Relationship between the Nutritional Status of Vitamin A per Trimester of Pregnancy with Maternal Anthropometry and Anemia after Roux-en-Y Gastric Bypass
Abstract
:1. Introduction
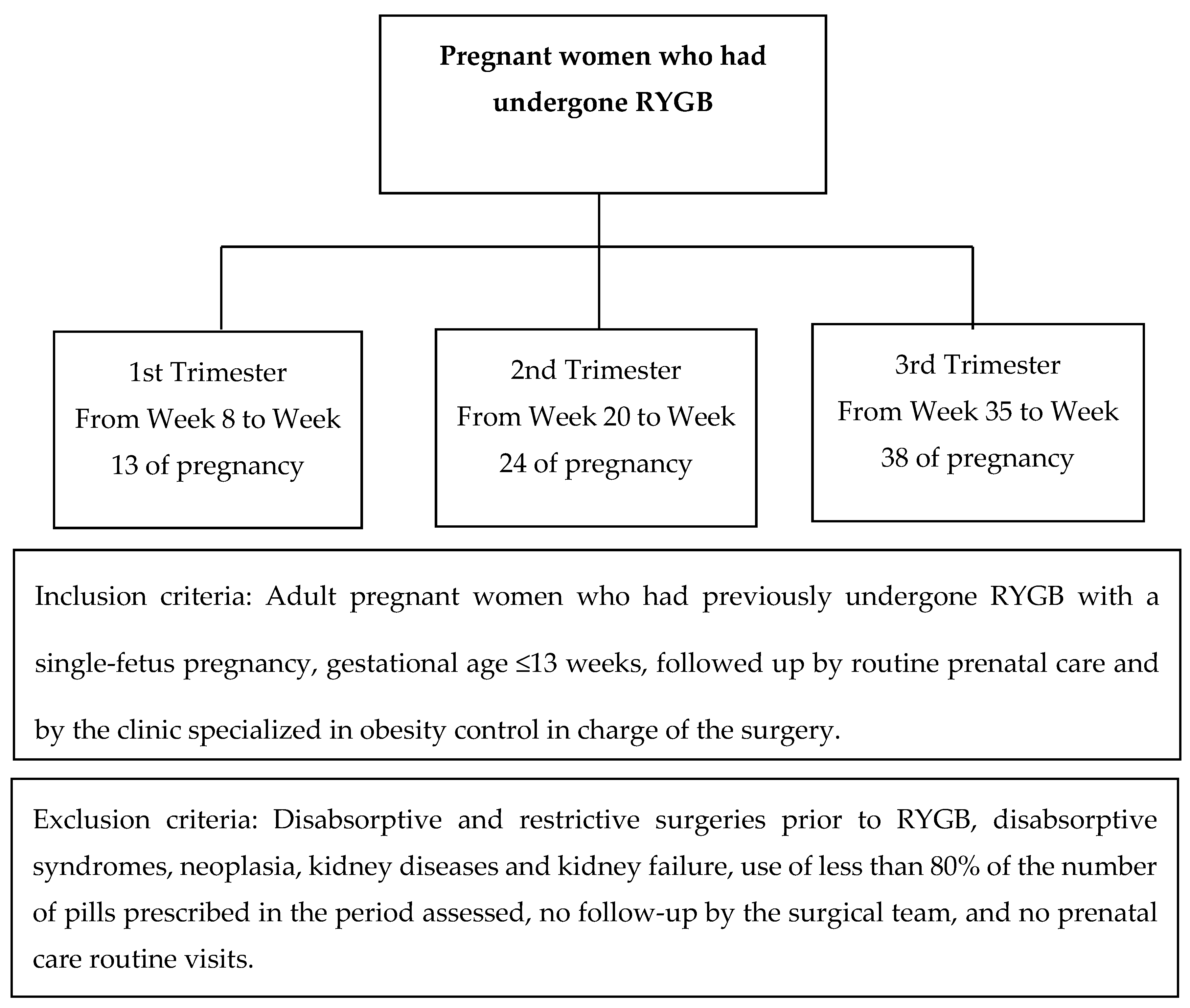
2. Methodology
3. Results
3.1. Sample Characterization
3.2. Biochemical and Functional Assessment of Vitamin A
3.3. Biochemical and Functional Assessment of Vitamin A According to Severity
3.4. Relationship between the Presence of Anemia and Vitamin A
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González, I.; Rubio, M.A.; Cordido, F.; Bretón, I.; Morales, M.J.; Vilarrasa, N.; Monereo, S.; Lecube, A.; Caixás, A.; Vinagre, I.; et al. Maternal and perinatal outcomes after bariatric surgery: A Spanish multicenter study. Obes. Surg. 2015, 25, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Benoit, S.C.; Hunter, T.D.; Francis, D.M.; De La Cruz-Munoz, N. Use of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgery. Obes. Surg. 2014, 24, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, B.; Bermano, G.; Rolland, C. Obesity and its health impact in Africa: A systematic review. Cardiovasc. J. Africa 2012, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Chaichian, S.; Moazzami, B.; Jesmi, F.; Pazouki, A.; Pishgahroudsari, M.; Mokhber, S.; Riazi, S. The controversy of the most proper time for pregnancy after bariatric surgery: A review of ten cases. Obes. Surg. 2016, 26, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Oien, D.M. Metabolic/bariatric surgery worldwide 2011. Obes. Surg. 2013, 23, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Fandiño, J.; Benchimol, A.K.; Coutinho, W.F.; Appolinário, J.C. Cirurgia bariátrica: Aspectos clínico-cirúrgicos e psiquiátricos. Rev. Psiquiatr. 2004, 26, 47–51. [Google Scholar] [CrossRef]
- Bloomberg, R.D.; Fleishman, A.; Nalle, J.E.; Kini, S. Nutritional deficiencies following bariatric surgery: What have we learned? Obes. Surg. 2005, 2, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Organização Mundial Da Saúde. Vitamin and Mineral Nutrition Information System (Vmnis). 2011. Available online: http://www.who.int/vmnis/en/index.html (accessed on 7 August 2015).
- Thorne-Lyman, A.L.; Fawzi, W.W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Jans, G.; Matthys, C.; Bogaerts, A.; Lannoo, M.; Verhaeghe, J.; Van der Schueren, B.; Devlieger, R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: A systematicreview. Adv. Nutr. 2015, 6, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, J.; Liu, Z.; Yun, C.; Piao, J.; Yang, X. Prevalence and influence factors of vitamin A deficiency of Chinese pregnant women. Nutr. J. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Devlieger, R.; Guelinckx, I.; Jans, G.; Voets, W.; Vanholsbeke, C.; Vansant, G. Micronutrient levels and supplement intake in pregnancy afterbariatric surgery: A prospective cohort study. PLoS ONE 2014, 9, 114–192. [Google Scholar] [CrossRef] [PubMed]
