A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases
Abstract
:1. Introduction
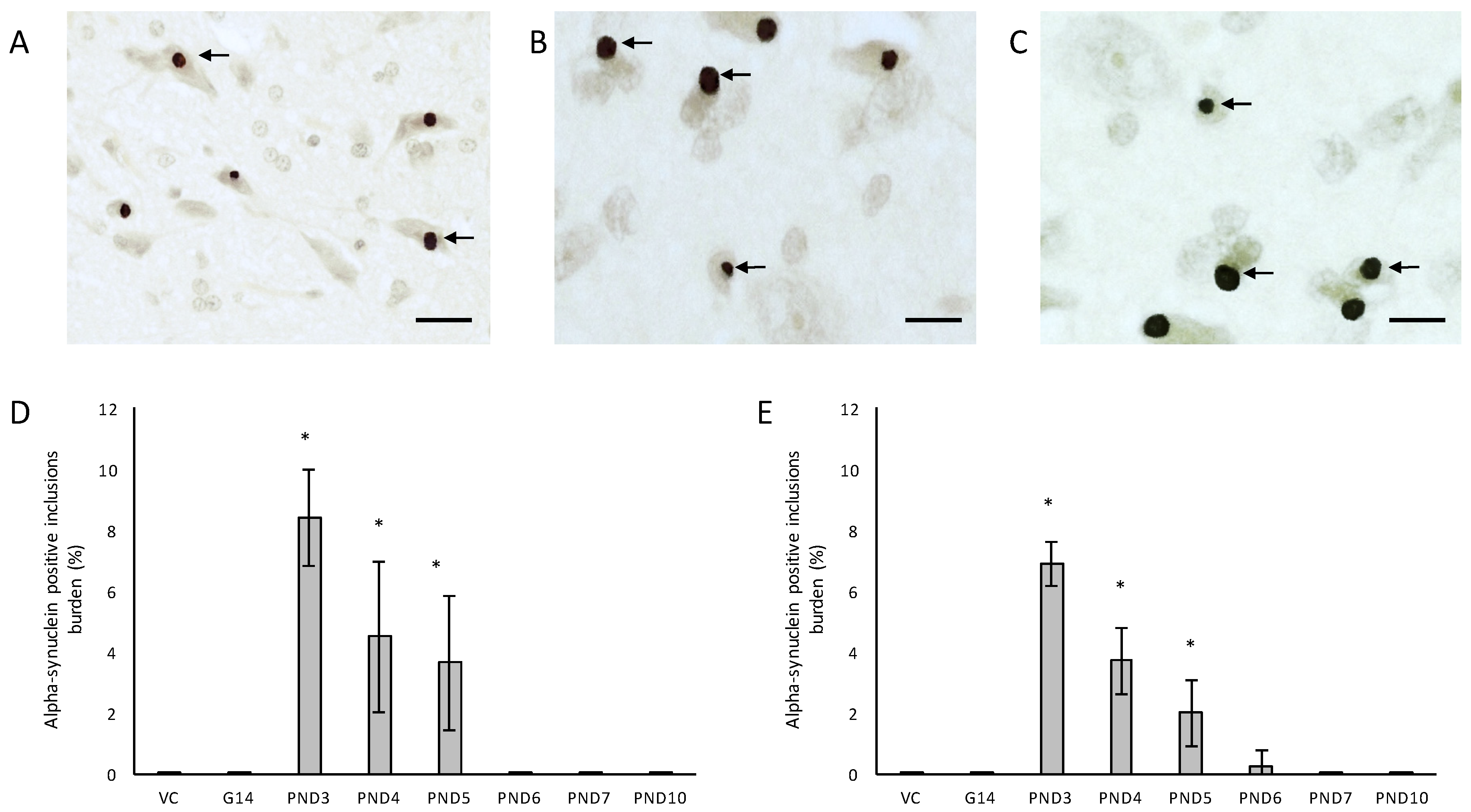
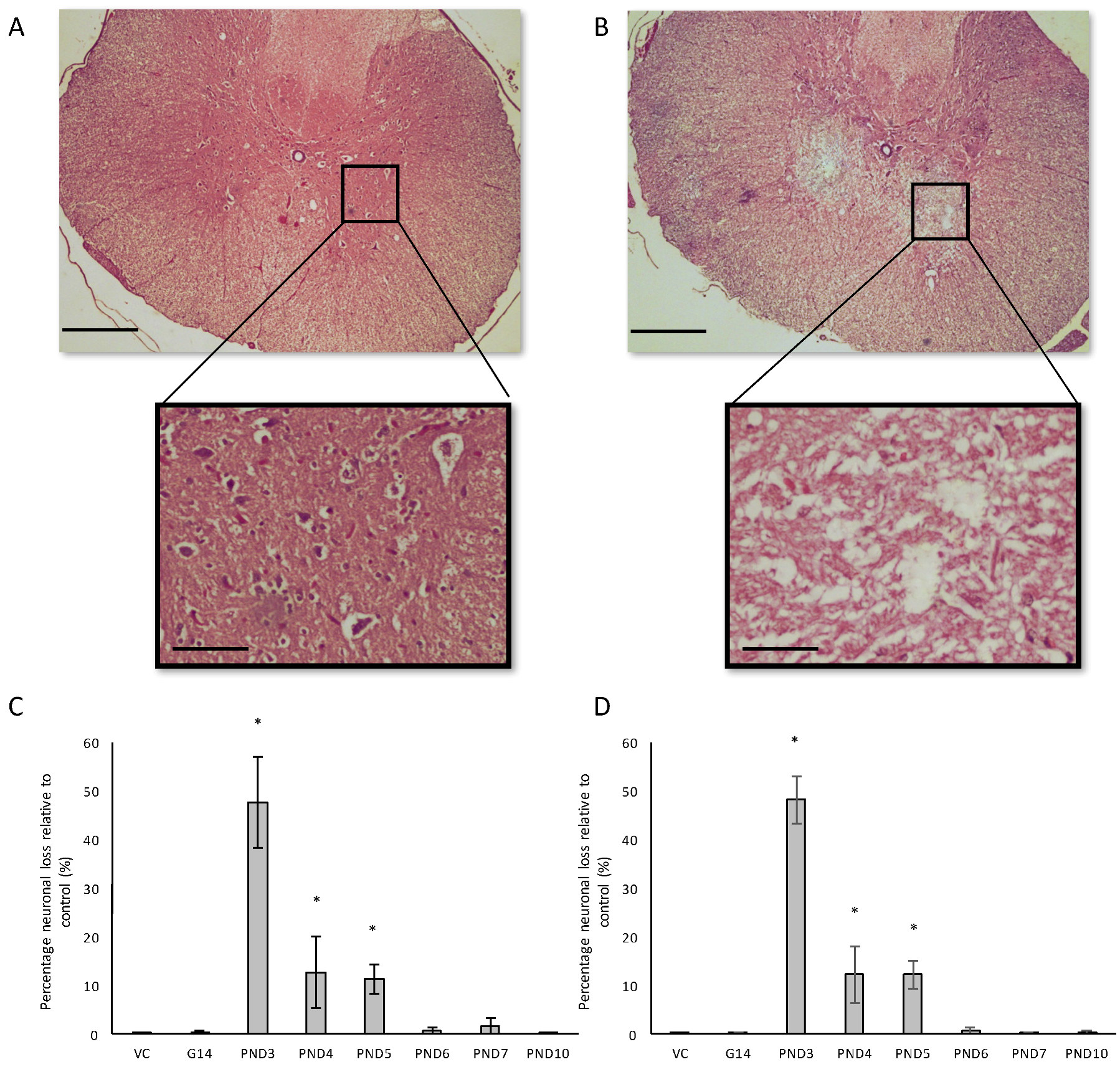
2. Results
2.1. General Findings
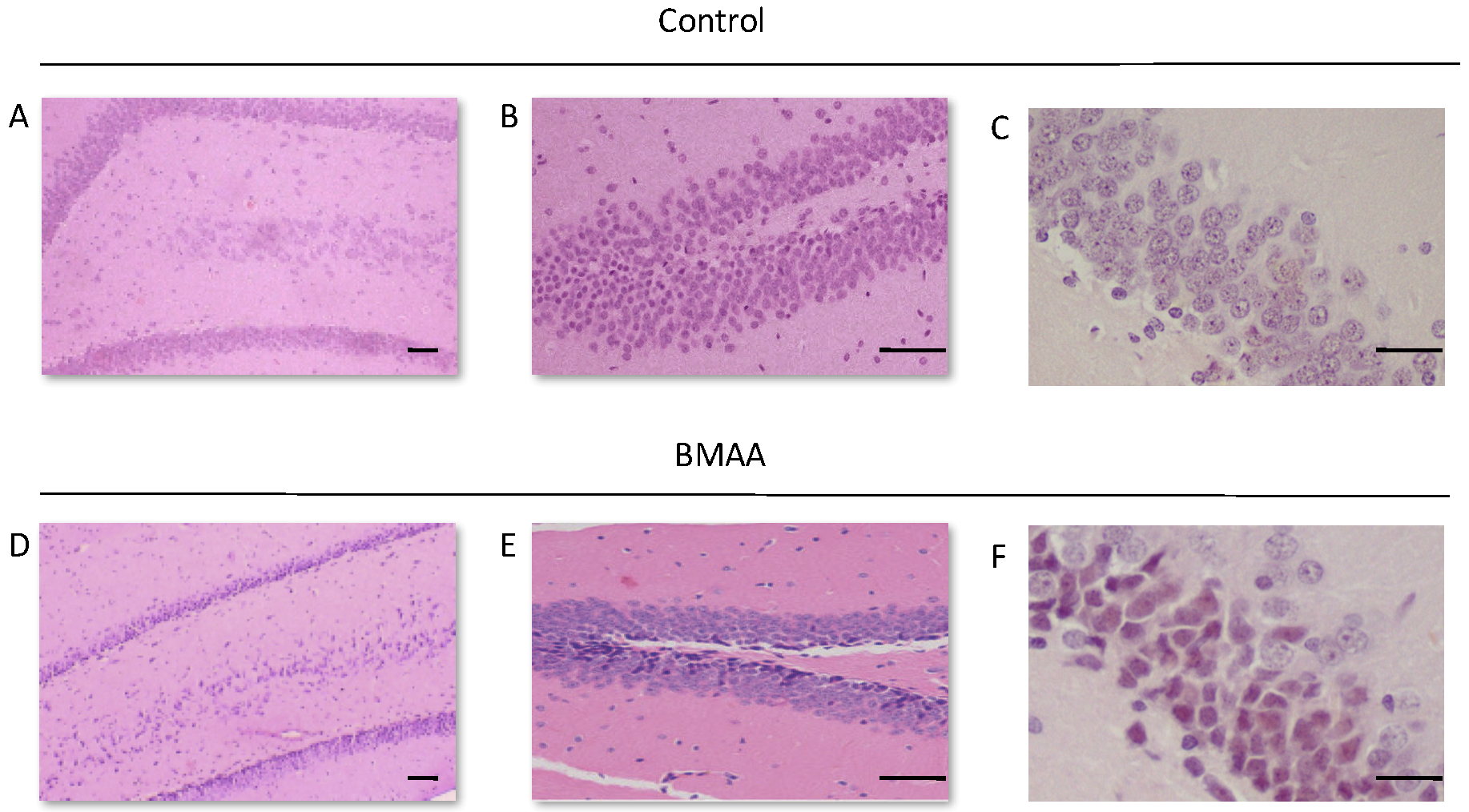
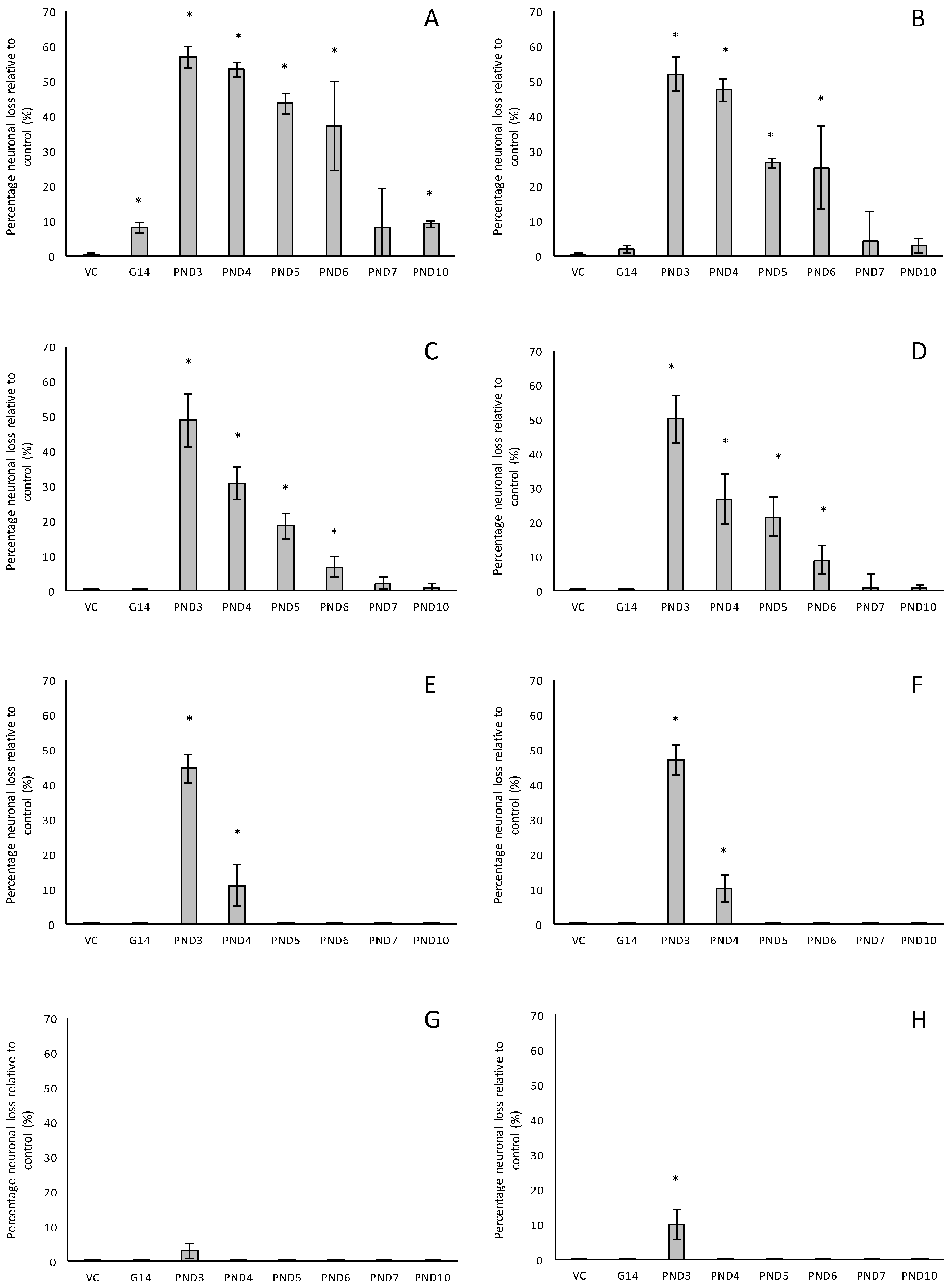
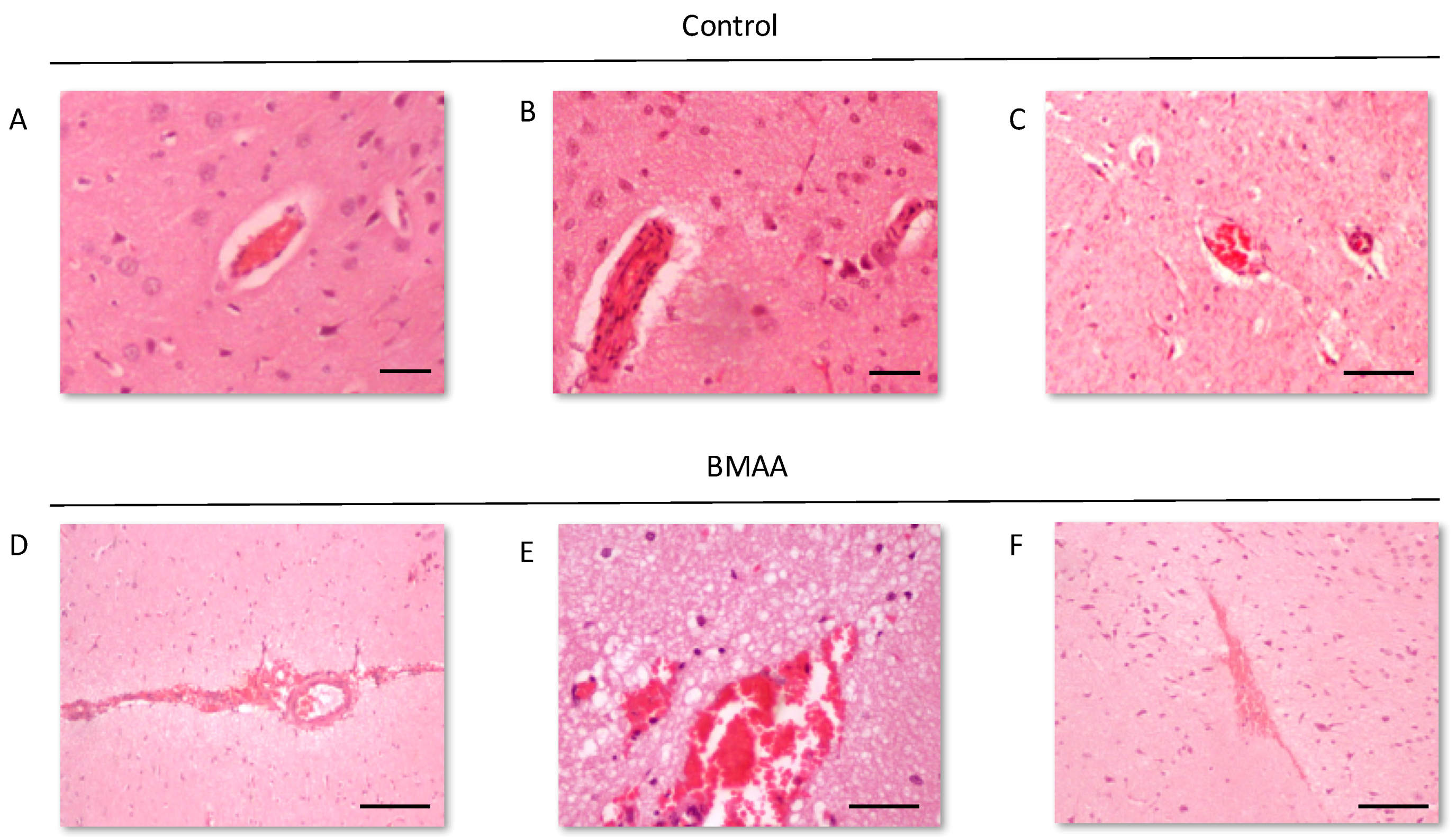
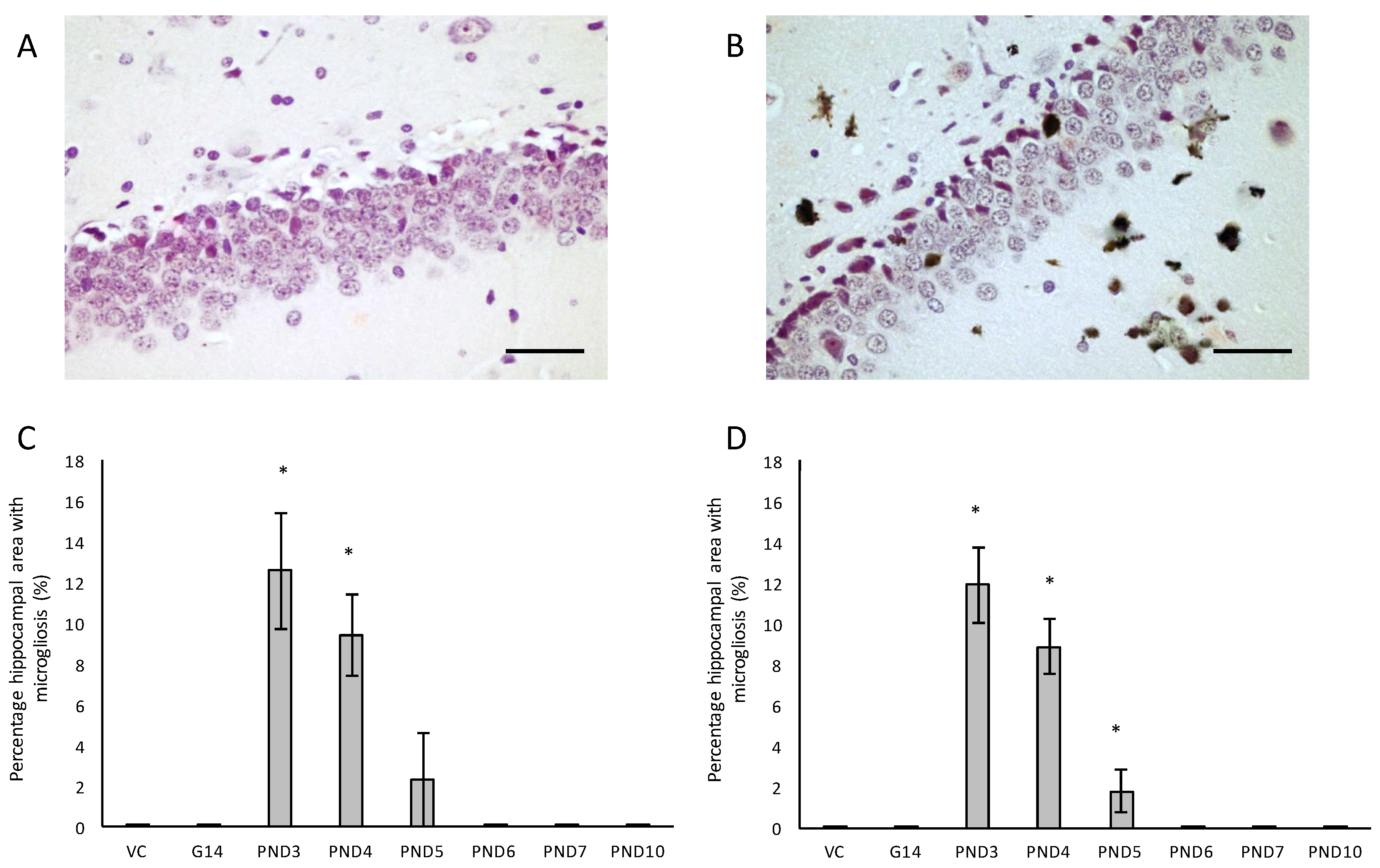
2.2. Brain Pathology
2.3. Spinal Cord Pathology
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Animal Maintenance
4.3. Exposure
4.4. Histology and Immunohistochemistry
4.5. Statistical Analysis
5. Patents
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirano, A.; Kurland, L.T.; Krooth, R.S.; Lessel, S. Parkinsonism-dementia complex, and endemic disease on the island of Guam. I. Clinical features. Brain 1961, 84, 642–661. [Google Scholar] [CrossRef]
- Hirano, A.; Malamud, N.; Elizan, T.S.; Kurland, L.T. Amyotrophic lateral sclerosis and parkinsonism-dementia complex on Guam: Further pathological studies. Arch. Neurol. 1966, 15, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Dembitzer, H.M.; Kurland, L.T.; Zimmerman, H.M. The fine structure of some intraganglionic alterations: Neurofibrillary tangles, granulovacuolar bodies, and “rod-like” structures in Guam amyotrophic lateral sclerosis and parkinsonism-dementia complex. J. Neuropathol. Exp. Neurol. 1968, 27, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Arumugasamy, N.; Zimmerman, H.M. Amyotrophic lateral sclerosis: A comparison of Guam and classical cases. Arch. Neurol. 1967, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.L.; Lee, V.M.; Saido, T.; Perl, D.; Schuck, T.; Iwatsubo, T.; Trojanowski, J.Q. Amyloid plaques in Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex contain species of A beta similar to those found in the amyloid plaques of Alzheimer’s disease and pathological aging. Acta Neuropathol. 2008, 95, 117–122. [Google Scholar] [CrossRef]
- Buee-Scherrer, V.; Buee, L.; Hof, P.R.; Leveugle, B.; Gilles, C.; Loerzel, A.J.; Perl, D.P.; Delacourte, A. Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam. Immunochemical characterization of tau proteins. Am. J. Pathol. 1995, 146, 924. [Google Scholar] [PubMed]
- Sebeo, J.; Hof, P.R.; Perl, D.P. Occurrence of α-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol. 2004, 107, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Geser, F.; Winton, M.J.; Kwong, L.K.; Xu, Y.; Xie, S.X.; Igaz, L.M.; Garruto, L.M.; Perl, D.P.; Galasko, D.; Lee, V.M.; et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008, 115, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Bell, E.A. α-Amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry 1967, 6, 759–762. [Google Scholar] [CrossRef]
- Spencer, P.S.; Nunn, P.B.; Hougon, J.; Ludolph, A.C.; Roy, D.N.; Ross, S.M.; Robertson, R.C. Guam amyotrophic lateral sclerosis-Parkinsonism-Dementia linked to a plant excitant neurotoxin. Science 2017, 237, 517. [Google Scholar] [CrossRef]
- Cox, P.A.; Sacks, O.W. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 2002, 58, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of β-N-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Murch, S.J.; Cox, P.A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 2006, 106, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Cox, P.A. Biomagnification of cycad neurotoxins in flying foxes: Implications for ALS-PDC in Guam. Neurology 2003, 61, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s Disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G.; Mash, D.C. Beyond Guam: The cyanobacteria/BMAA hypothesis of the cause of the cause of ALS and other neurodegenerative diseases. Amyotroph. Lateral Scler. 