Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design
2.2.1. Experiments 1 and 2
2.2.2. Experiment 3
2.3. Experimental Diets
2.4. Necropsy
2.5. Western Blot-Based Immuno-Nanocapillary Electrophoresis
2.6. Triglyceride Analysis
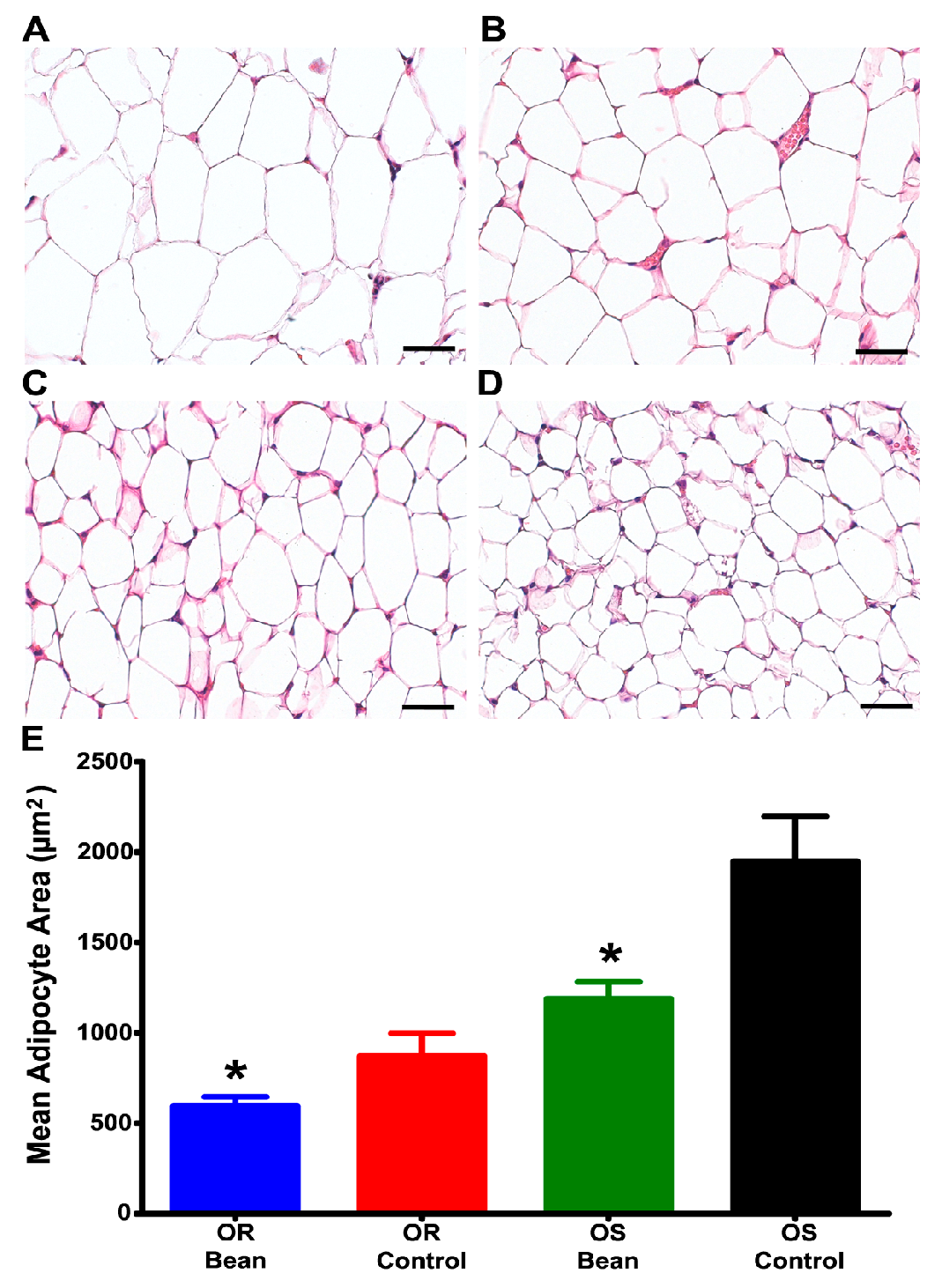
2.7. Morphometric Analysis of Adipose Tissue
2.8. Statistical Analyses
3. Results
3.1. Effect of Bean on Body Weight and Visceral Fat Deposition under Ad Libitum Feeding Conditions
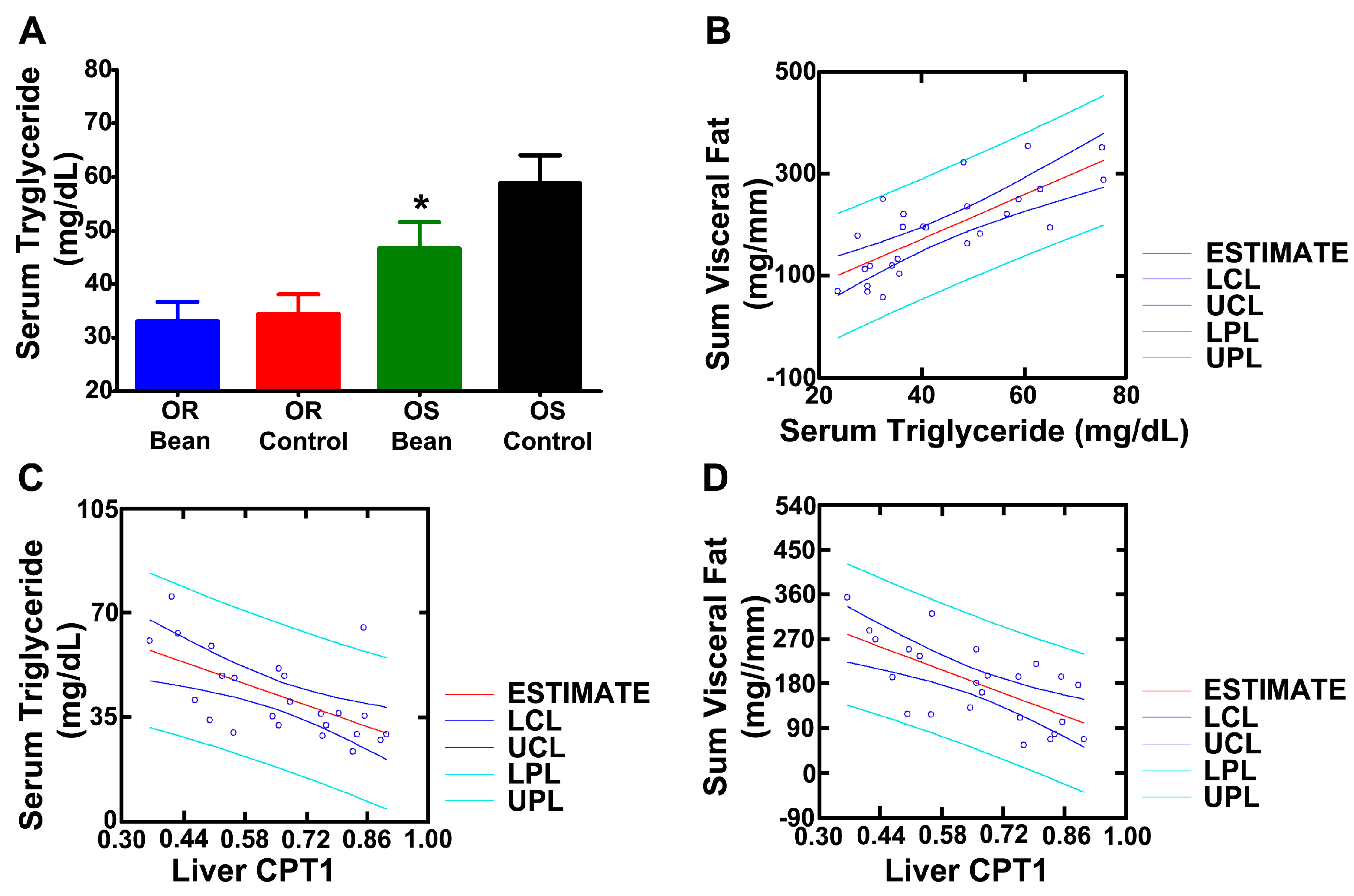
3.2. Effect of Bean on Feed Efficiency, Visceral Fat Disposition, and Hepatic Lipid Metabolism under Paired-Feeding Conditions
3.3. Investigation of Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACC | Acetyl CoA carboxylase |
| ACADL | Acyl CoA dehydrogenase |
| ACSL4 | Acyl CoA synthetase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| CD36 | Cluster of differentiation 36 (fatty acid translocase) |
| CPT1 | Carnitine palmitoyl transferase I |
| mTOR | Mammalian target of rapamycin |
| PPAR | Peroxisome proliferator-activated receptor |
| OR | Obesity resistant |
| OS | Obesity sensitive |
| SUMO32 | Sucrose and Moderate Fat 32% Diet |
| UCP1 | Uncoupling Protein 1 |
References
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary guidance for pulses: The challenge and opportunity to be part of both the vegetable and protein food groups. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations. Crop Statistics-Concepts, Definitions and Classifications. Available online: http://www.fao.org/economic/the-statistics-division-ess/methodology/methodology-systems/crops-statistics-concepts-definitions-and-classifications/en/ (accessed on 1 June 2017).
