Effects of Whey, Soy or Leucine Supplementation with 12 Weeks of Resistance Training on Strength, Body Composition, and Skeletal Muscle and Adipose Tissue Histological Attributes in College-Aged Males
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Screening of Participants
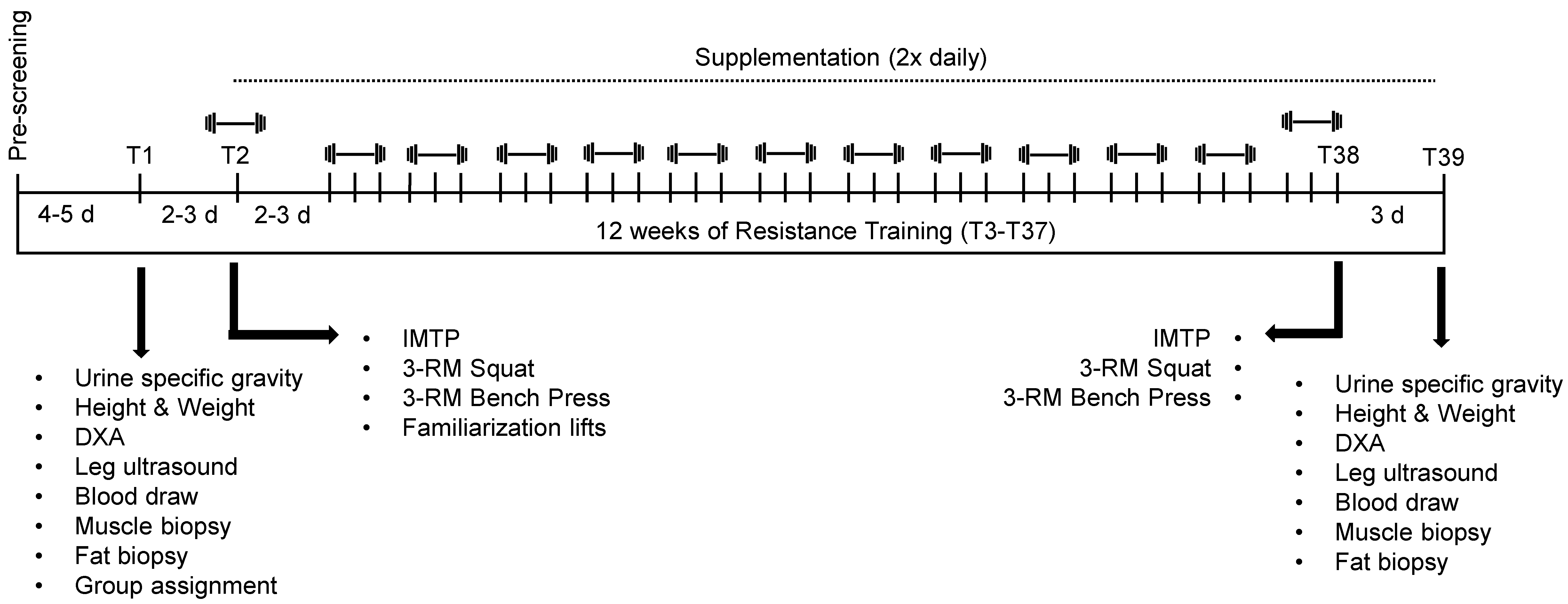
2.2. Study Design
2.3. Body Composition Testing
2.4. Venipuncture, and Percutaneous Skeletal Muscle and Fat Biopsies
2.5. Isometric Mid-Thigh Pull, Strength Testing, and Weightlifting Familiarization
2.6. Training Protocol
2.7. Supplementation
2.8. Nutritional Intake Monitoring
2.9. Immunofluorescent Histochemistry for Muscle Fiber Type-Specific Characteristics
2.10. SQ Fat CSA Analysis
2.11. A Priori Sample Size Calculations and Statistical Analyses
3. Results
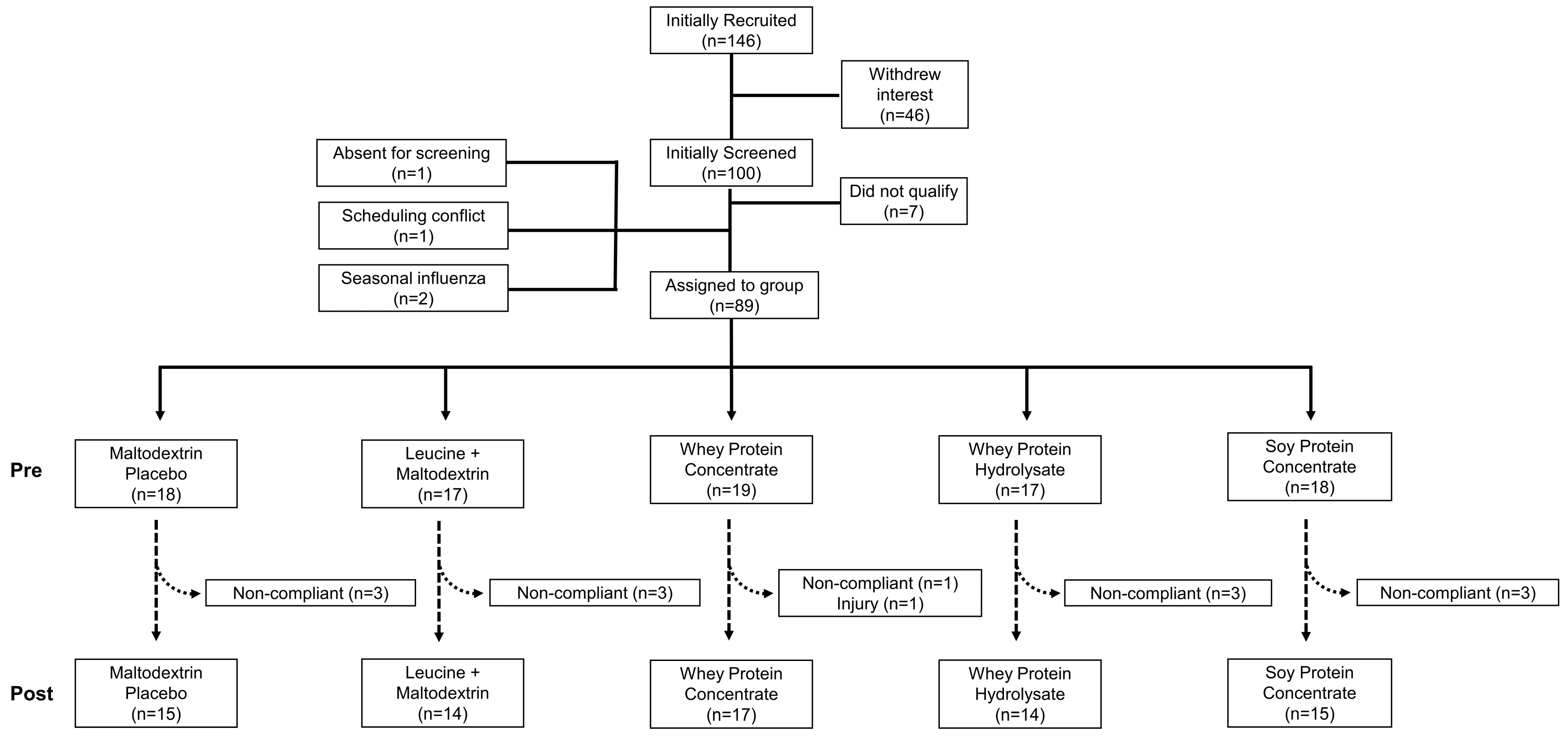
3.1. Subject Compliance and Baseline Characteristics
3.2. Self-Reported Nutritional Intakes
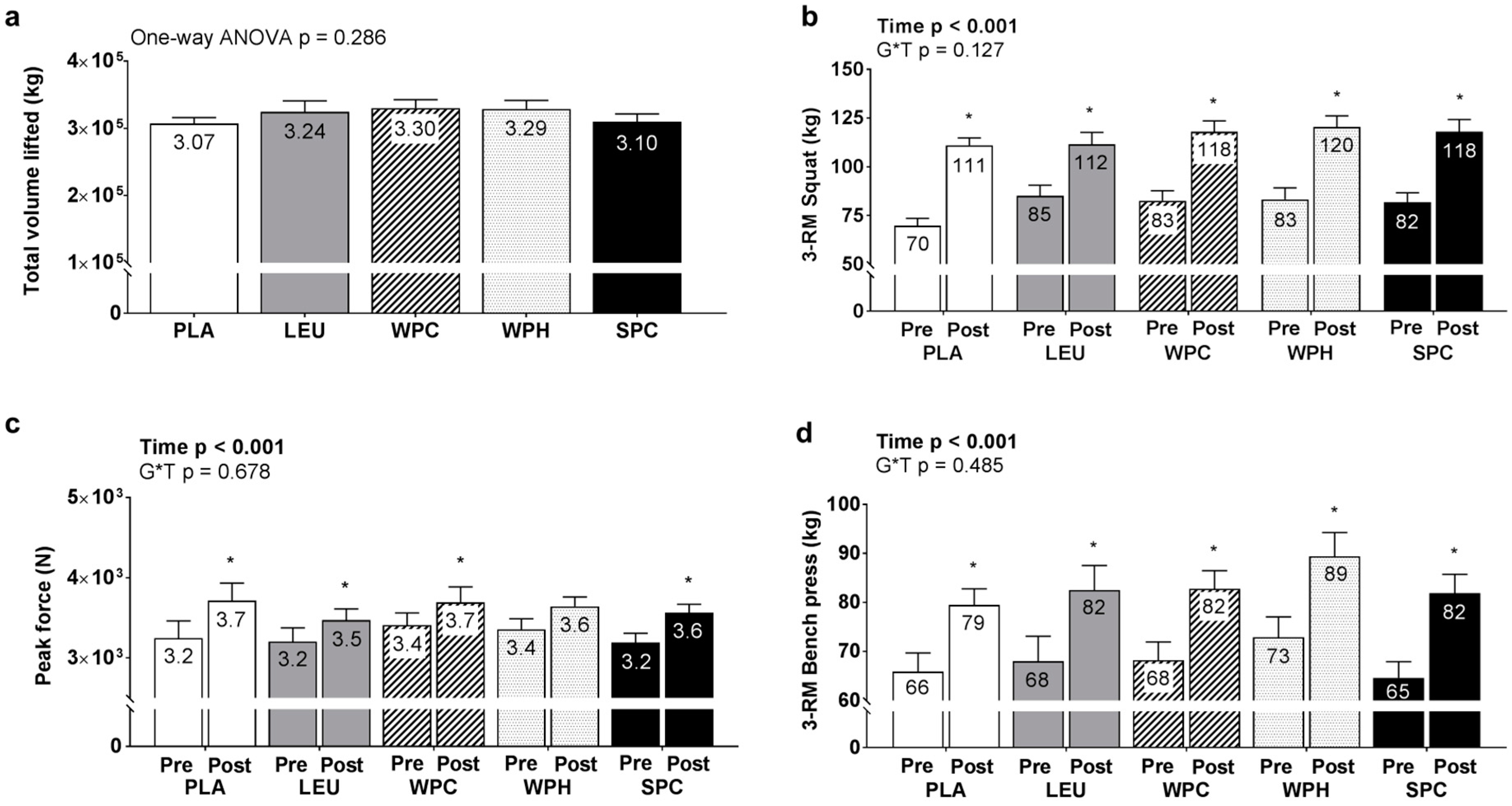
3.3. Training Volume, 3-RM Strength, IMTP
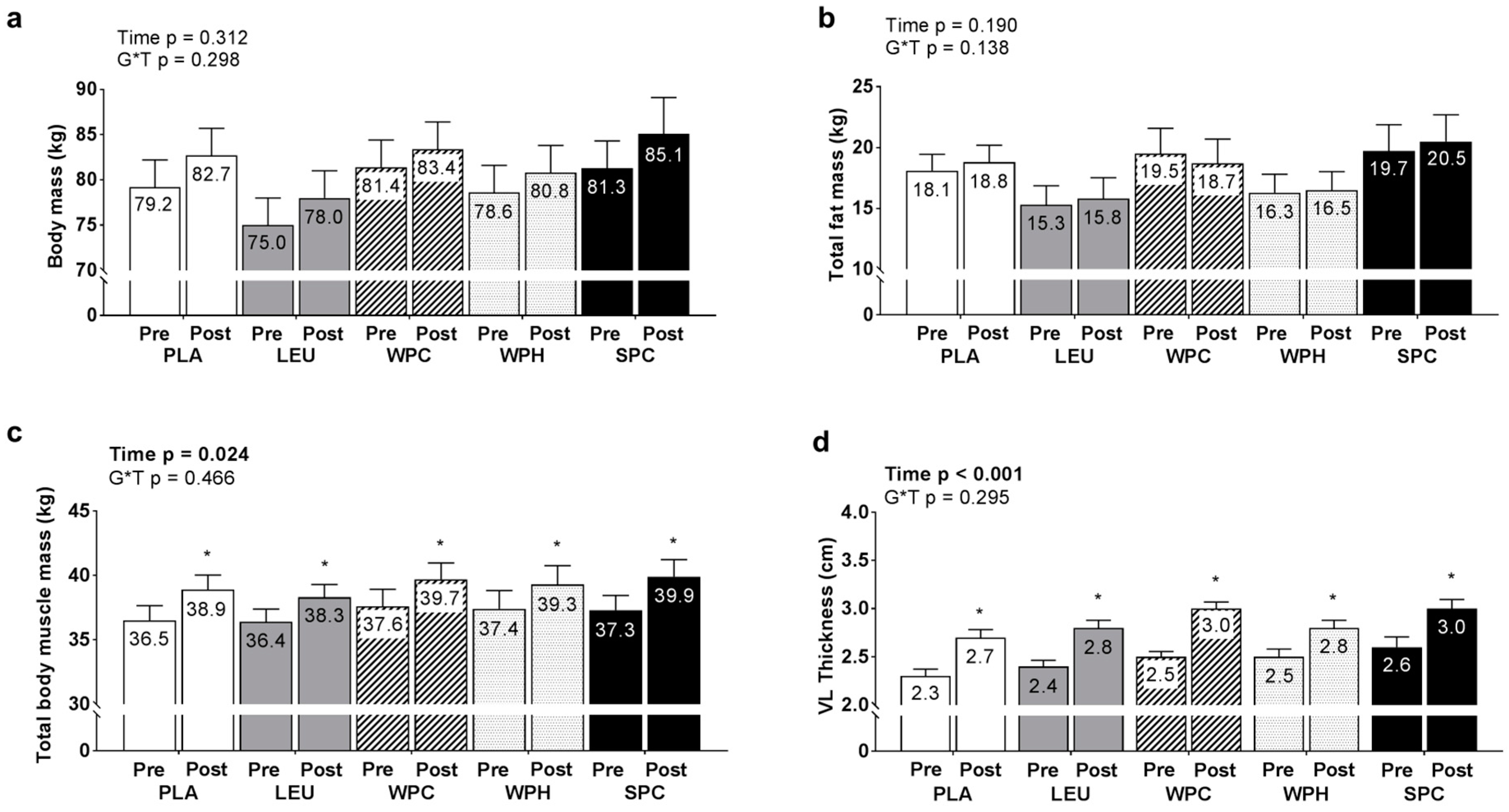
3.4. Changes in Body Mass, TBMM, Fat Mass, and Vastus Lateralis Muscle Thickness between Groups
3.5. Changes in Fiber Type-Specific CSA and Myonuclear Number as Well as Total Satellite Cell Number between Groups
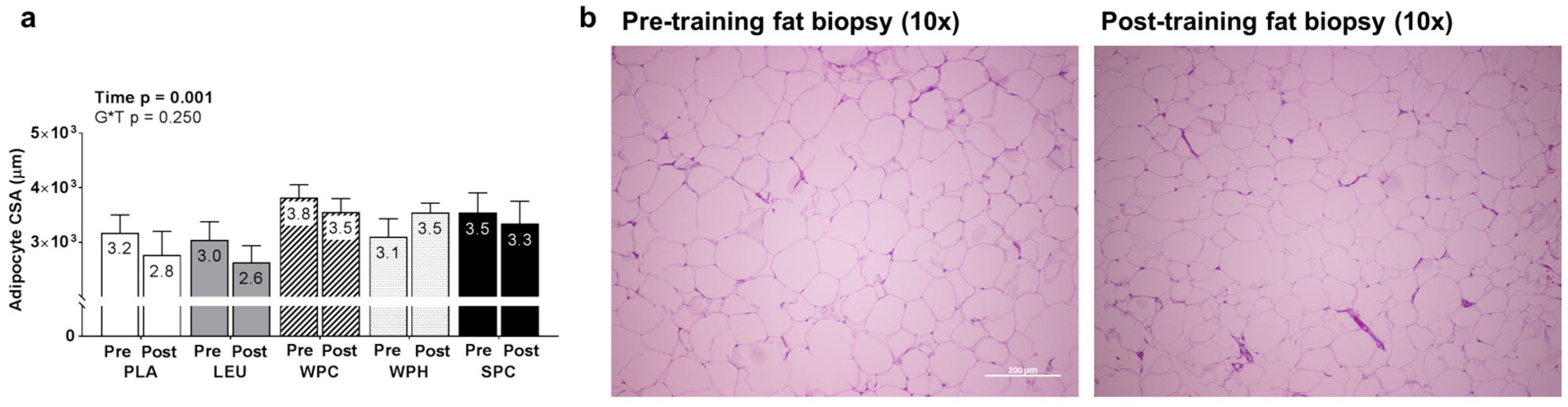
3.6. Changes in SQ Adipocyte CSA between Groups
4. Discussion
5. Experimental Considerations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cribb, P.J.; Williams, A.D.; Carey, M.F.; Hayes, A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; Chilibeck, P.D.; Davidson, K.S.; Candow, D.G.; Farthing, J.; Smith-Palmer, T. The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Burke, N.C.; Smith-Palmer, T.; Burke, D.G. Effect of whey and soy protein supplementation combined with resistance training in young adults. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Kovanen, V.; Selanne, H.; Kraemer, W.J.; Hakkinen, K.; Mero, A.A. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 2009, 37, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Volk, B.M.; Gomez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Aristizabal, J.C.; Saenz, C.; Dunn-Lewis, C.; Ballard, K.D.; et al. Whey protein supplementation during resistance training augments lean body mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Pacelli, Q.F.; Cancellara, P.; Toniolo, L.; Moro, T.; Canato, M.; Miotti, D.; Neri, M.; Morra, A.; Quadrelli, M.; et al. Protein supplementation does not further increase latissimus dorsi muscle fiber hypertrophy after eight weeks of resistance training in novice subjects, but partially counteracts the fast-to-slow muscle fiber transition. Nutrients 2016, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; Fry, C.S.; Igbinigie, S.; Deer, R.R.; Jennings, K.; Cope, M.B.; Mukherjea, R.; Volpi, E.; Rasmussen, B.B. Protein supplementation does not affect myogenic adaptations to resistance training. Med. Sci. Sports Exerc. 2017, 49, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; Borack, M.S.; Markofski, M.M.; Dickinson, J.M.; Deer, R.R.; Husaini, S.H.; Walker, D.K.; Igbinigie, S.; Robertson, S.M.; Cope, M.B.; et al. Protein supplementation has minimal effects on muscle adaptations during resistance exercise training in young men: A double-blind randomized clinical trial. J. Nutr. 2016, 146, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.M.; Roberts, M.D.; Dalbo, V.J.; Smith-Ryan, A.E.; Kendall, K.L.; Moon, J.R.; Stout, J.R. Effects of hydrolyzed whey versus other whey protein supplements on the physiological response to 8 weeks of resistance exercise in college-aged males. J. Am. Coll. Nutr. 2017, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.P.; Tarpenning, K.M.; Marino, F.E. Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur. J. Appl. Physiol. 2006, 97, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Vieillevoye, S.; Poortmans, J.R.; Duchateau, J.; Carpentier, A. Effects of a combined essential amino acids/carbohydrate supplementation on muscle mass, architecture and maximal strength following heavy-load training. Eur. J. Appl. Physiol. 2010, 110, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.; Grala, A.P.; da Silva, R.A.; Soares-Caldeira, L.F.; Pacagnelli, F.L.; Ribeiro, A.S.; da Silva, D.K.; de Andrade, W.B.; Balvedi, M.C.W. Free leucine supplementation during an 8-week resistance training program does not increase muscle mass and strength in untrained young adult subjects. Amino Acids 2017, 49, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in c2c12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.E.; Layman, D.K.; Bunpo, P.; Anthony, T.G.; Brana, D.V.; Garlick, P.J. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J. Nutr. 2009, 139, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.B.; Fox, C.D.; Ferguson, B.S.; Pascoe, C.A.; Healy, J.C.; McAdam, J.S.; Lockwood, C.M.; Roberts, M.D. Effects of protein type and composition on postprandial markers of skeletal muscle anabolism, adipose tissue lipolysis, and hypothalamic gene expression. J. Int. Soc. Sports Nutr. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Cruthirds, C.L.; Lockwood, C.M.; Pappan, K.; Childs, T.E.; Company, J.M.; Brown, J.D.; Toedebusch, R.G.; Booth, F.W. Comparing serum responses to acute feedings of an extensively hydrolyzed whey protein concentrate versus a native whey protein concentrate in rats: A metabolomics approach. Appl. Physiol. Nutr. Metab. 2014, 39, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wang, Z.; Heymsfield, S.B.; Baumgartner, R.N.; Gallagher, D. Total-body skeletal muscle mass: Estimation by a new dual-energy X-ray absorptiometry method. Am. J. Clin. Nutr. 2002, 76, 378–383. [Google Scholar] [PubMed]
- Kephart, W.C.; Wachs, T.D.; Mac Thompson, R.; Brooks Mobley, C.; Fox, C.D.; McDonald, J.R.; Ferguson, B.S.; Young, K.C.; Nie, B.; Martin, J.S.; et al. Ten weeks of branched-chain amino acid supplementation improves select performance and immunological variables in trained cyclists. Amino Acids 2016, 48, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Lohman, M.; Tallroth, K.; Kettunen, J.A.; Marttinen, M.T. Reproducibility of dual-energy X-ray absorptiometry total and regional body composition measurements using different scanning positions and definitions of regions. Metabolism 2009, 58, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Kephart, W.C.; Mobley, C.B.; Fox, C.D.; Pascoe, D.D.; Sefton, J.M.; Wilson, T.J.; Goodlett, M.D.; Kavazis, A.N.; Roberts, M.D.; Martin, J.S. A single bout of whole-leg, peristaltic pulse external pneumatic compression upregulates pgc-1alpha mrna and endothelial nitric oxide sythase protein in human skeletal muscle tissue. Exp. Physiol. 