Predicted Roles of the Uncharacterized Clustered Genes in Aflatoxin Biosynthesis
Abstract
:1. Introduction
2. General Considerations Concerning Aflatoxin Biosynthesis
2.1. Gene Clusters and Aflatoxin Biosynthesis

2.2. Problems in Gene Characterization
| Non-leaky mutations | Leaky mutations | Stable metabolites | Derailment products |
|---|---|---|---|
| aflR | hypB | Norsolorinic acid (NA) | Averufanin |
| aflJ | hypC | Averantin (AVN) | Averythrin |
| avnA | hypD | Hydroxyaverantin (HAVN) | Nidurufin |
| avfA? | hypE | Oxoaverantin (OAVN) | 6-Deoxyversicolorin A |
| estA | nor-1 | Averufin (AVF) | Versicolorone |
| pksA | norA | Hydroxyversicolorone (HVN) | Versicolorol |
| hexA/hexB | norB | Versicolorin hemiacetal acetate (VHA) | Versiconol |
| cypA | ordB | Versiconal hemiacetal (VAL) | Versiconal |
| cypX | nadA | Versicolorin B (VERB) | versiconol acetate |
| moxY | vbs | Versicolorin A (VERA) | Sterigmatin |
| ver-1 | Demethylsterigmatocystin (DMST) | Aflatoxicol | |
| verA | DihydroDMST (DHDMST) | Deoxyaflatoxin | |
| verB | Sterigmatocystin (ST) | ||
| omtA | DihydroST (DHDMST) | ||
| omtB | O-methylsterigmatocystin (OMST) | ||
| ordA | DihydroOMST (DHOMST) | ||
| aflY | 11-HydroxyOMST (HOMST) | ||
| 11-HydroxyDHOMST (DHHOMST) | |||
| Aflatoxin B1, B2 | |||
| Aflatoxin G1, G2 |
3. Formation of Norsolorinic Acid (NA), the First Stable Metabolite in AF Biosynthesis



4. Assignment of a Role for AvfA in Averufin Oxidation


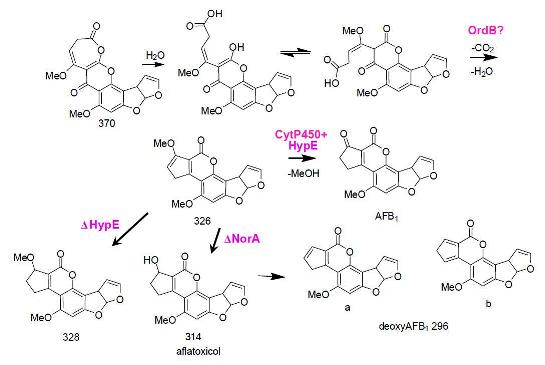
5. The Last Steps in AF Formation



6. Possible Involvement of HypD in AF Accumulation and Fungal Development



7. New Insights into the Involvement of AflJ in AF Biosynthesis Regulation
8. Conclusions
Acknowledgements
References
- Bhatnagar, D.; Cary, J.W.; Ehrlich, K.; Yu, J.; Cleveland, T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 2006, 162, 155–166. [Google Scholar] [PubMed]
- Chang, P.K.; Matsushima, K.; Takahashi, T.; Yu, J.; Abe, K.; Bhatnagar, D.; Yuan, G.F.; Koyama, Y.; Cleveland, T.E. Understanding nonaflatoxigenicity of Aspergillus sojae: A windfall of aflatoxin biosynthesis research. Appl. Microbiol. Biotechnol. 2007, 76, 977–984. [Google Scholar] [PubMed]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [PubMed]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [PubMed]
- Horn, B.W. Biodiversity of Aspergillus section Flavi in the United States: A review. Food Addit. Contam. 2007, 24, 1088–1101. [Google Scholar] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism-from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [PubMed]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses-an overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [PubMed]
- Yin, Y.N.; Yan, L.Y.; Jiang, J.H.; Ma, Z.H. Biological control of aflatoxin contamination of crops. J. Zhejiang Univ. Sci. B 2008, 9, 787–792. [Google Scholar] [PubMed]
- Yu, J.; Cleveland, T.E.; Nierman, W.C.; Bennett, J.W. Aspergillus flavus genomics: Gateway to human and animal health, food safety, and crop resistance to diseases. Rev. Iberoam. Micol. 2005, 22, 194–202. [Google Scholar] [PubMed]
- Geiser, D.M.; Dorner, J.W.; Horn, B.W.; Taylor, J.W. The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet. Biol. 2000, 31, 169–179. [Google Scholar] [PubMed]
- Cotty, P.J.; Mellon, J.E. Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 2006, 22, 110–117. [Google Scholar]
- Wicklow, D.T.; Wilson, D.M.; Nelsen, T.C. Survival of Aspergillus flavus sclerotia and conidia buried in soil in Illinois and Georgia. Phytopathology 1993, 83, 1141–1147. [Google Scholar]
- Gourama, H.; Bullerman, L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds: A review. J. Food Prot. 1995, 58, 1395–1404. [Google Scholar]
- Robens, J. The costs of mycotoxin management to the USA: Management of aflatoxins in the United States. APSnet Feature. 2001, pp. 2–8. Available online: http://www.apsnet.org/online/feature/mycotoxin/top.html.
