The Impact of Sex and 25(OH)D Deficiency on Metabolic Function in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dietary Intervention
2.2. Serum Vitamin D (25(OH)D) Concentration
2.3. Body Composition Changes
2.4. Histopathological Assessment of Liver Pathology
2.5. Hepatic Cytokine Expression
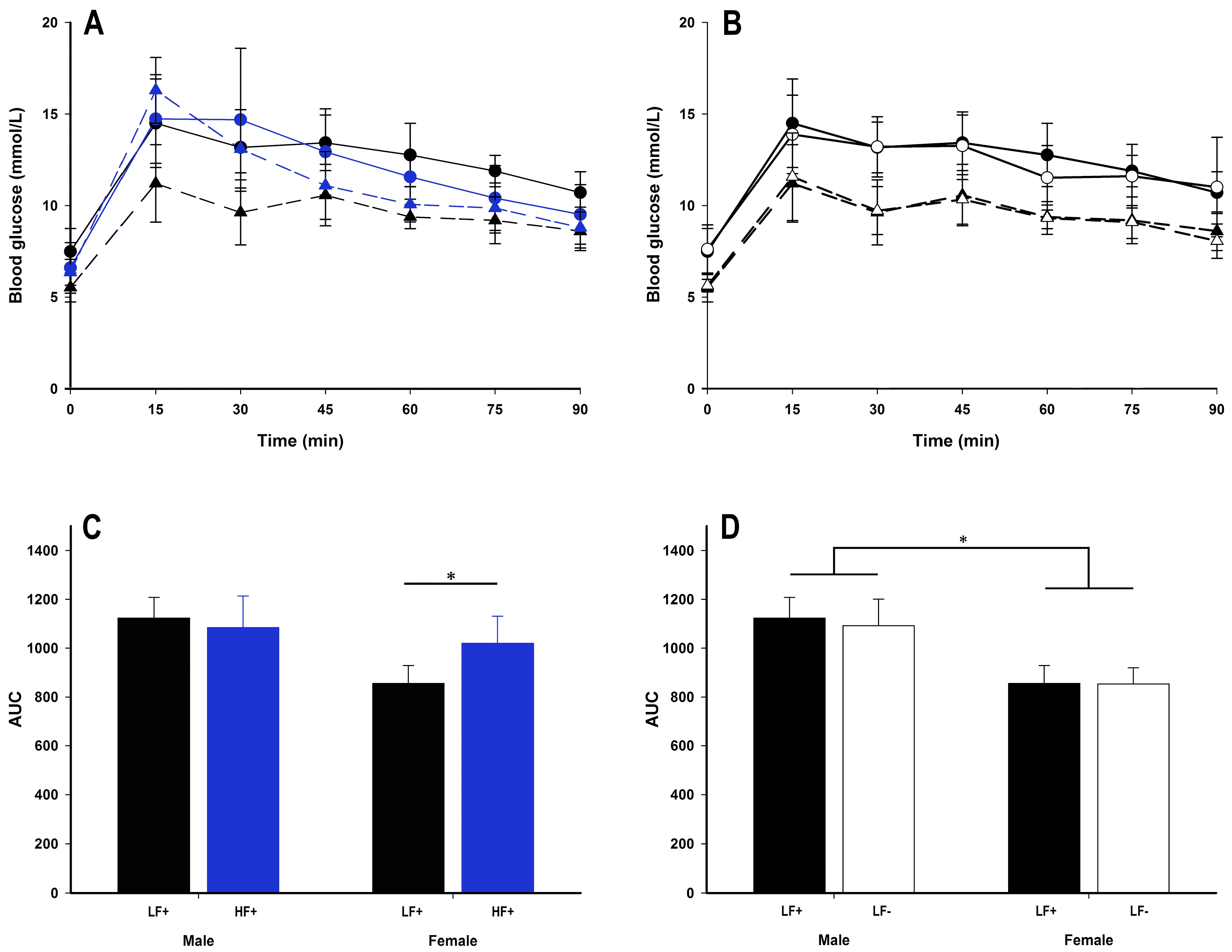
2.6. Glucose Tolerance Test
2.7. Serum Insulin Concentration
2.8. Statistical Analyses
3. Results
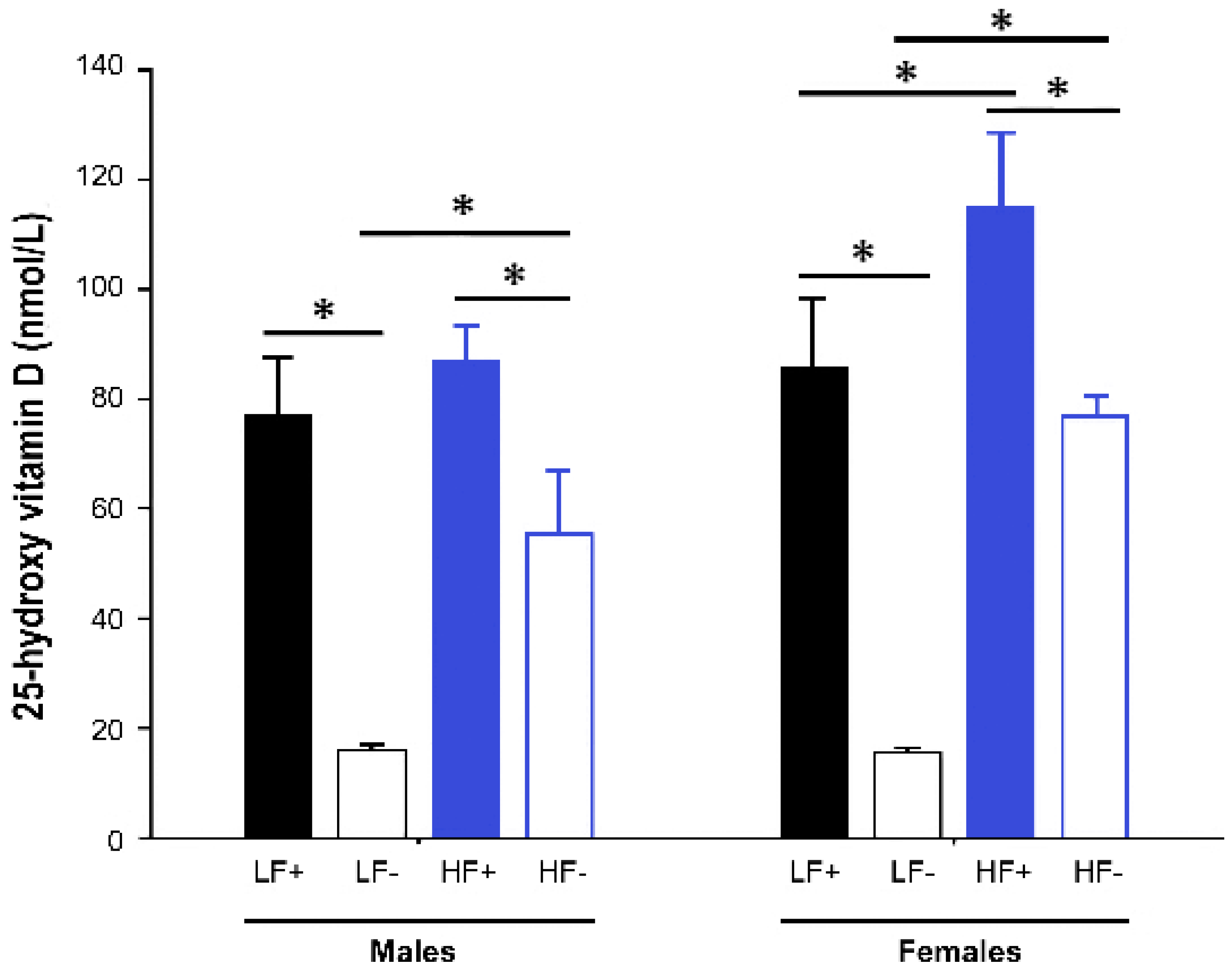
3.1. Serum 25-Hydroxy Vitamin D Concentration
- The effect of a high fat diet was assessed by comparing outcomes in the replete mice on high fat and low fat diets (LF+ vs. HF+)
- The effect of vitamin D deficiency was assessed by comparing outcomes in the low fat groups (LF+ vs. LF−)
- The modifying effect of vitamin D concentration on the high fat response was assessed by examining the relationship between vitamin D levels (as a continuous variable) and outcomes in mice on the high fat diets (both HF+ and HF−).