- Food and Agriculture Organization. Definition and Classification of Commodities: Pulses and Derived Products. Available online: http://www.fao.org/es/faodef/fdef04e.htm (accessed on 20 January 2017).
- Food and Agricultural Organization of the United Nations. International Year of Pulses: Nuritious Seeds for a Sustainable Future. Available online: http://www.fao.org/3/a-bb029e.pdf (accessed on 1 June 2017).
- U.S. Department of Agriculture and Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Available online: http://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf (accessed on 4 August 2017).
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Blanco, M.S.; Kendall, C.W.; Sievenpiper, J.L. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann. N. Y. Acad. Sci. 2017, 1392, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 1997, 273, R725–R730. [Google Scholar] [PubMed]
- Poiley, S.M. A systematic method of breeder rotation for non-inbred laboratory animal colonies. Proc. Anim. Care Panel 1960, 10, 159–166. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Thompson, H.J.; Jones, L.W.; Koch, L.G.; Britton, S.L.; Neil, E.S.; McGinley, J.N. Inherent aerobic capacity-dependent differences in breast carcinogenesis. Carcinogenesis 2017, 38, 920–928. [Google Scholar] [CrossRef]
- Fraulob, J.C.; Ogg-Diamantino, R.; Fernandes-Santos, C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J. Clin. Biochem. Nutr. 2010, 46, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Friedman, M.I. Fasting plasma triglyceride levels and fat oxidation predict dietary obesity in rats. Physiol. Behav. 2003, 78, 767–772. [Google Scholar] [CrossRef]
- Ji, H.; Outterbridge, L.V.; Friedman, M.I. Phenotype-based treatment of dietary obesity: Differential effects of fenofibrate in obesity-prone and obesity-resistant rats. Metabolism 2005, 54, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, W.; Thompson, H.J. Edible dry bean consumption (Phaseolus vulgaris L.) modulates cardiovascular risk factors and diet-induced obesity in rats and mice. Br. J. Nutr. 2012, 108 (Suppl. 1), S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Laforest, S.; Labrecque, J.; Michaud, A.; Cianflone, K.; Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 2015, 52, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Maynard, L.A.; Loosli, J.K. Feeding Experiments. The Determination of Digestability. In Animal Nutrition; McGraw-Hill: New York, NY, USA, 1969; pp. 339–341. [Google Scholar]
- Astell, K.J.; Mathai, M.L.; Su, X.Q. A review on botanical species and chemical compounds with appetite suppressing properties for body weight control. Plant Foods Hum. Nutr. 2013, 68, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Kendall, C.W.; Esfahani, A.; Wong, J.M.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.G.; Garnett, T. Plates, Pyramids and Planet. Available online: http://www.fao.org/3/a-i5640e.pdf (accessed on 4 August 2017).