2015, 100, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.S.; Kephart, W.C.; Mobley, C.B.; Wilson, T.J.; Goodlett, M.D.; Roberts, M.D. A single 60-min bout of peristaltic pulse external pneumatic compression transiently upregulates phosphorylated ribosomal protein s6. Clin. Physiol. Funct. Imaging 2016. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.S.; Kephart, W.C.; Haun, C.T.; McCloskey, A.E.; Shake, J.J.; Mobley, C.B.; Goodlett, M.D.; Kavazis, A.; Pascoe, D.D.; Zhang, L.; et al. Impact of external pneumatic compression target inflation pressure on transcriptome-wide rna expression in skeletal muscle. Physiol. Rep. 2016, 4, e13029. [Google Scholar] [CrossRef] [PubMed]
- Haun, C.T.; Roberts, M.D.; Romero, M.A.; Osburn, S.C.; Mobley, C.B.; Anderson, R.G.; Goodlett, M.D.; Pascoe, D.D.; Martin, J.S. Does external pneumatic compression treatment between bouts of overreaching resistance training sessions exert differential effects on molecular signaling and performance-related variables compared to passive recovery? An exploratory study. PLoS ONE 2017, 12, e0180429. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.L.; McBride, J.M.; Cormie, P.; McCaulley, G.O. Relationship between countermovement jump performance and multijoint isometric and dynamic tests of strength. J. Strength Cond. Res. 2008, 22, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hoffman, J.R.; Tanigawa, S.; Miramonti, A.A.; La Monica, M.B.; Beyer, K.S.; Church, D.D.; Fukuda, D.H.; Stout, J.R. Isometric mid-thigh pull correlates with strength, sprint, and agility performance in collegiate rugby union players. J. Strength Cond. Res. 2016, 30, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Beckham, G.; Mizuguchi, S.; Carter, C.; Sato, K.; Ramsey, M.; Lamont, H.; Hornsby, G.; Haff, G.; Stone, M. Relationships of isometric mid-thigh pull variables to weightlifting performance. J. Sports Med. Phys. Fit. 2013, 53, 573–581. [Google Scholar]
- Kraska, J.M.; Ramsey, M.W.; Haff, G.G.; Fethke, N.; Sands, W.A.; Stone, M.E.; Stone, M.H. Relationship between strength characteristics and unweighted and weighted vertical jump height. Int. J. Sports Physiol. Perform. 2009, 4, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Leary, B.K.; Statler, J.; Hopkins, B.; Fitzwater, R.; Kesling, T.; Lyon, J.; Phillips, B.; Bryner, R.W.; Cormie, P.; Haff, G.G. The relationship between isometric force-time curve characteristics and club head speed in recreational golfers. J. Strength Cond. Res. 2012, 26, 2685–2697. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.G.; Ruben, R.P.; Lider, J.; Twine, C.; Cormie, P. A comparison of methods for determining the rate of force development during isometric midthigh clean pulls. J. Strength Cond. Res. 2015, 29, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Rhea, M.R.; Ball, S.D.; Phillips, W.T.; Burkett, L.N. A comparison of linear and daily undulating periodized programs with equated volume and intensity for strength. J. Strength Cond. Res. 2002, 16, 250–255. [Google Scholar] [PubMed]
