Light-Irradiation Wavelength and Intensity Changes Influence Aflatoxin Synthesis in Fungi
Abstract
:1. Introduction
2. Results
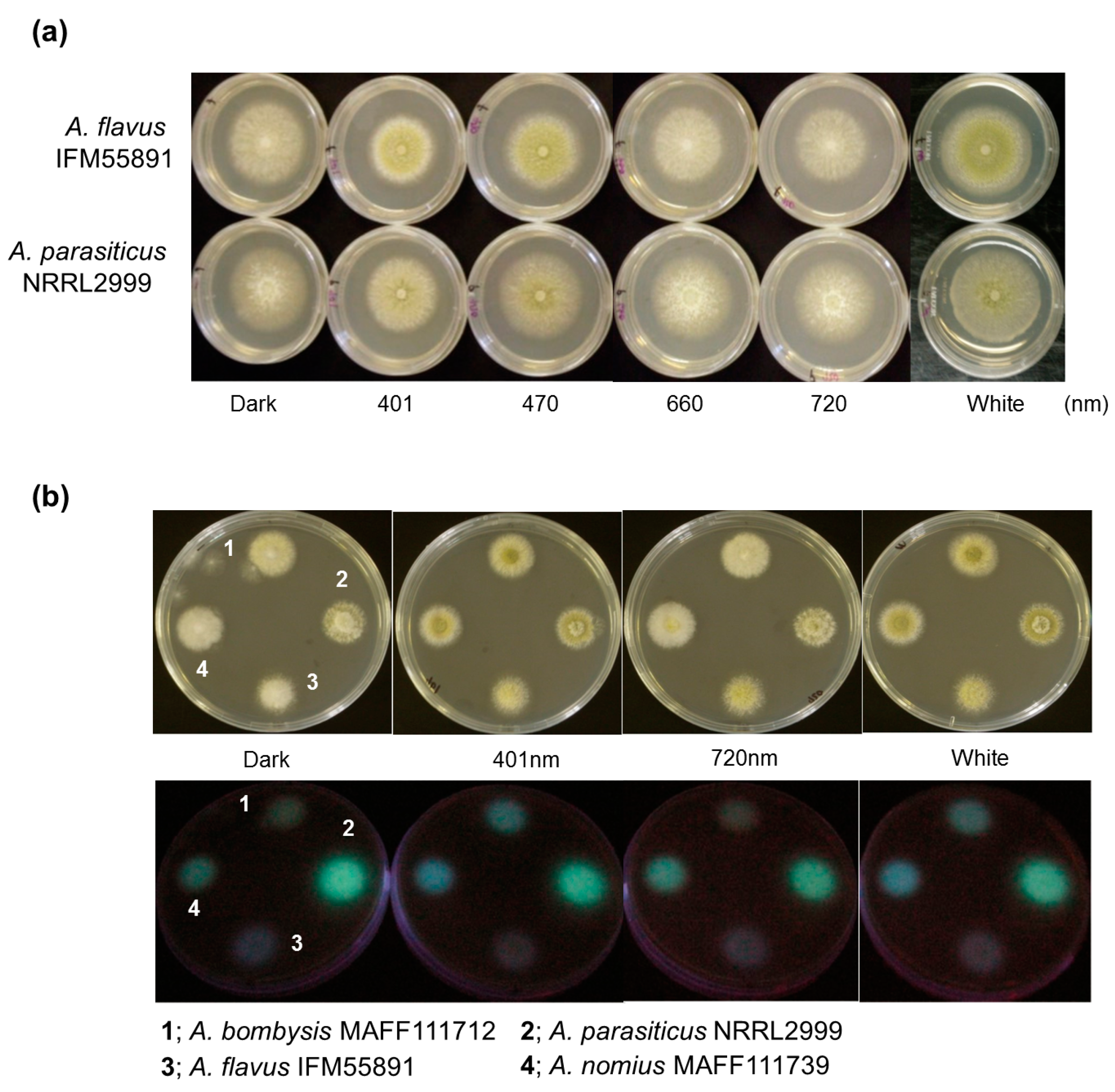
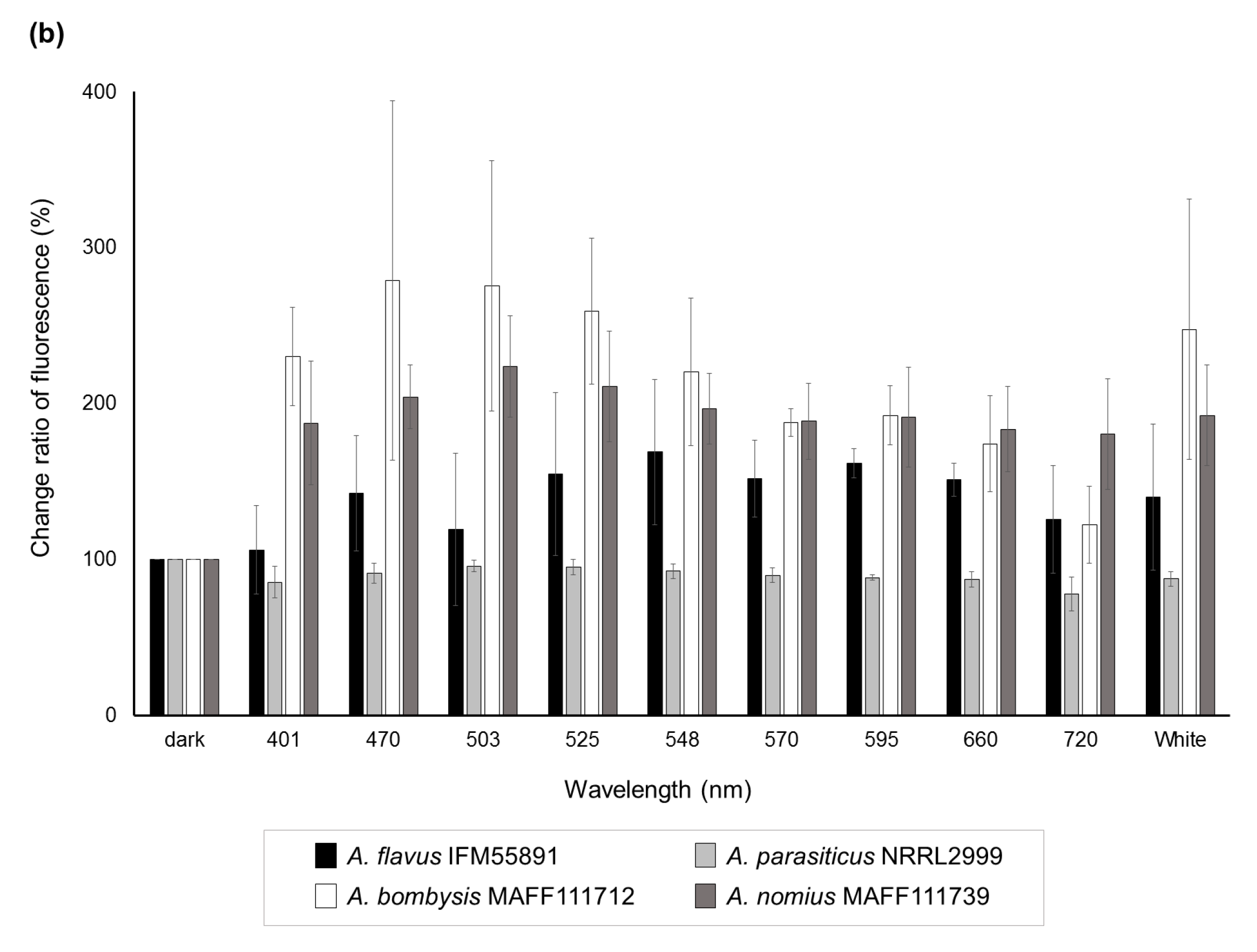