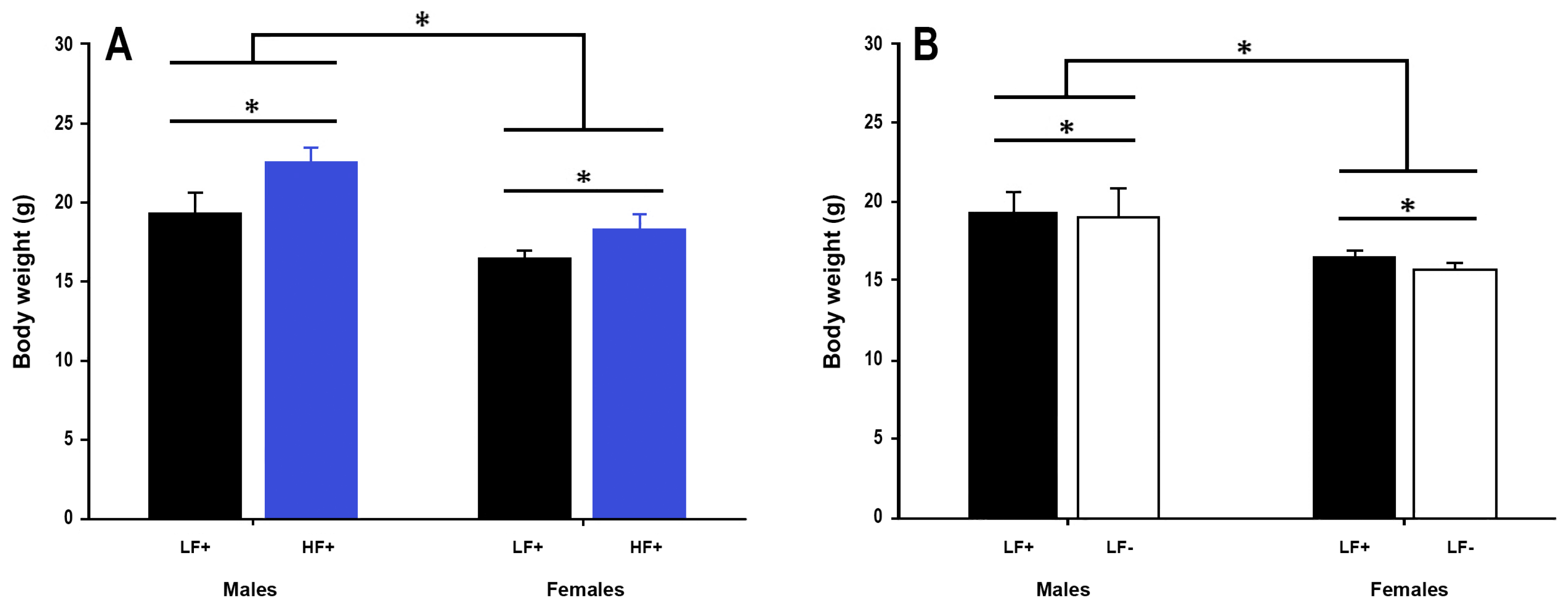
3.2. Body Weight and Length
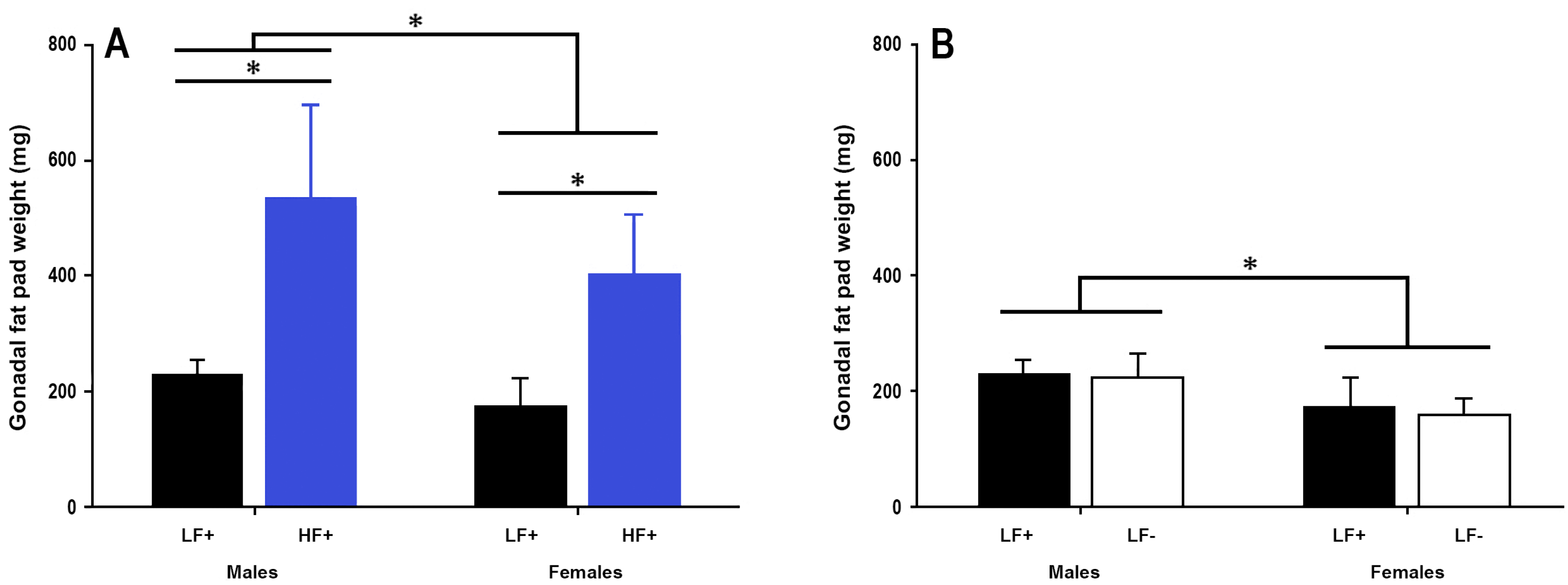
3.3. Gonadal Fat Pad Weight
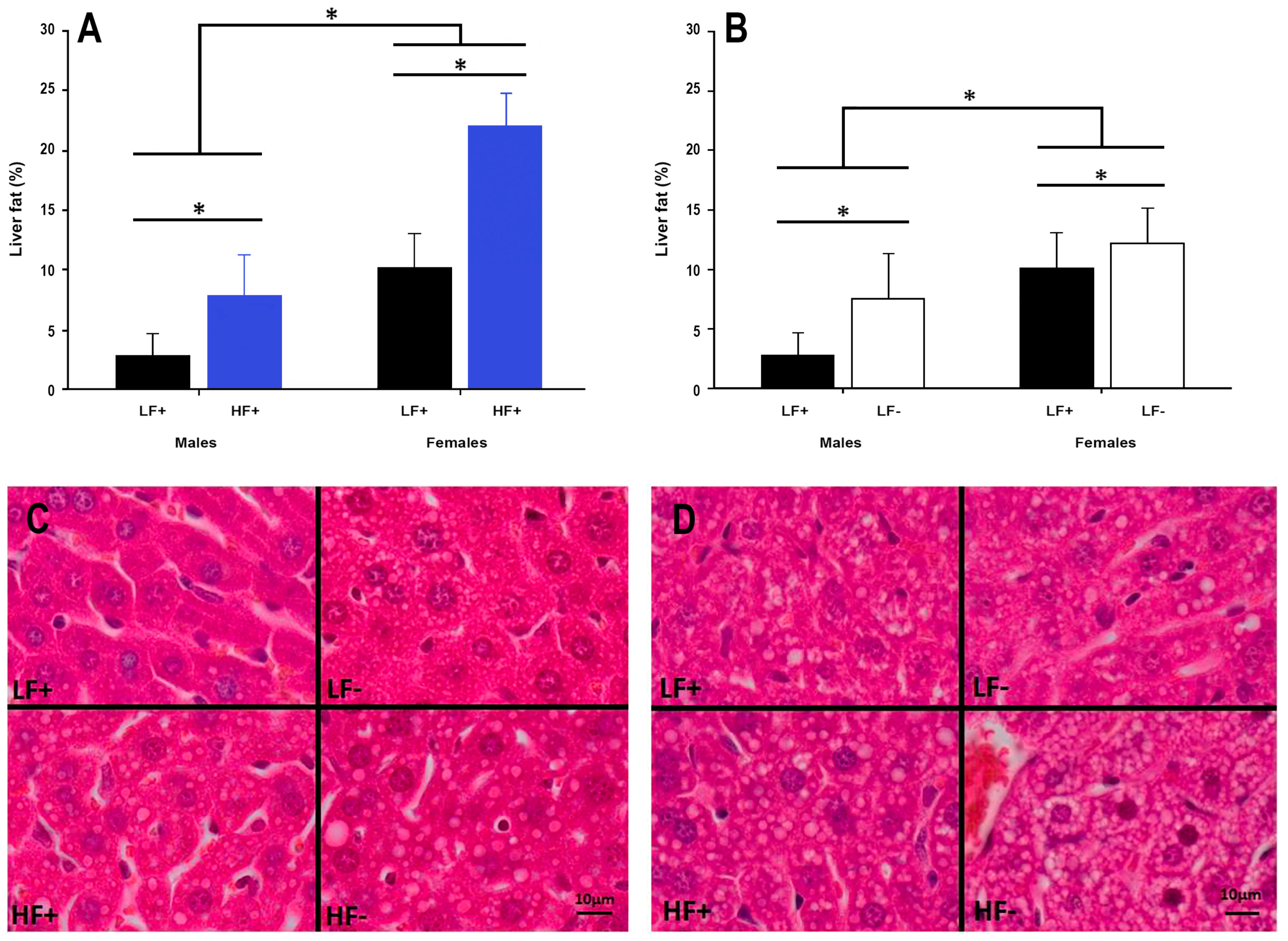
3.4. Hepatic Fat Content
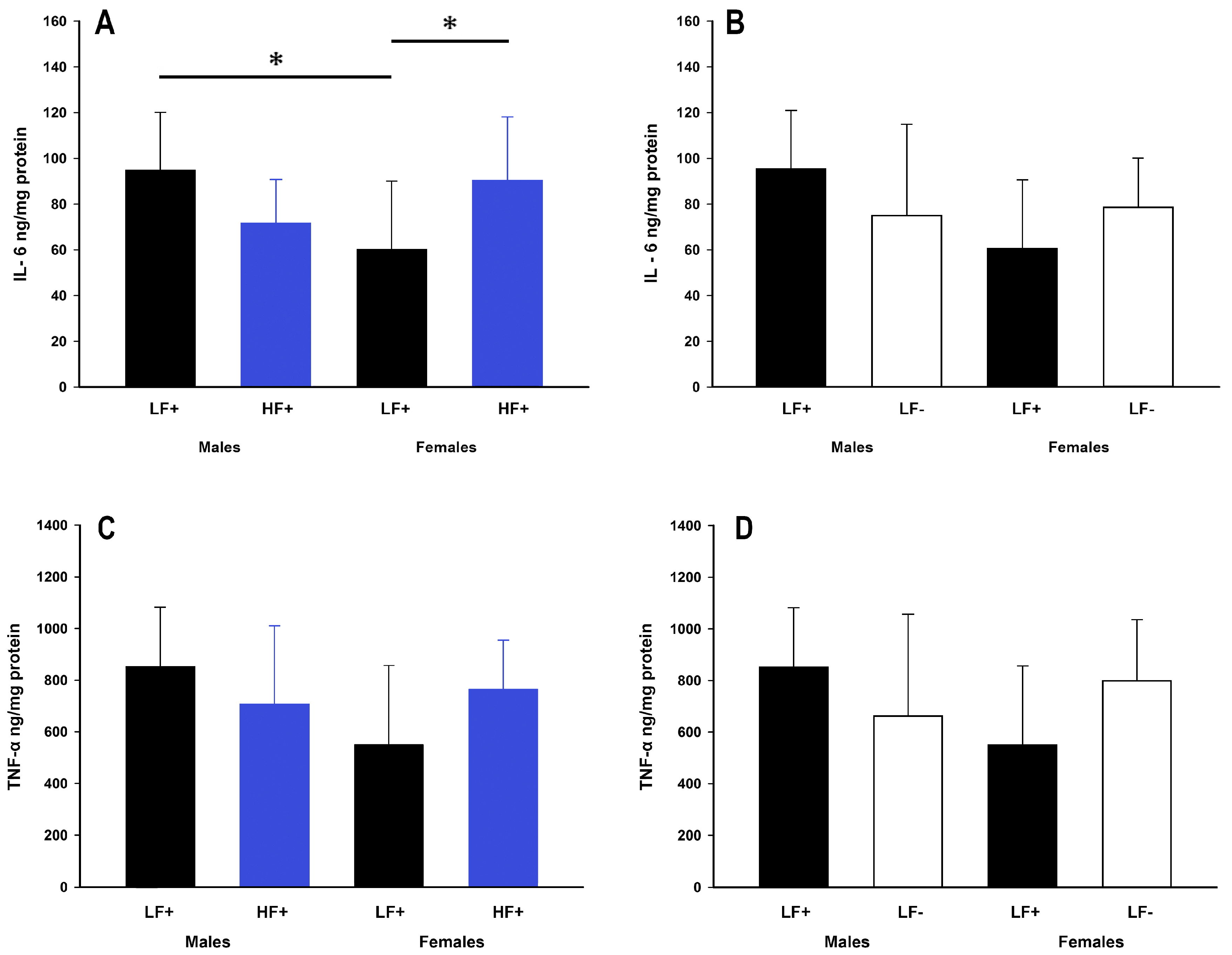
3.5. Hepatic Inflammation
3.6. Insulin Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkar, D.; Kamani, P.; Patel, N.; Gupte, P.; Kumar, P.; Agal, S.; Baijal, R.; Lala, S.; Chaudhary, D.; Deshpande, A. Prevalence of non-alcoholic fatty liver disease: Population based study. Ann. Hepatol. 2007, 6, 161–163. [Google Scholar] [PubMed]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Clegg, D.J.; Prossnitz, E.R.; Barton, M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiol. 2011, 203, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 147. [Google Scholar] [CrossRef]
- Williams, C.M. Lipid metabolism in women. Proc. Nutr. Soc. 2004, 63, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Paterson, J.; Shinyama, H.; Morton, N.M.; Mullins, J.J.; Seckl, J.R.; Flier, J.S. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001, 294, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Oron-Herman, M.; Kamari, Y.; Grossman, E.; Yeger, G.; Peleg, E.; Shabtay, Z.; Shamiss, A.; Sharabi, Y. Metabolic syndrome: Comparison of the two commonly used animal models. Am. J. Hypertens. 