- Prestes, J.; Frollini, A.B.; de Lima, C.; Donatto, F.F.; Foschini, D.; de Cassia Marqueti, R.; Figueira, A., Jr.; Fleck, S.J. Comparison between linear and daily undulating periodized resistance training to increase strength. J. Strength Cond. Res. 2009, 23, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Klemp, A.; Dolan, C.; Quiles, J.M.; Blanco, R.; Zoeller, R.F.; Graves, B.S.; Zourdos, M.C. Volume-equated high- and low-repetition daily undulating programming strategies produce similar hypertrophy and strength adaptations. Appl. Physiol. Nutr. Metab. 2016, 41, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Simao, R.; Spineti, J.; de Salles, B.F.; Matta, T.; Fernandes, L.; Fleck, S.J.; Rhea, M.R.; Strom-Olsen, H.E. Comparison between nonlinear and linear periodized resistance training: Hypertrophic and strength effects. J. Strength Cond. Res. 2012, 26, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Day, M.L.; McGuigan, M.R.; Brice, G.; Foster, C. Monitoring exercise intensity during resistance training using the session rpe scale. J. Strength Cond. Res. 2004, 18, 353–358. [Google Scholar] [PubMed]
- Impellizzeri, F.M.; Rampinini, E.; Coutts, A.J.; Sassi, A.; Marcora, S.M. Use of rpe-based training load in soccer. Med. Sci. Sports Exerc. 2004, 36, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Zourdos, M.C.; Klemp, A.; Dolan, C.; Quiles, J.M.; Schau, K.A.; Jo, E.; Helms, E.; Esgro, B.; Duncan, S.; Garcia Merino, S.; et al. Novel resistance training-specific rating of perceived exertion scale measuring repetitions in reserve. J. Strength Cond. Res. 2016, 30, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, K.R.; Young, W.B.; Fahrner, B.; Harvey, J.T. Different methods of training load quantification and their relationship to injury and illness in elite Australian football. J. Sci. Med. Sport 2016, 19, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Snijders, T.; Halson, S.L.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Pre-sleep dietary protein-derived amino acids are incorporated in myofibrillar protein during post-exercise overnight recovery. Am. J. Physiol. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.; Whelan, K.; Jeffries, O.; Burt, D.; Howe, L.; Patterson, S.D. The effects of acute branched-chain amino acid supplementation on recovery from a single bout of hypertrophy exercise in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Contreras, B.; Vigotsky, A.D.; Peterson, M. Differential effects of heavy versus moderate loads on measures of strength and hypertrophy in resistance-trained men. J. Sports Sci. Med. 2016, 15, 715–722. [Google Scholar] [PubMed]
- Jospe, M.R.; Fairbairn, K.A.; Green, P.; Perry, T.L. Diet app use by sports dietitians: A survey in five countries. JMIR mHealth uHealth 2015, 3, e7. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Orris, S.; Scheiner, M.; Gonzalez, A.; Peacock, C.A. A high protein diet (3.4 g/kg/day) combined with a heavy resistance training program improves body composition in healthy trained men and women—A follow-up investigation. J. Int. Soc. Sports Nutr. 2015, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Peacock, C.A.; Ellerbroek, A.; Fromhoff, B.; Silver, T. The effects of consuming a high protein diet (4.4 g/kg/day) on body composition in resistance-trained individuals. J. Int. Soc. Sports Nutr. 2014, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Peacock, C. The effects of a high protein diet on indices of health and body composition—A crossover trial in resistance-trained men. J. Int. Soc. Sports Nutr. 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Tamayo, A.; Buehn, R.; Peacock, C.A. A high protein diet has no harmful effects: A one-year crossover study in resistance-trained males. J. Nutr. Metab. 2016, 2016, 9104792. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Toedebusch, R.G.; Ruegsegger, G.; Mobley, C.B.; Fox, C.D.; McGinnis, G.R.; Quindry, J.C.; Booth, F.W.; Roberts, M.D.; Kavazis, A.N. Comparative adaptations in oxidative and glycolytic muscle fibers in a low voluntary wheel running rat model performing three levels of physical activity. Physiol. Rep. 2015, 3, e12619. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. Cellprofiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Mackey, A.L.; Kjaer, M.; Charifi, N.; Henriksson, J.; Bojsen-Moller, J.; Holm, L.; Kadi, F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 2009, 40, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.M.; Kephart, W.C.; Mumford, P.W.; Mobley, C.B.; Lowery, R.P.; Shake, J.J.; Patel, R.K.; Healy, J.C.; McCullough, D.J.; Kluess, H.A.; et al. Effects of a ketogenic diet on adipose tissue, liver, and serum biomarkers in sedentary rats and rats that exercised via resisted voluntary wheel running. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R337–R351. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.M.; Roberts, M.D.; Mumford, P.W.; Mobley, C.B.; Kephart, W.C.; Conover, C.F.; Beggs, L.A.; Balaez, A.; Otzel, D.M.; Yarrow, J.F.; et al. Testosterone inhibits expression of lipogenic genes in visceral fat by an estrogen-dependent mechanism. J. Appl. Physiol. 2016, 121, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Tufekovic, G.; Zebis, M.K.; Crameri, R.M.; Verlaan, G.; Kjaer, M.; Suetta, C.; Magnusson, P.; Aagaard, P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 2005, 54, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Stout, J.R.; Wilborn, C.D. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2007, 32, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Rahbek, S.K.; Vendelbo, M.H.; Matzon, A.; Hindhede, J.; Bejder, A.; Ringgard, S.; Vissing, K. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand. J. Med. Sci. Sports 2014, 24, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Cribb, P.J.; Williams, A.D.; Stathis, C.G.; Carey, M.F.; Hayes, A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med. Sci. Sports Exerc. 2007, 39, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Herda, A.A.; Herda, T.J.; Costa, P.B.; Ryan, E.D.; Stout, J.R.; Cramer, J.T. Muscle performance, size, and safety responses after eight weeks of resistance training and protein supplementation: A randomized, double-blinded, placebo-controlled clinical trial. J. Strength Cond. Res. 2013, 27, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Babault, N.; Paizis, C.; Deley, G.; Guerin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: A double-blind, randomized, placebo-controlled clinical trial vs. Whey protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Laakso, M.; Mero, A.A.; Hakkinen, K.; Ahtiainen, J.P.; Peltonen, H. The effects of whey protein with or without carbohydrates on resistance training adaptations. J. Int. Soc. Sports Nutr. 2015, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.K.; Kim, J.S.; Cross, J.M.; Kosek, D.J.; Bamman, M.M. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E937–E946. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Falvo, M.J.; Faigenbaum, A.D. Effect of protein intake on strength, body composition and endocrine changes in strength/power athletes. J. Int. Soc. Sports Nutr. 2006, 3, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Falvo, M.J.; Faigenbaum, A.D. Effects of protein supplementation on muscular performance and resting hormonal changes in college football players. J. Sports Sci. Med. 2007, 6, 85–92. [Google Scholar] [PubMed]