2009, 10, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Sammak, M.A.; Rogers, D.G.; Hoagland, K.D. Acute β-N-Methylamino-l-alanine Toxicity in a mouse model. J. Toxicol. 2015, 2015, 739746. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Saez, E.; de Munck Garcia, E.; Arahuetes Portero, R.M.; Martinez, A.; Solas Alados, M.T.; Miguel, B.G. Analysis of β-N-methylamino-l-alanine (l-BMAA) neurotoxicity in rat cerebellum. Neurotoxicology 2015, 48, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R., Jr.; Marschall, E.G.; Chan, K.C.; Millard, W.J.; Eppler, B.; Patterson, T.A. Neurochemical and neurobehavioural effects of neonatal administration of β-N-methylamino-l-alanine and 3,3′-iminodipropionitrile. Neurotoxicol. Teratol. 1998, 20, 181–192. [Google Scholar] [CrossRef]
- Seawright, A.A.; Brown, A.W.; Nolan, C.C.; Cavanagh, J.B. Selective degeneration of cerebellar cortical neurons caused by cycad neurotoxin l-β-methylaminoalanine (BMAA), in rats. Neuropathol. Appl. Neurobiol. 1990, 16, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Roman, E.; Brittebo, E.B. Long-term cognitive impairments in adult rats treated neonatally with beta-N-Methylamino-l-Alanine. Toxicol. Sci. 2009, 112, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Lindquist, N.G.; Brittebo, E.B.; Roman, E. Selective Brain Uptake and Behavioural Effects of the Cyanobacterial Toxin BMAA (β-N-Methylamino-l-alanine) following Neonatal Administration to Rodents. Toxicol. Sci. 2009, 109, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Polsky, F.I.; Nunn, P.B.; Bell, E.A. Distribution and toxicity of amino-β-methylaminopropionic acid. Fed. Proc. 1972, 31, 1473–1475. [Google Scholar] [PubMed]
- Perry, T.L.; Bergeron, C.; Biro, A.J.; Hansen, S. Chronic oral administration of β-N-methylamino-l-alanine is not neurotoxic to mice. J. Neurol. Sci. 1989, 94, 173–180. [Google Scholar] [CrossRef]
- Duncan, M.W.; Villacreses, N.E.; Pearson, P.G.; Wyatt, L.; Rapoport, S.I.; Kopin, I.J.; Markey, S.P.; Smith, Q.R. 2-Amino-3-(methylamino)-propanoic acid (BMAA) pharmacokinetics and blood-brain barrier permeability in the rat. J. Pharmacol. Exp. Ther. 1991, 258, 27–35. [Google Scholar] [PubMed]
- Cruz-Aguado, R.; Winkler, D.; Shaw, C.A. Lack of behavioural and neuropathological effects of dietary β-methylamino-l-alanine (BMAA) in mice. Pharmacol. Biochem. Behav. 2006, 84, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Staton, P.C.; Bristow, D.R. The Dietary Excitotoxins β-N-Methylamino-l-Alanine and f3-N-Oxalylamino-l-Alanine Induce Necrotic- and Apoptotic-Like Death of Rat Cerebellar Granule Cell. J. Neurochem. 1997, 69, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, K.; Yamazaki, M.; Takahashi, H.; Watabe, K.; Wada, M.; Komori, T.; Morita, T.; Mizutani, T. Spinal anterior horn cells in sporadic amyotrophic lateral sclerosis show ribosomal detachment from, and cisternal distention of the rough endoplasmic reticulum. Neuropathol. Appl. Neurobiol. 2008, 34, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Baloyannis, S.J. Golgi apparatus and protein trafficking in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42 (Suppl. 3), S153–S162. [Google Scholar]