- Osth, M.; Ost, A.; Kjolhede, P.; Stralfors, P. The concentration of β-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS ONE 2014, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Hammoud, A.O.; Davidson, L.E.; Laferrère, B.; Fraser, A.; Stanford, J.B.; Hashibe, M.; Greenwood, J.L.J.; Kim, J.; Taylor, D.; et al. Maternal and neonatal outcomes for pregnancies before and after gastric bypass surgery. Int. J. Obes. 2015, 39, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Song, W.O. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small-and large-for-gestational-age infants. J. Matern. Fetal. Neonatal. 2015, 28, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.P.; Syed, A.A. Pregnancy following bariatric surgery medical complications and management. Obes. Surg. 2016, 26, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.M.; Abdel Aleem, M.M.; El-Shazly, A.A. Maternal vitamin A deficiency during pregnancy and its relation with maternal and neonatal hemoglobin concentrations among poor Egyptian families. ISRN Pediatr. 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.L.; Kusner, R.F.; Sugerman, H.J.; Gonzalez-Campoy, J.M.; Collazo-Clavell, M.L.; Spitz, A.F.; Apovian, C.M.; Livingston, E.H.; Brolin, R.; Sarwer, D.B.; et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity 2009, 17, S1–S70. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of WHO Consultation on Obesity: Geneva, Switzerland, 1998. [Google Scholar]
- Institute of Medicine (IOM). Weight Gain during Pregnancy: Reexamining the Guidelines; National Academy Press: Washington, DC, USA, 2009. [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep. 1998, 47, 1–29. [Google Scholar]
- Sauberlich, H.E.; Hodges, R.E.; Wallace, D.L.; Kolder, H.; Canham, J.E.; Hood, J. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam. Horm. 1974, 32, 251–275. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Mclaren, D.S.; Frigg, M. Manual de ver y Vivir Sobre los Transtornos por Deficiencia de Vitamina A (VADD); Organização Panamericana de Saúde: Washington, DC, USA, 1999. [Google Scholar]
- Saunders, C.; Leal, M.C.; Gomes, M.M.; Campos, L.F.C.; Dos Santos Silva, B.A.; Thiapó de Lima, A.P.P.; Ramalho, R.A. Gestational nightblindness among women attending a public maternal hospital in Rio De Janeiro, Brazil. J. Health Popul. Nutr. 2004, 22, 348–356. [Google Scholar] [PubMed]
- Saunders, C.; Ramalho, R.A.; De Lima, A.P.P.T.; Gomes, M.M.; Campos, L.F.; Dos Santos Silva, B.A.; Gonçalves Soares, A.; Do Carmo Leal, M. Association between gestational night blindness and serum retinol in mother/newborn pairs in the city of Rio de Janeiro, Brazil. Nutrition 2005, 21, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C.; Troiand, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Marjanne, S.; Monti, D.; Stahl, W.; Sies, H. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- World Health Organization. Xerophthalmia and Night Blindness for the Assessment of Clinical Vitamin A Deficiency in Individuals and Populations; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Machado, S.N.; Pereira, S.; Saboya, C.; Saunders, C.; Ramalho, A. Influence of Roux-en-Y Gastric Bypass on the nutritional status of vitamin A in pregnant women: A comparative study. Obes. Surg. 2015, 26, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; West, K.P., Jr.; Khatry, S.K.; Katz, J.; Shrestha, S.R.; Pradhan, E.K.; Le Clerq, S.C.; Pokhrel, R.P. Night blindness of pregnancy in rural Nepal—nutritional and health risks. Int. J. Epidemiol. 