- Gillen, J.B.; Trommelen, J.; Wardenaar, F.C.; Brinkmans, N.Y.; Versteegen, J.J.; Jonvik, K.L.; Kapp, C.; de Vries, J.; van den Borne, J.J.; Gibala, M.J.; et al. Dietary protein intake and distribution patterns of well-trained dutch athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Moore, D.R.; Tang, J.E. A critical examination of dietary protein requirements, benefits, and excesses in athletes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S58–S76. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Caiozzo, V.J.; Haddad, F.; Baldwin, K.M. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am. J. Physiol. Cell Physiol. 2002, 283, C1182–C1195. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Gleeson, B.G.; Jonkers, R.A.; Meijer, K.; Savelberg, H.H.; Dendale, P.; van Loon, L.J. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Dalbo, V.J.; Hassell, S.E.; Brown, R.; Kerksick, C.M. Effects of preexercise feeding on markers of satellite cell activation. Med. Sci. Sports Exerc. 2010, 42, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Smeets, J.S.; van Kranenburg, J.; Kies, A.K.; van Loon, L.J.; Verdijk, L.B. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol. 2016, 216, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.K.; Kim, J.S.; Mayhew, D.L.; Cross, J.M.; Bamman, M.M. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: A cluster analysis. J. Appl. Physiol. 2008, 104, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Aagaard, P.; Kadi, F.; Tufekovic, G.; Verney, J.; Olesen, J.L.; Suetta, C.; Kjaer, M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J. Physiol. 2006, 573, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Rahbek, S.K.; Knudsen, I.S.; de Paoli, F.; Mackey, A.L.; Vissing, K. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 2014, 46, 2503–2516. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y. Role of matrix metalloproteinases in skeletal muscle: Migration, differentiation, regeneration and fibrosis. Cell Adhes. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef]
- Neidert, L.E.; Mobley, C.B.; Kephart, W.C.; Roberts, M.D.; Kluess, H.A. The serine protease, dipeptidyl peptidase iv as a myokine: Dietary protein and exercise mimetics as a stimulus for transcription and release. Physiol. Rep. 2016, 4, e12827. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Lippolis, J.D.; Nonnecke, B.J.; Sacco, R.E. Bovine milk exosome proteome. J. Proteom. 2012, 75, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [PubMed]
- Reidy, P.T.; Rasmussen, B.B. Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. J. Nutr. 2016, 146, 155–183. [Google Scholar] [CrossRef] [PubMed]

| Week | Training Paradigm | |
|---|---|---|
| 0 | Days 1–3 | Familiarization session, IMTP and 3-RM Testing |
| 1 | Day 1: 4 × 10 | 51% of Est. 1-RM |
| Day 2: 6 × 4 | 60% of Est. 1-RM | |
| Day 3: 5 × 6 | 56% of Est. 1-RM | |
| 2 | Day 1: 4 × 10 | 60% of Est. 1-RM |
| Day 2: 6 × 4 | 70% of Est. 1-RM | |
| Day 3: 5 × 6 | 65% of Est. 1-RM | |
| 3 | Day 1: 4 × 10 | 70% of Est. 1-RM |
| Day 2: 6 × 4 | 79% of Est. 1-RM | |
| Day 3: 5 × 6 | 74% of Est. 1-RM | |
| 4 | Day 1: 4 × 10 | 73% of Est. 1-RM |
| Day 2: 6 × 4 | 89% of Est. 1-RM | |
| Day 3: 5 × 6 | 84% of Est. 1-RM | |
| 5 | Day 1: 4 × 10 | 78% of Est. 1-RM |
| Day 2: 6 × 4 | 95% of Est. 1-RM | |