- Lindstrom, H.; Luthman, J.; Mouton, P.; Spencer, P.; Olson, L. Plant-derived neurotoxic amino acids (beta-N-oxalylamino-l-alanine and beta-N-methylamino-l-alanine): Effects on central monoamine neurons. J. Neurochem. 1990, 55, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Berg, A.-L.; Lindstrom, A.-K.; Arnerup, G.; Roman, E.; Bergquist, J.; Hanrieder, J.; Lindquist, N.G.; Brittebo, E.B.; Andersson, M. Neonatal exposure to the cyanobacterial toxin BMAA induces changes in protein expression, and neurodegeneration in adult hippocampus. Toxicol. Sci. 2012, 130, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Buenz, E.J.; Howe, C.L. Beta-methylamino-alanine (BMAA) injures hippocampal neurons in vivo. Neurotoxicology 2007, 28, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.J.; Bernal, F.; Andrés, N.; Malpesa, Y.; Mahy, N. Excitatory amino acids and neurodegeneration: A hypothetical role of calcium precipitation. Int. J. Dev. Neurosci. 2000, 18, 299–307. [Google Scholar] [CrossRef]
- Yin, H.Z.; Yu, S.; Hsu, C.-I.; Liu, J.; Acab, A.; Wu, R.; Tao, O.; Chiang, B.J.; Weiss, J.H. Intrathecal infusion of BMAA induces selective motor neuron damage and astrogliosis in the ventral horn of the spinal cord. Exp. Neurol. 2014, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. 2016, 283, 20152397. [Google Scholar] [CrossRef] [PubMed]
- Lemere, C.A.; Beierschmitt, A.; Iglesias, M.; Spooner, E.T.; Bloom, J.K.; Leverone, J.F.; Zheng, J.B.; Seabrook, T.J.; Louard, D.; Li, D.; et al. Alzheimer’s Disease Aβ Vaccine Reduces Central Nervous System Aβ Levels in a Non-Human Primate, the Caribbean Vervet. Am. J. Pathol. 2004, 165, 283–297. [Google Scholar] [CrossRef]
- Lemere, C.A.; Frost, J.L.; Djivre, I.; Butler, D.; Le, K.; Matthew, M.; Luo, E.; Fagan, A.M.; Ervin, F.R.; Palmour, R.M. Aging, biomarkers and behavior in Caribbean vervets. J. Alzheimer’s Assoc. 2010, 6, S78. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E.; Bohl, J.; Bratzke, H. Evolution of Alzheimer’s Disease related cortical lesions. In Alzheimer’s Disease—From Basic Research to Clinical Applications; Springer: Vienna, Austria, 1998; pp. 97–106. [Google Scholar]
- Mormino, E.C.; Kluth, J.T.; Madison, C.M.; Rabinovici, G.D.; Baker, S.L.; Miller, B.L.; Koeppe, R.A.; Mathis, C.A.; Weiner, M.W.; Jagust, W.J.; et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in eldery subjects. Brain 2009, 132, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosty, S.T.; Barberger-Gauteau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s Disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- Blesa, J.; Przedborski, S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Maries, E.; Dass, B.; Collier, T.J.; Kordower, J.H.; Steece-Collier, K. The role of alpha-synuclein in Parkinson’s disease: Insights from animal models. Nat. Rev. Neurosci. 2003, 4, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Gamber, K.M. Animal Models of Parkinson’s Disease: New models provide greater translational and predictive value. BioTechniques 2016, 61, 210–211. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Atlas of Prenatal Rat Brain Development; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Huang, H.; Liu, C.M.; Sun, J.; Hao, T.; Xu, C.M.; Wang, D.; Wu, Y.Q. Ketamine Affects the Neurogenesis of the Hippocampal Dentate Gyrus in 7-Day-Old Rats. Neurotox. Res. 2016, 30, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Voorn, P.; Kalsbeck, A.; Jorritsma-Byham, B.; Groenewegen, H.J. The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the stria-tum of the rat. Neuroscience 1988, 25, 857–887. [Google Scholar] [CrossRef]
- Tepper, J.M.; Damlama, M.; Trent, F. Postnatal changes in the dis-tribution and morphology of rat substantia nigra dopaminergic neurons. Neuroscience 1994, 60, 469–477. [Google Scholar] [CrossRef]