- Garnett, T. Changing What We Eat. Available online: http://www.fcrn.org.uk/sites/default/files/fcrn_wellcome_gfs_changing_consumption_report_final.pdf (accessed on 3 August 2017).
- Mitchell, D.C.; Lawrence, F.R.; Hartman, T.J.; Curran, J.M. Consumption of dry beans, peas, and lentils could improve diet quality in the US population. J. Am. Diet. Assoc. 2009, 109, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; Yu, N.; Hartman, T.J.; Mitchell, D.C.; Lawrence, F.R.; Aukema, H.M. Pulse consumption in Canadian adults influences nutrient intakes. Br. J. Nutr. 2012, 108 (Suppl. 1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; McLachlan, M.; Black, R.; Widders, I.; Manary, M. Collaboration among sectors to increase pulse consumption. Ann. N. Y. Acad. Sci. 2017, 1392, 3–5. [Google Scholar] [CrossRef] [PubMed]
| Diet | Initial Body Weight 1 (g) | Final Body Weight (g) | Weight Gain/d (g) | Tibia Length (mm) | Retro-Peritoneal Fat 2 (mg/mm) | Para-Metrial Fat (mg/mm) | Peri-Renal Fat (mg/mm) | Total Visceral Fat (mg/mm) |
|---|---|---|---|---|---|---|---|---|
| Control | 65.0 ± 2.2 | 164.2 ± 4.6 | 3.8 ± 0.1 | 28.5 ± 0.3 | 21.8 ± 2.0 | 31.4 ± 2.7 | 7.0 ± 0.6 | 60.1 ± 4.8 |
| Bean | 62.2 ± 2.6 | 147.7 ± 4.8 | 3.3 ± 0.1 | 27.5 ± 0.3 | 10.5 ± 0.7 | 15.8 ± 1.2 | 4.3 ± 0.3 | 30.5 ± 1.9 |
| p-value | 0.43 | 0.02 | 0.001 | 0.019 | 7 × 10−5 | 7 × 10−5 | 0.001 | 3 × 10−5 |
| Diet | Initial Body Weight 1 (g) | Final Body Weight (g) | Weight Gain/day (g) | Tibia Length (mm) | Retro-Peritoneal Fat 2 (mg/mm) | Para-Metrial Fat (mg/mm) | Peri-Renal Fat (mg/mm) | Total Visceral Fat (mg/mm) |
|---|---|---|---|---|---|---|---|---|
| Control | 66.6 ± 2.4 | 187.2 ± 4.0 | 4.2 ± 0.1 | 29.7 ± 0.3 | 27.1 ± 2.6 | 45.5 ± 4.9 | 12.3 ± 1.2 | 84.9 ± 7.7 |
| Bean | 66.8 ± 2.5 | 163.6 ± 3.2 | 3.3 ± 0.1 | 29.0 ± 0.3 | 17.3 ± 1.3 | 30.4 ± 3.1 | 7.4 ± 0.7 | 55.1 ± 4.7 |
| p-value | 0.95 | 1.3 × 10−4 | 2 × 10−5 | 0.107 | 0.003 | 0.017 | 0.002 | 0.004 |
| Diet 1 | Final Body Weight 1 (g) | Feed Efficiency Ratio (g) | Retro-Peritoneal Fat 2 (mg/mm) | Para-Metrial Fat (mg/mm) | Peri-Renal Fat (mg/mm) | Total Visceral Fat (mg/mm) |
|---|---|---|---|---|---|---|
| Obesity Sensitive (OS) | ||||||
| Control | 230 ± 3 | 0.321 ± 0.008 | 77.1 ± 6.8 | 185.6 ± 14.7 | 25.9 ± 2.3 | 288.6 ± 22.3 |
| Bean | 226 ± 4 | 0.323 ± 0.006 | 67.5 ± 5.2 | 136.9 ± 5.3 | 13.8 ± 1.9 | 218.2 ± 9.4 |