1998, 27, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.I.S.; Amancio, O.M.S.; Sarni, R.O.S.; Pitta, T.S.; Fernandes, A.P.; Fonseca, F.L.A.; Hix, S.; Ramalho, R.A. Non-alcoholic fatty liver disease in overweight children and its relationship with retinol serum levels. Int. J. Vitam. Nutr. Res. 2008, 78, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Villaça Chaves, G.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Non-alcoholic fatty liver disease and its relationship with the nutritional status of vitamin A in individuals with class III obesity. Obes. Surg. 2008, 18, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.E.; Saboya, C.J.; Saunders, C.; Ramalho, A. Serum levels and liver store of retinol and their association with night blindness in individuals with class III obesity. Obes. Surg. 2012, 22, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Tomedi, L.E.; Chang, C.C.; Newby, P.K.; Evans, R.W.; Luther, J.F.; Wisner, K.L.; Bodnar, L.M. Pre-pregnancyobesityandmaternalnutritionalbiomarkerstatusduringpregnancy: A factor analysis. Public Health Nutr. 2013, 16, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vajreswari, A. Vitamin A as a key regulator of obesity and its associated disorders: Evidences from an obese rat model. Indian. J. Med. Res. 2015, 141, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Yasmeen, R.; Reichert, B.; Ziouzenkova, O. Metabolism of vitamin A in white adipose tissue and obesity. In Carotenoids and Vitamin A in Translational Medicine; CRC Press: Boca Raton, FL, USA, 2013; pp. 23–51. [Google Scholar] [CrossRef]
- Gillis, L.J.; Kennedy, L.C.; Bar-Or, O. Overweight children reduce their activity levels earlier in life than healthy weight children. Clin. J. Sport. Med. 2006, 16, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, R.; Jeyakumar, S.M.; Reichert, B.; Yang, F.; Ziouzenkova, O. The contribution of vitamin A to autocrine regulation of fat depots. Biochim. Biophys. Acta 2011, 1821, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Baugh, N.; Harris, D.E.; Aboueissa, A.M.; Sarton, C.; Lichter, E. The impact of maternal obesity and excessive gestational weight gain on maternal and infant outcomes in Maine: Analysis of pregnancy risk assessment monitoring system results from 2000 to 2010. J. Pregnancy 2016. [Google Scholar] [CrossRef] [PubMed]
- Cañete, A.; Cano, E.; Muñoz-Chápuli, R.; Carmona, R. Role of vitamin A/retinoic acid in regulation of embryonic and adult hematopoiesis. Nutrients 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Benoist, B.; McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D. Worldwide Prevalence of Anemia 1993–2005; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Saltzman, E.; Karl, J.P. Nutrient deficiencies after gastric bypass surgery. Annu. Rev. Nutr. 2013, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.Y.; Zarshenas, N.; Jorgensen, J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition 2009, 25, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Moizé, V.; Deulofeu, R.; Torres, F.; Osaba, J.M.; Vidal, J. Nutritional intake and prevalence of nutritional deficiencies prior to surgery in a Spanish morbidly obese population. Obes. Surg. 2011, 21, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, N. Anaemia and Micronutrient Deficiencies. Br. Med. Bull. 2003, 67, 149–160. [Google Scholar] [CrossRef] [PubMed]