| Day 3: 5 × 6 | 90% of Est. 1-RM | |
| 6 | Day 1: 4 × 10 | 82% of Est. 1-RM |
| Day 2: 6 × 4 | 100% of Est. 1-RM | |
| Day 3: 5 × 6 | 94% of Est. 1-RM | |
| 7 | Day 1–3: 4 × 5 (de-load) | 60% of Est. 1-RM |
| 8 | Day 1: 4 × 10 | 74% of Est. 1-RM |
| Day 2: 6 × 4 | 90% of Est. 1-RM | |
| Day 3: 5 × 6 | 85% of Est. 1-RM | |
| 9 | Day 1: 4 × 10 | 83% of Est. 1-RM |
| Day 2: 6 × 4 | 101% of Est. 1-RM | |
| Day 3: 5 × 6 | 96% of Est. 1-RM | |
| 10 | Day 1: 4 × 10 | 87% of Est. 1-RM |
| Day 2: 6 × 4 | 107% of Est. 1-RM | |
| Day 3: 5 × 6 | 98% of Est. 1-RM | |
| 11 | Day 1: 4 × 10 | 90% of Est. 1-RM |
| Day 2: 6 × 4 | 109% of Est. 1-RM | |
| Day 3: 5 × 6 | 102% of Est. 1-RM | |
| 12 | Day 1–2: 4 × 5 (de-load) | 60% of Est. 1-RM |
| Day 3: IMTP and 3-RM Testing | 108% of Est. 1-RM | |
| Variable | PLA | LEU | WPC | WPH | SPC |
|---|---|---|---|---|---|
| Calories | 204 | 200 | 184 | 192 | 266 |
| Total Fat (g) | 2.8 | 2.0 | 3.5 | 4.6 | 4.5 |
| Saturated Fat (g) | 2.3 | 1.6 | 2.3 | 3.3 | 2.6 |
| Trans Fat (g) | 0.0 | 0.0 | 0.1 | 0.2 | 0.2 |
| Cholesterol (mg) | 3.8 | 2.9 | 74.0 | 74.3 | 5.3 |
| Total Carbohydrate (g) | 44.4 | 43.1 | 12.0 | 12.2 | 17.2 |
| Dietary Fiber (g) | 1.6 | 1.8 | 1.8 | 2.2 | 1.5 |
| Sugars (g) | 6.0 | 5.1 | 5.9 | 3.2 | 6.2 |
| Protein (g) | 0.4 | 2.3 | 26.3 | 25.4 | 39.2 |
| Alanine (mg) | 7 | 7 | 1397 | 1430 | 1646 |
| Arginine (mg) | 8 | 8 | 766 | 773 | 2969 |
| Aspartic Acid (mg) | 15 | 16 | 2881 | 3010 | 4537 |
| Cystine (mg) | 0 | 0 | 651 | 728 | 536 |
| Glutamic Acid (mg) | 36 | 35 | 4530 | 4730 | 7154 |
| Glycine (mg) | 6 | 6 | 489 | 543 | 1597 |
| Histidine (mg) | 0 | 0 | 470 | 477 | 910 |
| Isoleucine (mg) | 8 | 28 | 1736 | 1820 | 1842 |
| Leucine (mg) | 15 | 2871 | 2794 | 2910 | 2960 |
| Lysine (mg) | 11 | 79 | 2386 | 2640 | 2362 |
| Methionine (mg) | 0 | 8 | 598 | 611 | 540 |
| Phenylalanine (mg) | 8 | 9 | 861 | 908 | 1980 |
| Proline (mg) | 14 | 13 | 1630 | 1670 | 2029 |
| Serine (mg) | 10 | 9 | 1348 | 1400 | 1950 |
| Threonine (mg) | 7 | 7 | 1853 | 1900 | 1499 |
| Tryptophan | 0 | 0 | 482 | 525 | 501 |
| Tyrosine (mg) | 7 | 7 | 808 | 839 | 1480 |
| Valine (mg) | 11 | 14 | 1465 | 1530 | 1754 |
| Total EAAs (mg) | 60 | 3016 | 12,645 | 13,321 | 14,348 |
| Total BCAAs (mg) | 34 | 2913 | 5995 | 6260 | 6556 |
| Calcium (mg) | 15 | 15 | 155 | 152 | 165 |
| Iron (mg) | 0.38 | 0.35 | 0.63 | 1.04 | 5.21 |
| Potassium (mg) | 32 | 37 | 230 | 464 | 961 |
| Sodium (mg) | 91 | 105 | 133 | 310 | 217 |
| Vitamin D3 (IU) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Degree of hydrolysis (%) | N/A | N/A | N/A | 12.5 | N/A |
| M.W. range (%) | |||||
| >10.0 kD | - | - | 74.3 | 29.0 | 86.0 |
| 5.0–10.0 kD | - | - | 5.1 | 5.3 | 3.6 |
| 2.0–5.0 kD | - | - | 15.4 | 10.2 | 2.6 |
| 1.0–2.0 kD | - | - | 1.6 | 10.8 | 1.2 |
| 0.5–1.0 kD | - | - | 0.9 | 15.5 | 0.9 |
| <0.5 kD | - | - | 2.7 | 29.3 | 5.6 |
| Variable | PLA (n = 15) | LEU (n = 14) | WPC (n = 17) | WPH (n = 14) | SPC (n = 15) | ANOVA p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 21 ± 1 | 20 ± 1 | 21 ± 1 | 21 ± 1 | 21 ± 1 | 0.811 |
| Height (cm) | 183 ± 2 | 179 ± 1 | 179 ± 2 | 182 ± 2 | 182 ± 2 | 0.454 |
| Body Mass (kg) | 79 ± 3 | 75 ± 2 | 81 ± 3 | 79 ± 3 | 81 ± 3 | 0.600 |
| Lean body mass (kg) | 58 ± 4 | 57 ± 3 | 59 ± 4 | 59 ± 5 | 59 ± 4 | 0.899 |
| Total Fat Mass (kg) | 18 ± 3 | 15 ± 3 | 19 ± 5 | 16 ± 3 | 20 ± 5 | 0.378 |
| Strength 3-RM (kg) | ||||||
| Squat | 70 ± 8 | 83 ± 12 | 82 ± 11 | 79 ± 14 | 82 ± 10 | 0.369 |
