Intersex Tilapia (Oreochromis spp.) from a Contaminated River in Taiwan: A Case Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of the Sampled River and Collection Stations
| EJ-1 | EJ-2 | |
|---|---|---|
| Cd | <0.005 to <0.01 | <0.005 to <0.05 |
| Cr | <0.001 to 0.19* | <0.001 to 0.29* |
| Cu | 0.04 to 0.19* | 0.01 to 0.22* |
| Pb | 0.001 to 0.04 | 0.01 to 0.111* |
| Zn | 0.07 to 0.81* | 0.6 to 1.0 * |
| Hg | 15.94a* |
| EDC | Sample | Concentration | References |
|---|---|---|---|
| Dioxin¹ | Bottom mud(dry) | 0.369~66.9 pg-TEQ/g (Average:20.9 pg-TEQ/g n:4) | [21] |
| Total PCBs2 | Bottom mud(dry) (0-5 cm depth) | 871-13,615 ng/g | [20] |
| Tilapia (dry,5 fish) | 502 ng/g | ||
| Di(2.ethylhexyl) phthalate (DEHP) ³ | Bottom mud(dry) | 0.25 μg/g | [27] |
| Nonylphenol (NP) 4 | River water | 1.47-12.6 μg/L2 Average:7.01 μg/L | [28] |
| Bottom mud(dry) | 250-390 μg/Kg dry wt.3 |

2.2. Sampling Protocol
2.3. Histological Procedures
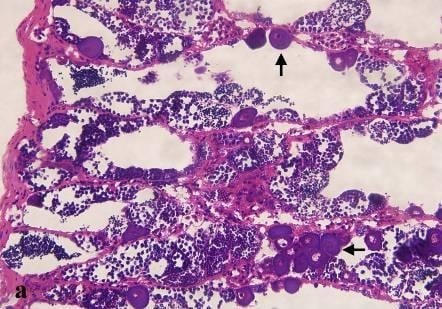
3. Results
| Item | Description | Percentage (%) of prevalence | Number of fish discovered among these 18 tilapia |
|---|---|---|---|
| Fin and body surface 1 | Fin split(s), body surface lesion and/or necrosis | 94.4 | 17 |
| Scale | deformity | 16.7 | 3 |
| Liver | Swollen, discoloration, necrosis, chocolate color | 61.1 | 11 |
| Spleen | Swollen, dark color | 44.4 | 8 |
| Body cavity | Edema, necrosis | 22.2 | 4 |
| Gill | Mucus secretion, necrosis, bleeding, proliferation, pale | 27.8 | 5 |
| Weight(g) | 215~400 g | ||
| Other condition ² |

4. Discussion
Acknowledgements
References and Notes
- Garaventa, F.; Centanni, E.; Pellizzato, F.; Faimali, M.; Terlizzi, A.; Pavoni, B. Imposex and accumulation of organotin compounds in populations of Hexaplex trunculus (Gastropoda, Muricidae) from the Lagoon of Venice (Italy) and Istrian Coast (Croatia). Mar. Pollut. Bull. 2007, 54, 602–625. [Google Scholar] [CrossRef] [PubMed]
- Swennen, C.; Sampantarak, U.; Ruttanadakul, N. TBT-pollution in the Gulf of Thailand: A re-inspection of imposex incidence after 10 years. Mar. Pollut. Bull. 2009, 58, 526–532. [Google Scholar] [PubMed]
- Rato, M.; Gaspar, M.B.; Takahashi, S.; Yano, S.; Tanabe, S.; Barroso, C. Inshore/offshore gradients of imposex and organotin contamination in Nassarius reticulatus (L.) along the Portuguese coast. Mar. Pollut. Bull. 2008, 56, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Jiang, G.; Zhou, Q.; Yang, R. Organotin pollution in China: an overview of the current state and potential health risk. J. Environ. Manage. 2009, 90, S16–S24. [Google Scholar]
- Matthiessen, P.; Allen, Y.; Bamber, S.; Craft, J.; Hurst, M.; Hutchinson, T.; Feist, S.; Katsiadaki, I.; Kirby, M.; Robinson, C.; Scott, S.; Thain, J.; Thomas, K. The impact of oestrogenic and androgenic contamination on marine organisms in the United Kingdom-summary of the EDMAR programme. Mar. Environ. Res. 2002, 54, 645–649. [Google Scholar] [PubMed]