- Yabe, K.; Nakajima, H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004, 64, 745–755. [Google Scholar] [PubMed]
- Ehrlich, K.C.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis gene clusters and flanking regions. J. Appl. Microbiol. 2005, 99, 518–527. [Google Scholar] [PubMed]
- Yu, J.; Cleveland, T.E. Aspergillus flavus genomics for discovering genes involved in aflatoxin biosynthesis. In Polyketides: Biosynthesis, Biological activity, and Genetic Engineering; Rimando, A.M., Baerson, S.R., Eds.; American Chemical Society: Washington, D.C., USA, 2007; pp. 246–260. [Google Scholar]
- Bhatnagar, D.; Ehrlich, K.C.; Cleveland, T.E. Oxidation-reduction reactions in biosynthesis of secondary metabolites. In Mycotoxins in Ecological Systems; Bhatnagar, D., Lillehoj, E.B., Arora, D.K., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 255–285. [Google Scholar]
- Cary, J.W.; Ehrlich, K.C. Aflatoxigenicity in Aspergillus: Molecular genetics, phylogenetic relationships and evolutionary implications. Mycopathologia 2006, 162, 167–177. [Google Scholar] [PubMed]
- Dutton, M.F. Enzymes and aflatoxin biosynthesis. Microbiol. Rev. 1988, 52, 274–295. [Google Scholar] [PubMed]
- Minto, R.E.; Townsend, C.A. Enzymology and molecular biology of aflatoxin biosynthesis. Chem. Rev. 1997, 97, 2537–2555. [Google Scholar] [PubMed]
- Yu, F.L. Mechanism of aflatoxin B1 inhibition of rat hepatic nuclear RNA synthesis. J. Biol. Chem. 1977, 252, 3245–3251. [Google Scholar] [PubMed]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [PubMed]
- Bok, J.W.; Balajee, S.A.; Marr, K.A.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Keller, N.P. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell 2005, 4, 1574–1582. [Google Scholar] [PubMed]
- Bok, J.W.; Hoffmeister, D.; Maggio-Hall, L.A.; Murillo, R.; Glasner, J.D.; Keller, N.P. Genomic mining for Aspergillus natural products. Chem. Biol. 2006, 13, 31–37. [Google Scholar] [PubMed]
- Bradshaw, R.E.; Zhang, S. Biosynthesis of dothistromin. Mycopathologia 2006, 162, 201–213. [Google Scholar] [PubMed]
- Brobst, S.W.; Townsend, C.A. The potential role of fatty-acid initiation in the biosynthesis of the fungal aromatic polyketide aflatoxin b-1. Can. J. Chem. 1994, 72, 200–207. [Google Scholar]
- Brown, D.W.; Yu, J.H.; Kelkar, H.S.; Fernandes, M.; Nesbitt, T.C.; Keller, N.P.; Adams, T.H.; Leonard, T.J. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 1996, 93, 1418–1422. [Google Scholar]
- Carbone, I.; Ramirez-Prado, J.H.; Jakobek, J.L.; Horn, B.W. Gene duplication, modularity and adaptation in the evolution of the aflatoxin gene cluster. BMC Evol. Biol. 2007, 7, 111. [Google Scholar] [PubMed]
- Cary, J.W.; Ehrlich, K.C.; Beltz, S.B.; Harris-Coward, P.; Klich, M.A. Characterization of the Aspergillus ochraceoroseus aflatoxin/sterigmatocystin biosynthetic gene cluster. Mycologia 2009, 101, 352–362. [Google Scholar] [PubMed]
- Jenke-Kodama, H.; Sandmann, A.; Muller, R.; Dittmann, E. Evolutionary implications of bacterial polyketide synthases. Mol. Biol. Evol. 2005, 22, 2027–2039. [Google Scholar] [PubMed]
- Ehrlich, K.C.; Scharfenstein, L.L.; Montalbano, B.G.; Chang, P.-K. Are the genes nadA and norB involved in formation of aflatoxin G1. Int. J. Mol. Sci. 2008, 9, 1717–1729. [Google Scholar] [PubMed]
- Maggon, K.K.; Gupta, S.K.; Venkitasubramanian, T.A. Biosynthesis of aflatoxins. Bacteriol. Rev. 1977, 41, 822–855. [Google Scholar] [PubMed]
- Bennett, J.W.; Chang, P.K.; Bhatnagar, D. One gene to whole pathway: The role of norsolorinic acid in aflatoxin research. Adv. Appl. Microbiol. 1997, 45, 1–15. [Google Scholar] [PubMed]