- Schmidt, U.; Beyer, C.; Oestreicher, A.B.; Reisert, I.; Schilling, K.; Pilgrim, C. Activation of dopaminergic D1 receptors promotes morphogenesis of developing striatal neurons. Neuroscience 1996, 74, 453–460. [Google Scholar] [CrossRef]
- Spencer, G.E.; Klumperman, J.; Syed, N.I. Neurotransmitters and neurodevelopment. Role of dopamine in neurite outgrowth, target selection and specific synapse formation. Perspect. Dev. Neurobiol. 1998, 5, 451–467. [Google Scholar] [PubMed]
- Stanwood, G.; Levitt, P. The effects of cocaine on the developing nervous system. In Handbook of Developmental Cognitive Neuroscience; Nelson, C.A., Luciana, M., Eds.; MIT Press: Cambridge, MA, USA, 2001; pp. 519–536. [Google Scholar]
- Bellone, C.; Mameli, M.; Luscher, C. In Utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat. Neurosci. 2011, 14, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.M.; Zhang, X.; Darnell, S.B.; Sangrey, G.R.; Yanagawa, Y.; Sadri-Vakili, G. Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J. Neurosci. 2011, 31, 13400–13411. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.L.; Downing, T.G. β-N-methylamino-l-alanine (BMAA) toxicity is gender and exposure-age dependent in rats. Toxins. 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Opitz, B. Memory function and the hippocampus. Front. Neurol. Neurosci. 2014, 34, 51–59. [Google Scholar] [PubMed]
- Desikan, R.S.; Sabuncu, M.R.; Schmansky, N.J.; Reuter, M.; Cabral, H.J.; Hess, C.P.; Weiner, M.W.; Biffi, A.; Anderson, C.D.; Rosand, J.; et al. Alzheimer’s Disease Neuroimaging Initiative. Selective disruption of the cerebral neocortex in Alzheimer’s disease. PLoS ONE 2010, 5, 12853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, C.M.; Perez, S.E.; Overk, C.R.; Wynick, D.; Mufson, E.J. Effect of Neocortical and Hippocampal Amyloid Deposition upon Galaninergic and Cholinergic Neurites in AβPPswe/PS1∆E9 Mice. J. Alzheimer’s Dis. 2011, 25, 491–504. [Google Scholar]
- Simic, G.; Kostovic, I.; Winblad, B.; Bogdanovic, N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J. Comp. Neurol. 1997, 379, 482–494. [Google Scholar] [CrossRef]
- West, M.J.; Coleman, P.D.; Flood, D.G.; Troncoso, J.C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 1994, 344, 769–772. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Mavroudis, J.; Fotiou, D.; Baloyannis, S. Hippocampal Neuronal Loss in the Ca1 And Ca3 Areas Of Alzheimer’s Disease Patients. Psychiatr. Danub. 2012, 24, 152–158. [Google Scholar] [PubMed]
- Adachi, M.; Kawakatsu, S.; Hosoya, T.; Otani, K.; Honma, T.; Shibata, A.; Sugai, Y. Morphology of the inner structure of the hippocampal formation in Alzheimer disease. Am. J. Neuroradiol. 2003, 24, 1575–1581. [Google Scholar] [PubMed]
- Fukutani, Y.; Cairns, N.J.; Shiozawa, M.; Sasaki, K.; Sudo, S.; Isaki, K.; Lantos, P.L. Neuronal loss and neurofibrillary degeneration in the hippocampal cortex in late-onset sporadic Alzheimer’s disease. Psychiatry Clin. Neurosci. 2000, 54, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Ordóñez, C.; del Valle, E.; Navarro, A.; Tolivia, J. Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, G.A.; Hess, C.P.; Hammond-Rosenbluth, K.E.; Xu, D.; Rabinovici, G.D.; Kelley, D.A.; Vigneron, D.B.; Nelson, S.J.; Miller, B.L. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology 2010, 75, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, M.; Prots, I.; Winner, B. Adult Hippocampal Neurogenesis in Parkinson’s Disease: Impact on Neuronal Survival and Plasticity. Neural Plast. 2014, 2014, 454696. [Google Scholar] [CrossRef] [PubMed]