| 1st Trimester | 2nd Trimester | 3rd Trimester | p-Value | |
|---|---|---|---|---|
| Retinol | ||||
| Mean/Standard deviation | 0.99 ± 0.29 | 1.07 ± 0.18 | 1.09 ± 0.31 | 0.266 |
| % of Inadequacy | 63.3 | 63.3 | 65.5 | 0.886 |
| β-Carotene | ||||
| Mean/Standard deviation | 31.06 ± 16.12 | 32.83 ± 16.12 | 31.03 ± 16.03 | 0.871 |
| % of Inadequacy | 80.0 | 66.7 | 75.9 | 0.643 |
| Night Blindness | ||||
| % Present | 56.7 | 56.7 | 58.6 | 0.708 |
| Retinol | 1st T | 2nd T | 3rd T | p-Value | β-carotene | 1st T | 2nd T | 3rd T | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Normal weight | 42.9 | 85.7 | 71.4 | 0.223 | Normal weight | 85.7 | 85.7 | 100 | 0.575 |
| Overweight | 62.5 | 56.3 | 68.8 | 0.766 | Overweight | 75 | 62.5 | 62.5 | 0.687 |
| Class I Obesity | 85.7 | 57.1 | 50 | 0.424 | Class I Obesity | 85.7 | 57.1 | 83.3 | 0.398 |
| p-value | 0.249 | 0.373 | 0.663 | p-value | 0.765 | 0.460 | 0.137 | ||
| Normal weight | 42.9 | 85.7 | 71.4 | 0.223 | Normal weight | 85.7 | 85.7 | 100 | 0.575 |
| Overweight | 69.6 | 56.5 | 60.9 | 0.656 | Overweight | 78.3 | 60.9 | 68.2 | 0.440 |
| p-value | 0.372 | 0.215 | 1 | p-value | 0.66 | 0.372 | 0.087 |
| TGWG | Retinol | p-Value | β-carotene | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1st T | 2nd T | 3rd T | 1st T | 2nd T | 3rd T | |||
| Adequate | 0.92 ± 0.21 | 1.05 ± 0.13 | 1.04 ± 0.19 | 0.387 | 35.20 ± 18.28 | 40.20 ± 18.18 | 37.60 ± 17.86 | 0.741 |
| Below | 1.02 ± 0.37 | 1.07 ± 0.22 | 1.12 ± 0.22 | 0.549 | 29.46 ± 14.11 | 27.80 ± 12.83 | 28.33 ± 16.58 | 0.627 |
| Above | 1.06 ± 0.15 | 1.11 ± 0.19 | 1.09 ± 0.07 | 0.819 | 24.5 ± 20.69 | 31 ± 20.28 | 24.75 ± 4.03 | 0.472 |
| p-value | 0.779 | 0.472 | 0.097 | 0.779 | 0.368 | 0.097 | ||
| % Inadequacy of retinol | p-value | % Inadequacy of β-carotene | p-value | |||||
| 1st T | 2nd T | 3rd T | 1st T | 2nd T | 3rd T | |||
| Adequate | 60 | 50 | 80 | 0.366 | 70 | 40 | 60 | 0.387 |
| Below | 66.7 | 73.3 | 66.7 | 0.897 | 86.7 | 86.7 | 80 | 0.844 |
| Above | 50 | 75 | 25 | 0.368 | 75 | 75 | 100 | 0.549 |
| p-value | 0.819 | 0.442 | 0.146 | 0.586 | 0.045 * | 0.248 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, S.; Matos, A.; Da Cruz, S.P.; Pereira, S.; Saboya, C.; Ramalho, A. Relationship between the Nutritional Status of Vitamin A per Trimester of Pregnancy with Maternal Anthropometry and Anemia after Roux-en-Y Gastric Bypass. Nutrients 2017, 9, 989. https://doi.org/10.3390/nu9090989
Cruz S, Matos A, Da Cruz SP, Pereira S, Saboya C, Ramalho A. Relationship between the Nutritional Status of Vitamin A per Trimester of Pregnancy with Maternal Anthropometry and Anemia after Roux-en-Y Gastric Bypass. Nutrients. 2017; 9(9):989. https://doi.org/10.3390/nu9090989
Chicago/Turabian StyleCruz, Sabrina, Andréa Matos, Suelem Pereira Da Cruz, Silvia Pereira, Carlos Saboya, and Andréa Ramalho. 2017. "Relationship between the Nutritional Status of Vitamin A per Trimester of Pregnancy with Maternal Anthropometry and Anemia after Roux-en-Y Gastric Bypass" Nutrients 9, no. 9: 989. https://doi.org/10.3390/nu9090989