- Bortone, S.A.; Cody, R.P. Morphological masculinization in poeciliid females from a paper mill effluent receiving tributary of the St, Johns River, Florida, USA. Bull. Environ. Contam. Toxicol. 1999, 63, 0150–0156. [Google Scholar] [CrossRef]
- Bortone, S.A.; Davis, W.P. Fish intersexuality as indicator of environmental stress. BioScience 1994, 44, 165–172. [Google Scholar]
- Barnhoorn, I.E.J.; Bornman, M.S.; Pieterse, G.M.; van Vuren, J.H.J. Histological evidence of intersex in feral sharptooth catfish (Clarias gariepinus) from an estrogen-polluted water source in Gauteng, South Africa. Environ Toxicol. 2004, 19, 603–608. [Google Scholar] [PubMed]
- Viganò, L.; Mandich, A.; Benfenati, E.; Bertolotti, R.; Bottero, S.; Porazzi, E.; Agradi, E. Investigating the estrogenic risk along the River Po and its intermediate section. Arch. Environ. Contam. Toxicol. 2006, 51, 641–651. [Google Scholar] [PubMed]
- Blazer, V.S.; Iwanowicz, L.R.; Iwanowicz, D.D.; Smith, D.R.; Young, J.A.; Hedrick, J.D.; Foster, S.W.; Reeser, S.J. Intersex (testicular oocytes) in smallmouth bass from the Potomac River and selected nearby drainages. J. Aquat. Anim. Health. 2007, 19, 242–253. [Google Scholar] [PubMed]
- Gercken, J.; Sordyl, H. Intersex in feral marine and freshwater fish from northeastern Germany. Mar. Environ. Res. 2002, 54, 651–655. [Google Scholar] [PubMed]
- Liu, L.L.; Suen, I.J. Prosobranch gastropod imposex in the west coast of Taiwan. Venus 1996, 55, 207–214. [Google Scholar]
- Liu, L.L.; Chen, S.J.; Peng, W.Y.; Hung, J.J. Organotin concentrations in three intertidal neogastropods from the coastal waters of Taiwan. Environ. Pollut. 1997, 98, 113–118. [Google Scholar] [PubMed]
- Hung, T.C.; Hsu, W.K.; Mang, P.J.; Chuang, A. Organotins and imposex in the rock shell, Thais clavigera, from oyster mariculture areas in Taiwan. Environ. Pollut. 2001, 112, 145–152. [Google Scholar] [PubMed]
- Sun, H.W.; Dai, S.G.; Huang, G.L. Advances in research of organotin compounds in environment (in Chinese). In Advances in Environmental Chemistry; Daios, G., Ed.; Chemical Industry Press: Beijing, China, 2005; pp. 247–263. [Google Scholar]
- Liu, W.H.; Chiu, Y.W.; Huang, D.J.; Liu, M.Y.; Lee, C.C.; Liu, L.L. Imposex in the golden apple snail Pomacea canaliculata in Taiwan. Sci. Total Environ. 2006, 371, 138–143. [Google Scholar] [PubMed]
- Meng, P.J.; Lin, J.; Liu, L.L. Aquatic organotin pollution in Taiwan. J. Environ. Manage. 2009, 90, S8–S15. [Google Scholar]
- Water Resources Bureau, Hydrological Yearbook of Taiwan 1996; R.O.C. Water Resources Bureau, Ministry of Economic Affairs (in Chinese with English abstract): Taipei, Taiwan, 1997.