| Obesity Resistant (OR) | ||||||
| Control | 166 ± 4 | 0.251 ± 0.005 | 38.5 ± 4.4 | 88.2 ± 9.3 | 14.2 ± 0.8 | 140.8 ± 12.7 |
| Bean | 164 ± 4 | 0.260 ± 0.006 | 25.1 ± 4.3 | 59.9 ± 11.2 | 5.7 ± 0.8 | 90.7 ± 16.0 |
| Factorial Analysis of Variance (p-Values) 3 | ||||||
| Strain | 1.8 × 10−11 | 1.3 × 10−9 | 2.1 × 10−7 | 5.1 × 10−8 | 1.0 × 10−5 | 2.1 × 10−8 |
| Diet | 0.463 | 0.394 | 0.047 | 0.002 | 4.8 × 10−6 | 0.001 |
| Interaction | 0.837 | 0.571 | 0.734 | 0.354 | 0.309 | 0.537 |
| Obesity Sensitive (OS) | Obesity Resistant (OR) | p-Values 2 | |||||
|---|---|---|---|---|---|---|---|
| Protein 1 | Control | Bean | Control | Bean | Strain | Diet | Interaction |
| Ser79pACC | 1.32 ± 0.07 | 1.02 ± 0.10 | 1.08 ± 0.09 | 0.67 ± 0.04 | 0.001 | 2.2 × 10−4 | 0.486 |
| ACC | 1.17 ± 0.08 | 0.73 ± 0.05 | 0.81 ± 0.06 | 0.66 ± 0.11 | 0.014 | 0.001 | 0.083 |
| ACC Ratio | 1.14 ± 0.06 | 1.40 ± 0.08 | 1.34 ± 0.09 | 1.12 ± 0.13 | 0.671 | 0.839 | 0.018 |
| ACADL | 7.00 ± 0.50 | 7.91 ± 0.44 | 5.54 ± 0.20 | 6.08 ± 0.29 | 3.2 × 10−4 | 0.070 | 0.630 |
| ACSL4 | 1.49 ± 0.15 | 1.68 ± 0.03 | 1.12 ± 0.06 | 1.19 ± 0.06 | 4.0 × 10−5 | 0.119 | 0.468 |
| Thr172pAMPK | 0.93 ± 0.06 | 0.95 ± 0.08 | 0.95 ± 0.07 | 1.17 ± 0.10 | 0.144 | 0.137 | 0.245 |
| AMPK | 2.06 ± 0.10 | 2.25 ± 0.07 | 1.95 ± 0.06 | 2.27 ± 0.13 | 0.613 | 0.012 | 0.490 |
| AMPK Ratio | 0.46 ± 0.05 | 0.43 ± 0.04 | 0.49 ± 0.03 | 0.52 ± 0.04 | 0.162 | 0.969 | 0.452 |
| CD36 | 1.27 ± 0.19 | 1.87 ± 0.50 | 1.53 ± 0.13 | 1.69 ± 0.33 | 0.912 | 0.273 | 0.523 |
| CPT1 | 0.50 ± 0.05 | 0.67 ± 0.06 | 0.67 ± 0.06 | 0.81 ± 0.03 | 0.007 | 0.006 | 0.753 |
| Diet 1 | Liver Triglyceride (mg/mg Protein × 10−2) 2 |
|---|---|
| Obesity Sensitive (OS) | |
| Control | 3.8 ± 0.5 |
| Bean | 2.4 ± 0.3 |
| Obesity Resistant (OR) | |
| Control | 3.4 ± 0.2 |
| Bean | 3.2 ± 0.2 |
| Factorial Analysis of Variance (p-Values) 3 | |
| Strain | 0.541 |
| Diet | 0.036 |
| Interaction | 0.076 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. https://doi.org/10.3390/nu9090998
Thompson HJ, McGinley JN, Neil ES, Brick MA. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients. 2017; 9(9):998. https://doi.org/10.3390/nu9090998
Chicago/Turabian StyleThompson, Henry J., John N. McGinley, Elizabeth S. Neil, and Mark A. Brick. 2017. "Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism" Nutrients 9, no. 9: 998. https://doi.org/10.3390/nu9090998