- Camicioli, R.M.; Moore, M.; Kinney, A.; Corbridge, E.; Glassberg, K.; Kaye, G.A. Parkinson’s disease is associated with hippocampal atrophy. Mov. Disord. 2003, 18, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Lajud, N.; Torner, L. Early life stress and hippocampal neurogenesis in the neonate: Sexual dimorphism, long term consequences and possible mediators. Front. Mol. Neurosci. 2015, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Kikusui, T.; Mori, Y. Behavioural and neurochemical consequences of early weaning in rodents. J. Neuroendocrinol. 2009, 21, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Koricka, S.; Lucassen, P.J.; Joëls, M. Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Front. Endocrinol. 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Uhl, G.R.; Hedreen, J.C.; Price, D.L. Parkinson’s disease: Loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology 1985, 35, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Healy-Stoffel, M.; Ahmad, S.O.; Stanford, J.A.; Levant, B. Differential Effects of Intrastriatal 6-Hydroxydopamine On Cell Number And Morphology In Midbrain Dopaminergic Subregions of the RaT. Brain Res. 2014, 1574, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Phani, S.; Gonye, G.; Iacovitti, L. VTA neurons show a potentially protective transcriptional response to MPTP. Brain Res. 2010, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.L.; Atienza, J.G.; Langston, J.W.; Di Monte, D.A. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience 2002, 141, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Dopeso-Reyes, I.G.; Rico, A.J.; Roda, E.; Sierra, S.; Pignataro, D.; Lanz, M.; Lanciego, J.L. Calbindin content and differential vulnerability of midbrain efferent dopaminergic neurons in macaques. Front. Neuroanat. 2014, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kim, K.Y.; Kim, J.H.; Kim, J.H.; Park, M.S.; Bahk, J.Y.; Kim, M.O. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neurosci. Lett. 2004, 363, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Yeterian, E.H.; Pandya, D.N. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J. Comp. Neurosci. 1991, 312, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, A.; Provost, J.-B.; Monchi, O. Neuroimaging studies of striatum in cognition part II: Parkinson’s disease. Neurosci. Front. Syst. 2015, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-C.; Ulane, C.M.; Burke, R. Clinical Progression in Parkinson’s Disease and the Neurobiology of Axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.P.; Partanen, K.; Riekkinen, P.; Lehtovirta, M.; Helkala, E.L.; Hallikainen, M.; Hanninen, T.; Vainio, P.; Soininen, H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia An MRI study. Neurology 1996, 46, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Zainal, N.H.; Narasimhalu, K.; Chander, R.J.; Ng, A.; Mak, E.; Au, W.L.; Sitoh, Y.Y.; Nadkarni, N.; Tan, L.C. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.J.; McFarland, N.R.; Price, C.C. Striatal and Hippocampal Atrophy in Idiopathic Parkinson’s Disease Patients without Dementia: A Morphometric Analysis. Front. Neurol. 2017, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Goldman-Rakic, P.S. Segregation of working memory functions within the dorsolateral prefrontal cortex. In Executive Control and the Frontal Lobe: Current Issues; Schneider, W.X., Owen, A.M., Duncan, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Kulisevsky, J.; García-Sánchez, C.; Berthier, M.L.; Barbanoj, M.; Pascual-Sedano, B.; Gironell, A.; Estévez-González, A. Chronic Effects of Dopaminergic Replacement on Cognitive Function in Parkinson’s Disease: A Two-Year Follow-Up Study of Previously Untreated Patients. Mov. Disord. 