ↓ COX-2 protein expression, ↓ BACE-1 ([PJ] ≥ 50 μg/mL), ↓ amyloid-β ([PJ] ≥ 100 μg/mL), ↓ IκBα phosphorylation ([PJ] ≥ 50 μg/mL) | Velagapudi et al., 2016 [63] | |
| Primary astrocytes and BV-2 | PUNI (10, 20, and 50 μM) for 1 h | LPS (1 mg/mL) | ↓ iNOS and COX-2 protein expression, ↓ APP and BACE-1 protein expression, ↓ IκBα phosphorylation | Kim et al., 2017 [53] | |
| Rheumatoid arthritis cells | MH7A | Delphinidin (10 and 30 μM) for 24 h or 2 h (for ELISA) | TNF-α (20 ng/mL) | ↓ IL-1β and IL-6 expression, ↓ COX-2 expression, ↓ p65 acetylation, ↓ NF-κB DNA binding activity | Seong et al., 2011 [66] |
| Cancer cells | DU145 and PC3 | PJ (1% or 5%) for 18 h | - | ↓ IL-6 and IL-12 secretion, ↓ IL-1β secretion (DU145 only), ↓ CCL5 secretion (PC3 only) | Wang et al., 2011 [70] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danesi, F.; Ferguson, L.R. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. https://doi.org/10.3390/nu9090958
Danesi F, Ferguson LR. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients. 2017; 9(9):958. https://doi.org/10.3390/nu9090958
Chicago/Turabian StyleDanesi, Francesca, and Lynnette R. Ferguson. 2017. "Could Pomegranate Juice Help in the Control of Inflammatory Diseases?" Nutrients 9, no. 9: 958. https://doi.org/10.3390/nu9090958