- Trail, F.; Chang, P.K.; Cary, J.; Linz, J.E. Structural and functional analysis of the nor-1 gene involved in the biosynthesis of aflatoxins by Aspergillus parasiticus. Appl. Environ. Microbiol. 1994, 60, 4078–4085. [Google Scholar] [PubMed]
- Weissman, K.J. Biochemistry. Anatomy of a fungal polyketide synthase. Science 2008, 320, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.R.; Yu, J.; Abbas, H.K.; Scheffler, B.E.; Kim, H.S.; Nierman, W.C.; Bhatnagar, D.; Cleveland, T.E. Aflatoxin formation and gene expression in response to carbon source media shift in Aspergillus parasiticus. Food Addit. Contam. 2007, 24, 1051–1060. [Google Scholar] [PubMed]
- Yabe, K.; Yan, P.S.; Song, Y.; Ichinomiya, M.; Nakagawa, H.; Shima, Y.; Ando, Y.; Sakuno, E.; Nakajima, H. Isolation of microorganisms and substances inhibitory to aflatoxin production. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1111–1117. [Google Scholar] [PubMed]
- Chung, J.Y.; Fujii, I.; Harada, S.; Sankawa, U.; Ebizuka, Y. Expression, purification, and characterization of AknX anthrone oxygenase, which is involved in aklavinone biosynthesis in Streptomyces galilaeus. J. Bacteriol. 2002, 184, 6115–6122. [Google Scholar] [PubMed]
- Sciara, G.; Kendrew, S.G.; Miele, A.E.; Marsh, N.G.; Federici, L.; Malatesta, F.; Schimperna, G.; Savino, C.; Vallone, B. The structure of ActVA-Orf6, a novel type of monooxygenase involved in actinorhodin biosynthesis. Embo. J. 2003, 22, 205–215. [Google Scholar] [PubMed]
- Chen, Z.-G.; Fujii, I.; Ebizuka, Y.; Sankawa, U. Purification and characterization of emodinanthrone oxygenase from Aspergillus terreus. Phytochemistry 1995, 38, 299–305. [Google Scholar]
- Fujii, I.; Chen, Z.G.; Ebizuka, Y.; Sankawa, U. Identification of emodinanthrone oxygenase in fungus Aspergillus terreus. Biochem. Int. 1991, 25, 1043–1049. [Google Scholar] [PubMed]
- Sakuno, E.; Wen, Y.; Hatabayashi, H.; Arai, H.; Aoki, C.; Yabe, K.; Nakajima, H. Aspergillus parasiticus cyclase catalyzes two dehydration steps in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2005, 71, 2999–3006. [Google Scholar] [PubMed]
- Townsend, C.A.; Christensen, S.B.; Davis, S.G. Synthesis of averufin and its role in aflatoxin-B1 biosynthesis. 1988. [Google Scholar]
- Wen, Y.; Hatabayashi, H.; Arai, H.; Kitamoto, H.K.; Yabe, K. Function of the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 2005, 71, 3192–3198. [Google Scholar] [PubMed]
- Yabe, K.; Chihaya, N.; Hamamatsu, S.; Sakuno, E.; Hamasaki, T.; Nakajima, H.; Bennett, J.W. Enzymatic conversion of averufin to hydroxyversicolorone and elucidation of a novel metabolic grid involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2003, 69, 66–73. [Google Scholar] [PubMed]
- Yu, J.; Woloshuk, C.P.; Bhatnagar, D.; Cleveland, T.E. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000, 248, 157–167. [Google Scholar] [PubMed]
- Townsend, C.A.; Isomura, Y.; Davis, S.G.; Hodge, J.A. Reaction models of the oxidative rearrangement of averufin to 1'-hydroxyversicolorone - the 1st step in dihydrobisfuran formation in aflatoxin biosynthesis. Tetrahedron 1989, 45, 2263–2276. [Google Scholar]
- Townsend, C.A.; Christensen, S.B. Concerning the role of nidurufin in aflatoxin biosynthesis. J. Am. Chem. Soc. 1985, 107, 270–271. [Google Scholar]
- Cary, J.W.; Ehrlich, K.; Bland, J.M.; Montalbano, B. The aflatoxin biosynthesis cluster gene aflX, encodes an oxidoreductase involved in conversion of versicolorin A to demethylsterigmatocystin. Appl. Environ. Microbiol. 2006, 72, 1096–1101. [Google Scholar] [PubMed]