2000, 15, 613–626. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trend Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Geyer, M.; Couillard, S.; Aigner, R.; Bogdahn, U.; Aigner, L.; Kuhn, G.; Winkler, J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp. Neurol. 2006, 197, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisén, J. Neurogenesis in the Striatum of the Adult Human Brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Triarhou, L.C. Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2000–2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6271/#_NBK6271_pubdet (accessed on 20 October 2017).
- Fitzgerald, J.L.; Reid, J.J. Effects of methylenedioxymethamphetamine on the release of monoamines from rat brain slices. Eur. J. Pharmacol. 1990, 191, 217–220. [Google Scholar] [CrossRef]
- Eiden, L.E.; Weihe, E. VMAT2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci. 2011, 1216, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Granado, N.; O’Shea, E.; Bove, J.; Vila, J.; Colado, I.; Moratalla, R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J. Neurochem. 2008, 107, 1102–1112. [Google Scholar] [PubMed]
- Quinton, M.S.; Yamamoto, B.K. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2008, 8, E337–E339. [Google Scholar] [CrossRef]
- Scott, J.C. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol. Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Ares-Santos, S.; Granado, N.; Espadas, I.; Martinez-Murillo, R.; Moratalla, R. Methamphetamine Causes Degeneration of Dopamine Cell Bodies and Terminals of the Nigrostriatal Pathway Evidenced by Silver Staining. Neuropsychopharmacology 2014, 39, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Granado, N.; Ares-Santos, S.; Moratalla, R. Methamphetamine and Parkinson’s disease. Parkinson’s Disord 2013. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Matarrdona, E.R.; Machado, A.; Cano, J. Acute perfusion of BMAA in the rat’s striatum by in vivo microdialysis. Toxicol. Lett. 2006, 167, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Herlenius, E.; Langercrantz, H. Development of neurotransmitter systems during critical periods. Exp. Neurol. 2004, 190 (Suppl. 1), S8–S21. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Clearing the brain’s amyloid cobwebs. Neuron 2001, 32, 177–180. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E.J. Alzheimer’s disease: Striatal amyloid deposits and neurofibrillary changes. Neuropathol. Exp. Neurol. 1990, 49, 215–224. [Google Scholar] [CrossRef]
- Gershoni-Baruch, R.; Brik, R.; Zacks, N.; Shinawi, M.; Lidar, M.; Livneh, A. The contribution of genotypes at the MEFV and SAA1 loci to amyloidosis and disease severity in patients with familial Mediterranean fever. Arthritis Rheumatol. 2009, 48, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Carrión, O.; Freundlieb, N.; Oertel, W.H.; Höglinger, G.U. Adult neurogenesis and Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2007, 6, 326–335. [Google Scholar] [CrossRef]