2008, 21, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; McGuire, L.C.; Liu, S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care 2005, 28, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Laaksonen, M.; Mattila, C.; Härkänen, T.; Marniemi, J.; Heliövaara, M.; Rissanen, H.; Montonen, J.; Reunanen, A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008, 19, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Chu, A.; Go, V.L.W.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [PubMed]
- Barchetta, I.; Angelico, F.; Del Ben, M.; Baroni, M.G.; Pozzilli, P.; Morini, S.; Cavallo, M.G. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25 (OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.; Potter, J.; Koteish, A.; Clark, J.; Guallar, E.; Hernaez, R. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; DePeter, K.C.; Feldman, H.A.; Grace, E.; Emans, S.J. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004, 158, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Elfers, C.T.; Figlewicz, D.P.; Melhorn, S.J.; Morton, G.J.; Hoofnagle, A.; Yeh, M.M.; Nelson, J.E.; Kowdley, K.V. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and toll-like receptor activation. Hepatology 2012, 55, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, S.; Hart, P.H.; Endersby, R.; Jacoby, P.; Feelisch, M.; Weller, R.B.; Matthews, V.; Gorman, S. Ultraviolet radiation suppresses obesity and symptoms of metabolic syndrome independently of vitamin D in mice fed a high-fat diet. Diabetes 2014, 63, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Berry, L.J.; Elliot, J.G.; James, A.L.; Gorman, S.; Hart, P.H. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am. J. Respir. Crit. Care Med. 2011, 183, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Catta-Preta, M.; Mendonca, L.S.; Fraulob-Aquino, J.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A critical analysis of three quantitative methods of assessment of hepatic steatosis in liver biopsies. Virchows Arch. 2011, 459, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Franzén, L.E.; Ekstedt, M.; Kechagias, S.; Bodin, L. Semiquantitative evaluation overestimates the degree of steatosis in liver biopsies: A comparison to stereological point counting. Mod. Pathol. 2005, 18, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Del Ben, M.; Angelico, F.; Di Martino, M.; Fraioli, A.; La Torre, G.; Saulle, R.; Perri, L.; Morini, S.; Tiberti, C.; et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. BMC Med. 2016, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Moosazadeh, M.