- Ehrlich, K.C.; Montalbano, B.; Boue, S.M.; Bhatnagar, D. An aflatoxin biosynthesis cluster gene encodes a novel oxidase required for conversion of versicolorin a to sterigmatocystin. Appl. Environ. Microbiol. 2005, 71, 8963–8965. [Google Scholar] [PubMed]
- Prieto, R.; Woloshuk, C.P. ord1, an oxidoreductase gene responsible for conversion of O-methylsterigmatocystin to aflatoxin in Aspergillus flavus. Appl. Environ. Microbiol. 1997, 63, 1661–1666. [Google Scholar] [PubMed]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Cary, J.W.; Montalbano, B.; Dyer, J.M.; Bhatnagar, D.; Cleveland, T.E. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2. Appl. Environ. Microbiol. 1998, 64, 4834–4841. [Google Scholar] [PubMed]
- Udwary, D.W.; Casillas, L.K.; Townsend, C.A. Synthesis of 11-hydroxy-O-methylsterigmatocystin and the role of a cytochrome P-450 in the final step of aflatoxin biosynthesis. J. Am. Chem. Soc. 2002, 124, 5294–5303. [Google Scholar] [PubMed]
- Ehrlich, K.C.; Chang, P.-K.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004, 70, 6518–6524. [Google Scholar] [PubMed]
- Holmes, R.A. Characterization of an Aflatoxin Biosynthetic Gene and Resistance in Maize Seeds to Aspergillus flavus. PhD thesis, North Carolina State University, Raleigh, NC, USA, 2008. [Google Scholar]
- Chauvaux, S.; Chevalier, F.; Le Dantec, C.; Fayolle, F.; Miras, I.; Kunst, F.; Beguin, P. Cloning of a genetically unstable cytochrome P-450 gene cluster involved in degradation of the pollutant ethyl tert-butyl ether by Rhodococcus ruber. J. Bacteriol. 2001, 183, 6551–6557. [Google Scholar] [PubMed]
- Cary, J.W.; Wright, M.; Bhatnagar, D.; Lee, R.; Chu, F.S. Molecular characterization of an Aspergillus parasiticus gene, norA, located on the aflatoxin biosynthesis gene cluster. Appl. Environ. Microbiol. 1996, 62, 360–366. [Google Scholar] [PubMed]
- Lau, H.P.; Chu, F.S. Preparation and characterization of acid dehydration products of aflatoxicol. J. Assoc. Off. Anal. Chem. 1983, 66, 98–101. [Google Scholar] [PubMed]
- Chan, S.I.; Yu, S.S. Controlled oxidation of hydrocarbons by the membrane-bound methane monooxygenase: The case for a tricopper cluster. Acc. Chem. Res. 2008, 41, 969–979. [Google Scholar] [PubMed]
- Pereira, M.; Felipe, M.S.; Brigido, M.M.; Soares, C.M.; Azevedo, M.O. Molecular cloning and characterization of a glucan synthase gene from the human pathogenic fungus Paracoccidioides brasiliensis. Yeast 2000, 16, 451–462. [Google Scholar] [PubMed]
- Szczesna-Skorupa, E.; Kemper, B. Influence of protein-protein interactions on the cellular localization of cytochrome P450. Expert Opin. Drug Metab. Toxicol. 2008, 4, 123–136. [Google Scholar] [PubMed]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [PubMed]
- Reiss, J. Development of Aspergillus parasiticus and formation of aflatoxin B1 under the influence of conidiogenesis affecting compounds. Arch. Microbiol. 1982, 133, 236–238. [Google Scholar] [PubMed]
- Wieser, J.; Yu, J.H.; Adams, T.H. Dominant mutations affecting both sporulation and sterigmatocystin biosynthesis in Aspergillus nidulans. Curr. Genet. 1997, 32, 218–224. [Google Scholar] [PubMed]
- Chang, P.K.; Yu, J.; Yu, J.H. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet. Biol. 2004, 41, 911–920. [Google Scholar] [PubMed]
- Amaike, S.; Keller, N.P. Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot. Cell 2009, 8, 1051–1060. [Google Scholar] [PubMed]
- Roze, L.V.; Calvo, A.M.; Gunterus, A.; Beaudry, R.; Kall, M.; Linz, J.E. Ethylene modulates development and toxin biosynthesis in Aspergillus possibly via an ethylene sensor-mediated signaling pathway. J. Food Prot. 2004, 67, 438–447. [Google Scholar] [PubMed]