- Ellis, R.J.; Olichney, J.M.; Thal, L.J.; Mirra, S.S.; Morris, J.C.; Beekly, D.; Heyman, A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: The CERAD experience, Part XV. Neurology 1996, 46, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, B.J.; Rog, J.-H. Silent microbleeds are associated with volume of primary intracerebral haemorrhage. Neurology 2006, 66, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Wang, Z.; Fan, L.; Zhang, M.; Chu, Z.; Zuo, C.; Liu, L.; Haacke, E.; Guo, W.; Shen, W.; et al. Increased Number and Distribution of Cerebral Microbleeds Is a Risk Factor for Cognitive Dysfunction in Hemodialysis Patients: A Longitudinal Study. Medicine 2016, 95, e2974. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2001, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Sturchler-Pierrat, C.; Abramowski, D.; Duke, M.; Wiederhold, K.H.; Mistl, C.; Rothacher, S.; Ledermann, B.; Bürki, K.; Frey, P.; Paganetti, P.A.; et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 1997, 94, 13287–13292. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Hof, P.R. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Slunt, H.H.; Ratovitski, T.; Jenkins, N.A.; Copeland, N.G.; Borchelt, D.R. Co-expression of multiple transgenes in mouse CNS: A comparison of strategies. Biomol. Eng. 2001, 17, 157–165. [Google Scholar] [CrossRef]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Borthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Kazim, S.F.; Grundke-Iqbal, I.; Garruto, R.M.; Iqbal, K. Tau pathology involves protein phosphatase 2A in parkinsonism-dementia of Guam. Proc. Natl. Acad. Sci. USA 2014, 111, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Shaikh, S.; Wang, J.Z.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Phosphatase activity toward abnormally phosphorylated tau: Decrease in Alzheimer disease brain. J. Neurochem. 1995, 65, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Stalder, M.; Phinney, A.; Probst, A.; Sommer, B.; Staufenbiel, M.; Jucker, M. Association of Microglia with Amyloid Plaques in Brains of APP23 Transgenic Mice. Am. J. Pathol. 1999, 154, 1673–1684. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; de Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Paresce, D.M.; Chung, H.; Maxfield, F.R. Slow degradation of aggregates of the Alzheimer’s disease amyloid beta-protein by microglial cells. J. Biol. Chem. 1997, 272, 29390–29397. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, I.; Pérez, M.; Hernández, F.; Avila, J.; Moreno, F.J. Characteristics of the binding of thioflavin S to tau paired helical filaments. J. Alzheimer’s Disord. 2006, 9, 279–285. [Google Scholar] [CrossRef]
- Rajamohamedsait, H.B.; Sigurdsson, E.M. Histological Staining of Amyloid and Pre-Amyloid Peptides and Proteins in Mouse Tissue. Methods Mol. Biol. 2012, 849. [Google Scholar] [CrossRef]
- Cagnin, A.; Rossor, M.; Sampson, E.L.; Mackinnon, T.; Banati, R.B. In vivo detection of microglial activation in frontotem-poral dementia. Ann. Neurol. 2004, 56, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Versijpt, J.; Debruyne, J.C.; Van Laere, K.J.; De Vos, F.; Keppens, J.; Strijckmans, K.; Achten, E.; Slegers, G.; Dierckx, R.A.; Korf, J.; et al. Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: A correlative study. Mult. Scler. 2005, 11, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Bonito-Oliva, A.; Fisone, G.; Brambilla, R. Understanding cognitive deficits in Parkinson’s disease: Lessons from preclinical animal models. Learn. Mem. 2013, 20, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2015, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Vega, I.E.; Collier, T.J. Editorial: Unraveling Neuroprotective and Neurodegenerative Signals in Neurodegeneration. Front. Neurosci. 