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Kolahdooz, F.; Samimi, M.; Asemi, Z. The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Yasoshima, A.; Doi, K.; Nakayama, H.; Uetsuka, K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp. Anim. 2007, 56, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E. Vitamin D: A new player in non-alcoholic fatty liver disease? World J. Gastroenterol. WJG 2015, 21, 1718. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef] [PubMed]

| Diet | Carbohydrate | Protein | Fat | Micronutrients * | |||
|---|---|---|---|---|---|---|---|
| Sucrose | Starch | Fiber | Casein | Canola Oil | Lard | ||
| Low Fat | 10.0 | 53.4 | 5.0 | 20.0 | 5.0 | - | 6.6 |
| High Fat | 10.0 | 30.6 | 5.0 | 20.0 | 2.9 | 20.7 | 10.8 |
| Outcome | 25-Hydroxy Vitamin D | Sex | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Body weight | 0.007 (−0.021 0.035) | 0.62 | 5.7 (4.5 7.0) | <0.001 |
| Body length | 0.000 (−0.050 0.051) | 0.99 | 5.1 (2.8 7.4) | <0.001 |
| Gonadal fad pad | 2.507 (0.012 5.002) | 0.049 | 234.3 (120.9 347.6) | <0.001 |
| Liver fat % | −0.162 (−0.272 −0.051) | 0.006 | −15.9 (−20.9 −10.9) | <0.001 |
| GTT peak | 0.021 (−0.029 0.071) | 0.40 | 1.9 (−0.4 4.2) | 0.10 |
| AUC | −1.051 (−3.560 1.458) | 0.40 | 134.7 (20.7 248.6) | 0.02 |
| Fasting glucose | 0.030 (−0.007 0.068) | 0.11 | 1.3 (−0.4 3.0) | 0.12 |
| Fasting insulin | 0.013 (0.006 0.019) | 0.007 | 0.6 (0.2 1.0) | 0.004 |
| HOMA-IR | 0.009 (0.001 0.018) | 0.04 | 0.4 (0.0 0.8) | 0.04 |
| IL-6 protein | 0.199 (−0.520 0.919) | 0.06 | −32.7 (−65.6 0.2) | 0.05 |