- Chang, P.K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genomics. 2003, 268, 711–719. [Google Scholar] [PubMed]
- Chang, P.K. Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae. J. Biotechnol. 2004, 107, 245–253. [Google Scholar] [PubMed]
- Du, W.; Obrian, G.R.; Payne, G.A. Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Addit. Contam. 2007, 24, 1043–1050. [Google Scholar] [PubMed]
- Meyers, D.M.; Obrian, G.; Du, W.L.; Bhatnagar, D.; Payne, G.A. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Environ. Microbiol. 1998, 64, 3713–3717. [Google Scholar] [PubMed]
- Ehrlich, K.C.; Cotty, P.J. Variability in nitrogen regulation of aflatoxin production by Aspergillus flavus strains. Appl. Microbiol. Biotechnol. 2002, 60, 174–178. [Google Scholar] [PubMed]
- Keller, N.; Bok, J.; Chung, D.; Perrin, R.M.; Keats Shwab, E. LaeA, a global regulator of Aspergillus toxins. Med. Mycol. 2006, 44 Suppl., 83–85. [Google Scholar]
- Bok, J.W.; Noordermeer, D.; Kale, S.P.; Keller, N.P. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol. Microbiol. 2006, 61, 1636–1645. [Google Scholar] [PubMed]
- Perrin, R.M.; Fedorova, N.D.; Bok, J.W.; Cramer, R.A.; Wortman, J.R.; Kim, H.S.; Nierman, W.C.; Keller, N.P. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007, 3, e50. [Google Scholar]
- Kale, S.P.; Milde, L.; Trapp, M.K.; Frisvad, J.C.; Keller, N.P.; Bok, J.W. Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet. Biol. 2008, 45, 1422–1429. [Google Scholar] [PubMed]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; Braus, G.H. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [PubMed]
- Chiou, C.H.; Miller, M.; Wilson, D.L.; Trail, F.; Linz, J.E. Chromosomal location plays a role in regulation of aflatoxin gene expression in Aspergillus parasiticus. Appl. Environ. Microbiol. 2002, 68, 306–315. [Google Scholar] [PubMed]
- Liang, S.H.; Wu, T.S.; Lee, R.; Chu, F.S.; Linz, J.E. Analysis of mechanisms regulating expression of the ver-1 gene, involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1997, 63, 1058–1065. [Google Scholar] [PubMed]
- Busch, S.; Eckert, S.E.; Krappmann, S.; Braus, G.H. The COP9 signalosome is an essential regulator of development in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2003, 49, 717–730. [Google Scholar] [PubMed]
- Lima, J.F.; Malavazi, I.; von Zeska Kress Fagundes, M.R.; Savoldi, M.; Goldman, M.H.; Schwier, E.; Braus, G.H.; Goldman, G.H. The csnD/csnE signalosome genes are involved in the Aspergillus nidulans DNA damage response. Genetics 2005, 171, 1003–1015. [Google Scholar] [PubMed]
- Chiba, T.; Tanaka, K. Cullin-based ubiquitin ligase and its control by NEDD8-conjugating system. Curr. Protein Pept. Sci. 2004, 5, 177–184. [Google Scholar] [PubMed]
- He, Q.; Cheng, P.; He, Q.; Liu, Y. The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 2005, 19, 1518–1531. [Google Scholar] [PubMed]
- Wimuttisuk, W.; Singer, J.D. The Cullin3 ubiquitin ligase functions as a Nedd8-bound heterodimer. Mol. Biol. Cell 2007, 18, 899–909. [Google Scholar] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ehrlich, K.C. Predicted Roles of the Uncharacterized Clustered Genes in Aflatoxin Biosynthesis. Toxins 2009, 1, 37-58. https://doi.org/10.3390/toxins1010037
Ehrlich KC. Predicted Roles of the Uncharacterized Clustered Genes in Aflatoxin Biosynthesis. Toxins. 2009; 1(1):37-58. https://doi.org/10.3390/toxins1010037
Chicago/Turabian StyleEhrlich, Kenneth C. 2009. "Predicted Roles of the Uncharacterized Clustered Genes in Aflatoxin Biosynthesis" Toxins 1, no. 1: 37-58. https://doi.org/10.3390/toxins1010037