- Hsu, S.K. The vision of sustainable development of water resources and international non-boundary corporation (in Chinese). In Personnel Development Program for Environmental Diplomacy Major Theme: The Vision in Taiwan Water Resources, Taiwan Vision Council; National Taiwan University: Taipei, Taiwan, 2000; pp. 118–133. [Google Scholar]
- Ling, Y.C.; Soong, D.K.; Lee, M.K. PCDD/DFS and coplanar PCBs in sediment and fish samples from the Er-Jen river in Taiwan. Chemosphere 1995, 31, 2863–2872. [Google Scholar] [PubMed]
- Pan, F.H. An investigation on the present status of dioxin and planar polychlorinated biphenyls concentration in bottom mud of Era-Jiin and Kao-Ping River and the pollution background (in Chinese). Annual Report NIEA Taiwa, R.O.C. 2002, 9, 89–110. [Google Scholar]
- Chyan, J.M.; Wan, M.W.; Huang, H.C.; Kuo, H.W.; Chen, I.M. Investigation of polychlorinated biphenyls contamination in Erh-Jen river sediment (In Chinese with English abstract). Chia-Nan Annual Bulletin (Taiwan) 2006, 32, 54–63. [Google Scholar]
- Chang, B.V.; Yu, C.H.; Yuan, S.Y. Degradation of nonylphenol by anaerobic microorganisms from river sediment. Chemosphere 2004, 55, 493–500. [Google Scholar] [PubMed]
- Lin, C.; Lee, C.J.; Mao, W.M.; Nadim, F. Identifying the potential sources of di-(2-ethylhexyl) phthalate contamination in the sediment of the Houjing River in southern Taiwan. J. Hazard. Mater. 2009, 161, 270–275. [Google Scholar] [PubMed]
- Environmental Protection Department of Taiwan Province Government, Annual Water Quality Analyzed Results of Taiwan Rivers of 1993-1996 (in Chinese). Taiwan Province Environmental Protection Agency of Taiwan Provincial Government: Taipei, Taiwan, 1994-1997.
- Kaohsiung Irrigation Association, The Investigation and Toxicity Identification Evaluation of Irrigation Water in Wan-Li Canal and Ee-Ren Stream System of Kaohsiung Irrigation Association (in Chinese), Kaohsiung Irrigation Association Press: Kaohsiung, Taiwan, 1996.
- Lee, C.C. The Establishment of the Environmental Distributions Survey of Toxic Chemicals (in Chinese with English abstract). Project no. EPA-94-J103-02-102, National Science Council of Taiwan: Taiwan, 2005. [Google Scholar]
- Wang, C.H.; Chang, S.P.; Huang, R.K.; Lee, Y.H.; Wang, S.K.; Hung, W.T.; Chen, P.S. Residues survey of nonylphenol and its biological effect on male Crap. Ann Rept. NIEA Taiwan R.O.C 2002, 9, 291–312. [Google Scholar]
- Sun, P.L.; Brown-Peterson, N.J.; Hawkins, W.E.; Overstreet, R.M.; Krol, R.M.; Tsaio, S.H.; Zhu, Y. Morphological and histological abnormalities in tilapia (Oreochromis spp.) from two contaminated rivers in southern Taiwan. Environ. Sci. 1998, 6, 129–152. [Google Scholar]
- Wu, D.R. Chemical characteristics and microbial diversity in water body of the Erh-jeh River (in Chinese). Soil Environ. 2003, 6, 59–70. [Google Scholar]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Tech. 1998, 32, 2498–2506. [Google Scholar]
- Cheek, A.N.; McLachlan, J.A. Environmental hormones and the male reproductive system. J. Androl. 1998, 19, 5–10. [Google Scholar] [PubMed]
- Goksøyr, A.; Arukwe, A.; Larsson, J.; Cajaraille, M.P.; Hauser, L.; Nilsen, B.M.; Lowe, D.; Matthiessen, P. Chapter 5: Molecular/cellular processes and the impact on reproduction. In Effects of Pollution on Fish; Lawrence, A.J., Hemingway, K.L., Eds.; Blackwell Publishing: Oxford, UK, 2003; pp. 179–220. [Google Scholar]
- Cardinali, M.; Maradonna, F.; Olivotto, I.; Bortoluzzi, G.; Mosconi, G.; Polzonetti-Magni, A.M.; Carnevali, O. Temporary impairment of reproduction in freshwater teleost exposed to nonylphenol. Reprod. Toxicol. 2004, 18, 597–604. [Google Scholar] [PubMed]
- Heath, A.G. Water Pollution and Fish Physiology; CRC Press: Boca Raton, FL, USA, 1995; p. 384. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, P.L.; Tsai, S.-S. Intersex Tilapia (Oreochromis spp.) from a Contaminated River in Taiwan: A Case Study. Toxins 2009, 1, 14-24. https://doi.org/10.3390/toxins1010014
Sun PL, Tsai S-S. Intersex Tilapia (Oreochromis spp.) from a Contaminated River in Taiwan: A Case Study. Toxins. 2009; 1(1):14-24. https://doi.org/10.3390/toxins1010014
Chicago/Turabian StyleSun, Peter Lin, and Shinn-Shoung Tsai. 2009. "Intersex Tilapia (Oreochromis spp.) from a Contaminated River in Taiwan: A Case Study" Toxins 1, no. 1: 14-24. https://doi.org/10.3390/toxins1010014