| Bench press | 66 ± 8 | 67 ± 11 | 68 ± 8 | 73 ± 9 | 65 ± 7 | 0.650 |
| IMTP (N) | 3247 ± 215 | 3205 ± 170 | 3476 ± 141 | 3461 ± 130 | 3192 ± 117 | 0.488 |
| Variable | Baseline | Week 6 | Week 12 | ANCOVA p-Values |
|---|---|---|---|---|
| Group | ||||
| Energy intake (kcal/day) | Time p < 0.001 G*T p = 0.865 | |||
| PLA | 2109 ± 166 | 2756 ± 236 * | 2812 ± 232 * | |
| LEU | 1835 ± 116 | 2303 ± 165 * | 2488 ± 132 * | |
| WPC | 1866 ± 115 | 2305 ± 116 * | 2389 ± 177 * | |
| WPH | 2039 ± 149 | 2611 ± 156 * | 2617 ± 144 * | |
| SPC | 1853 ± 136 | 2461 ± 129 * | 2611 ± 158 * | |
| Protein intake (g/day; g/kg/day) | ||||
| PLA | 94 ± 7; 1.2 ± 0.1 | 109 ± 8c; 1.3 ± 0.1 b | 111 ± 11b; 1.3 ± 0.1 b | Time p < 0.001 G*T p < 0.001 |
| LEU | 87 ± 6; 1.2 ± 0.1 | 96 ± 8c; 1.3 ± 0.1 b | 108 ± 10 *,b; 1.4 ± 0.1 b | |
| WPC | 88 ± 6; 1.1 ± 0.1 | 142 ± 5 *,a; 1.8 ± 0.1 *,a | 145 ± 6 *,a; 1.8 ± 0.1 *,a,b | |
| WPH | 94 ± 8; 1.2 ± 0.1 | 160 ± 7 *,a,b; 2.0 ± 0.1 *,a | 153 ± 7 *,a; 1.9 ± 0.1 *,a | |
| SPC | 88 ± 6; 1.1 ± 0.1 | 176 ± 7 *,b; 2.1 ± 0.1 *,a | 179 ± 10; 2.1 ± 0.1 *,a | |
| Carbohydrate intake (g/day; g/kg/day) | ||||
| PLA | 244 ± 23; 3.1 ± 0.3 | 337 ± 21 *,a; 4.2 ± 0.3 *,a,b | 348 ± 29 *,a; 4.2 ± 0.3 *,a | Time p < 0.001 G*T p = 0.002 |
| LEU | 206 ± 17; 2.8 ± 0.3 | 303 ± 24 *,a,b; 4.0 ± 0.4 *,a | 310 ± 21 *,a,b; 4.1 ± 0.4 *,a,b | |
| WPC | 215 ± 14; 2.8 ± 0.3 | 231 ± 18b; 2.9 ± 0.3 b | 244 ± 16 *,b; 3.0 ± 0.2 b | |
| WPH | 208 ± 20; 2.7 ± 0.3 | 247 ± 15 *,b; 3.1 ± 0.3 *,a,b | 255 ± 16 *,b; 3.3 ± 0.3 *,a,b | |
| SPC | 203 ± 18; 2.6 ± 0.3 | 238 ± 16 *,b; 2.9 ± 0.2 b | 256 ± 20 *,b; 3.1 ± 0.3 *,a,b | |
| Fat intake (g/day; g/kg/day) | ||||
| PLA | 83 ± 9; 1.1 ± 0.1 | 106 ± 12 *; 1.3 ± 0.1 | 110 ± 12 *; 1.3 ± 0.1 * | Time p < 0.001 G*T p = 0.549 |
| LEU | 73 ± 5; 1.0 ± 0.1 | 79 ± 5; 1.0 ± 0.1 | 92 ± 6; 1.2 ± 0.1 | |
| WPC | 71 ± 5; 0.9 ± 0.1 | 87 ± 5 *; 1.1 ± 0.1 * | 93 ± 12; 1.2 ± 0.1 | |
| WPH | 81 ± 6; 1.1 ± 0.1 | 102 ± 9 *; 1.3 ± 0.2 | 101 ± 7; 1.3 ± 0.1 | |
| SPC | 73 ± 6; 0.9 ± 0.1 | 90 ± 7 *; 1.1 ± 0.1 * | 101 ± 10 *; 1.2 ± 0.1 * | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mobley, C.B.; Haun, C.T.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Kephart, W.C.; Anderson, R.G.; Vann, C.G.; Osburn, S.C.; Pledge, C.D.; et al. Effects of Whey, Soy or Leucine Supplementation with 12 Weeks of Resistance Training on Strength, Body Composition, and Skeletal Muscle and Adipose Tissue Histological Attributes in College-Aged Males. Nutrients 2017, 9, 972. https://doi.org/10.3390/nu9090972
Mobley CB, Haun CT, Roberson PA, Mumford PW, Romero MA, Kephart WC, Anderson RG, Vann CG, Osburn SC, Pledge CD, et al. Effects of Whey, Soy or Leucine Supplementation with 12 Weeks of Resistance Training on Strength, Body Composition, and Skeletal Muscle and Adipose Tissue Histological Attributes in College-Aged Males. Nutrients. 2017; 9(9):972. https://doi.org/10.3390/nu9090972
Chicago/Turabian StyleMobley, C. Brooks, Cody T. Haun, Paul A. Roberson, Petey W. Mumford, Matthew A. Romero, Wesley C. Kephart, Richard G. Anderson, Christopher G. Vann, Shelby C. Osburn, Coree D. Pledge, and et al. 2017. "Effects of Whey, Soy or Leucine Supplementation with 12 Weeks of Resistance Training on Strength, Body Composition, and Skeletal Muscle and Adipose Tissue Histological Attributes in College-Aged Males" Nutrients 9, no. 9: 972. https://doi.org/10.3390/nu9090972