| TNF-α protein | 4.117 (−2.368 10.602) | 0.20 | −152.5 (−449.1 144.1) | 0.30 |
| Sex | Diet | Fasting Blood Glucose | Fasting Insulin | HOMA-IR |
|---|---|---|---|---|
| (mmol/L) | (mU/L) | |||
| Males | LF+ | 10.6 ± 1.9 | 4.06 ± 0.74 | 1.9 ± 0.5 |
| HF+ | 10.6 ± 1.8 | 4.06 ± 0.54 | 1.9 ± 0.5 | |
| Females | LF+ | 8.9 ± 1.8 | 3.96 ± 0.79 | 1.5 ± 0.2 |
| HF+ | 9.7 ± 1.6 | 3.90 ± 0.57 | 1.7 ± 0.4 |
| Sex | Diet | Fasting Blood Glucose | Fasting Insulin | HOMA-IR |
|---|---|---|---|---|
| (mmol/L) | (mU/L) | |||
| Males | LF+ | 10.6 ± 1.9 | 4.06 ± 0.74 | 1.9 ± 0.5 * |
| LF− | 9.8 ± 2.7 | 3.73 ± 0.41 | 1.6 ± 0.6 * | |
| Females | LF+ | 8.9 ± 1.8 | 3.96 ± 0.79 | 1.5 ± 0.2 |
| LF− | 8.4 ± 1.0 | 3.56 ± 0.29 | 1.4 ± 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giblin, R.J.; Bennett, E.J.; Zosky, G.R.; Dwyer, R.M. The Impact of Sex and 25(OH)D Deficiency on Metabolic Function in Mice. Nutrients 2017, 9, 985. https://doi.org/10.3390/nu9090985
Giblin RJ, Bennett EJ, Zosky GR, Dwyer RM. The Impact of Sex and 25(OH)D Deficiency on Metabolic Function in Mice. Nutrients. 2017; 9(9):985. https://doi.org/10.3390/nu9090985
Chicago/Turabian StyleGiblin, Ryan J., Ellen J. Bennett, Graeme R. Zosky, and Renée M. Dwyer. 2017. "The Impact of Sex and 25(OH)D Deficiency on Metabolic Function in Mice" Nutrients 9, no. 9: 985. https://doi.org/10.3390/nu9090985