2016, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Rockenstein, E.; Veinbergs, I.; Mallory, M.; Hashimoto, M.; Takeda, A.; Sagara, Y.; Sisk, A.; Mucke, L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science 2000, 287, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Nasimudeen, R.J.; Shakil, S.; Nigel, H.G.; Alam, Q.; Adel, M.A.; Ghazi, A.D.; Mohammad, A.M. A Synopsis on the Role of Tyrosine Hydroxylase in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2012, 11, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Yavich, L.; Tanila, H.; Vepsäläinen, S.; Jäkälä, P. Role of alpha-synuclein in presynaptic dopamine recruitment. J. Neurosci. 2004, 24, 11165–11170. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Iseki, E.; Nakamura, S.; Kasanuki, K.; Chiba, Y.; Ota, K.; Murayama, N.; Sato, K. Dementia with Lewy bodies: Early diagnostic challenges. Psychogeriatrics 2013, 13, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Yu, L.; Capuano, A.W.; Wilson, R.; Leurgans, S.E.; Bennett, D.A.; Schneider, J.A. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann. Neurol. 2015, 77, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Murray, M.E.; Whitwell, J.L.; Parisi, J.E.; Petrucelli, L.; Jack, C.R.; Petersen, R.C.; Dickson, D.W. Staging TDP-43 Pathology in Alzheimer’s Disease. Acta Neuropathol. 2017, 127, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic Lateral Sclerosis. Orphanet J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Fuccillo, M.; Joyner, A.L.; Fishell, G. Morphogen to mitogen: The multiple roles of hedgehog signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006, 7, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoids and spinal cord development. J. Neurobiol. 2006, 66, 726–738. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.L.; Fetcho, J.R. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 2004, 480, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.M.; Norris, A.; Ohnmacht, J.; Patani, R.; Zhong, Z.; Dias, T.B.; Kuscha, V.; Scott, A.L.; Chen, Y.C.; Rozov, S.; et al. Dopamine from the Brain Promotes Spinal Motor Neuron Generation during Development and Adult Regeneration. Dev. Cell 2013, 25, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Borta, A.; Hoglinger, G.U. Dopamine and adult neurogenesis. J. Neurochem. 2007, 100, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Josephs, K.A.; Amador-Ortiz, C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007, 114, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.H.; Kril, J.R.; Fatima, M.; McGeachie, A.; McCann, H.; Shepherd, C.; Forrest, S.L.; Affleck, A.; Kwok, J.B.; Hodges, J.R.; et al. TDP-43 proteinopathies: Pathological identification of brain regions differentiating clinical phenotypes. Brain 2015, 138, 3110–3122. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Kiernan, M.C.; Kril, J.J. TDP-43 in the hypoglossal nucleus identifies amyotrophic lateral sclerosis in behavioral variant frontotemporal dementia. J. Neurosci. 2016, 15, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Metcalf, J.S.; Spáčil, Z.; Downing, T.G.; Downing, S.; Long, A.; Nunn, P.B.; Cox, P.A. Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from other diamino acids. Toxicon 2011, 57, 730–738. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, L.L.; Downing, T.G. A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases. Toxins 2018, 10, 22. https://doi.org/10.3390/toxins10010022
Scott LL, Downing TG. A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases. Toxins. 2018; 10(1):22. https://doi.org/10.3390/toxins10010022
Chicago/Turabian StyleScott, Laura Louise, and Timothy Grant Downing. 2018. "A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases" Toxins 10, no. 1: 22. https://doi.org/10.3390/toxins10010022