2.1. Visible Changes of Plate Cultures Caused by Conidiation and Fluorescence
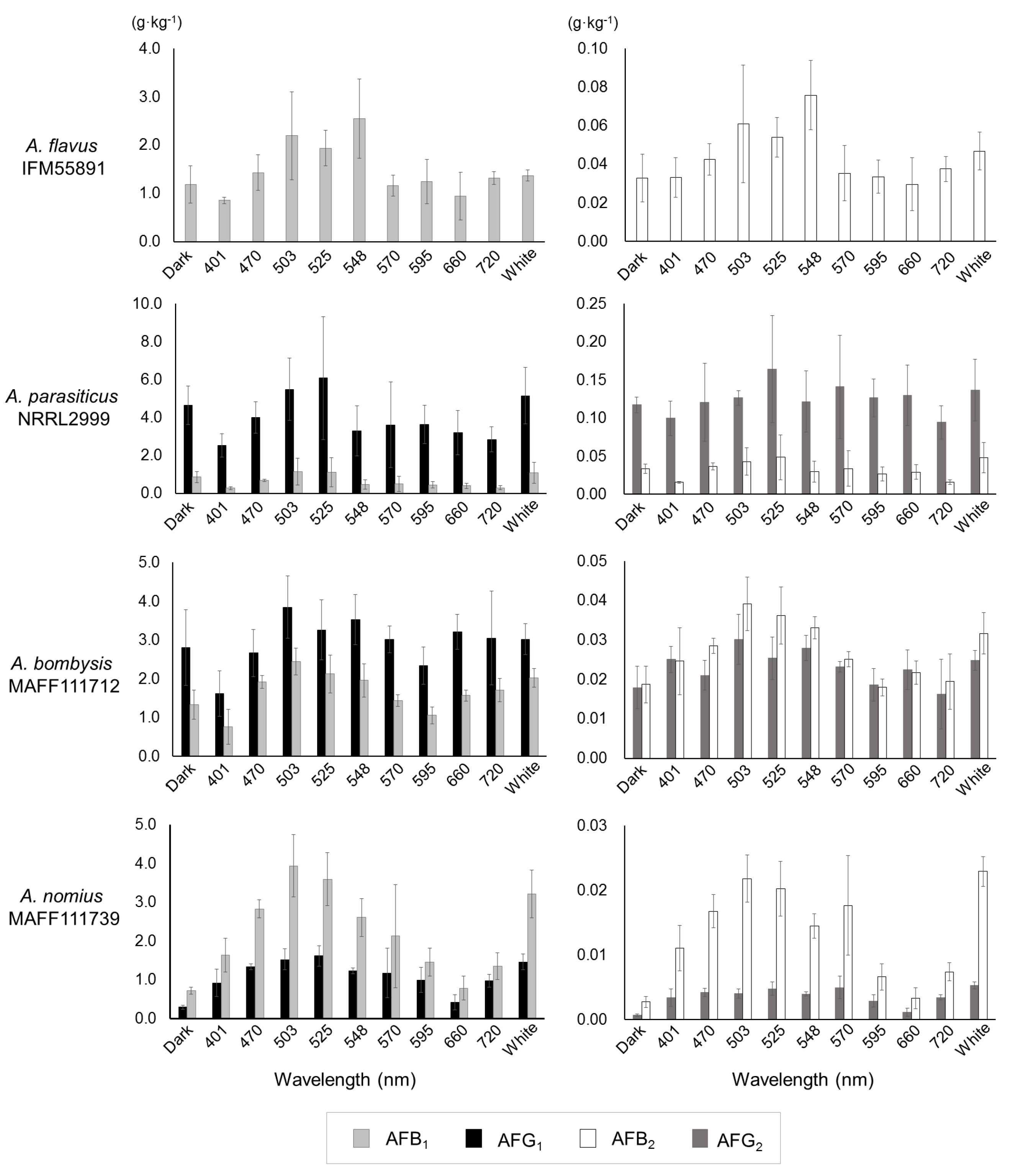
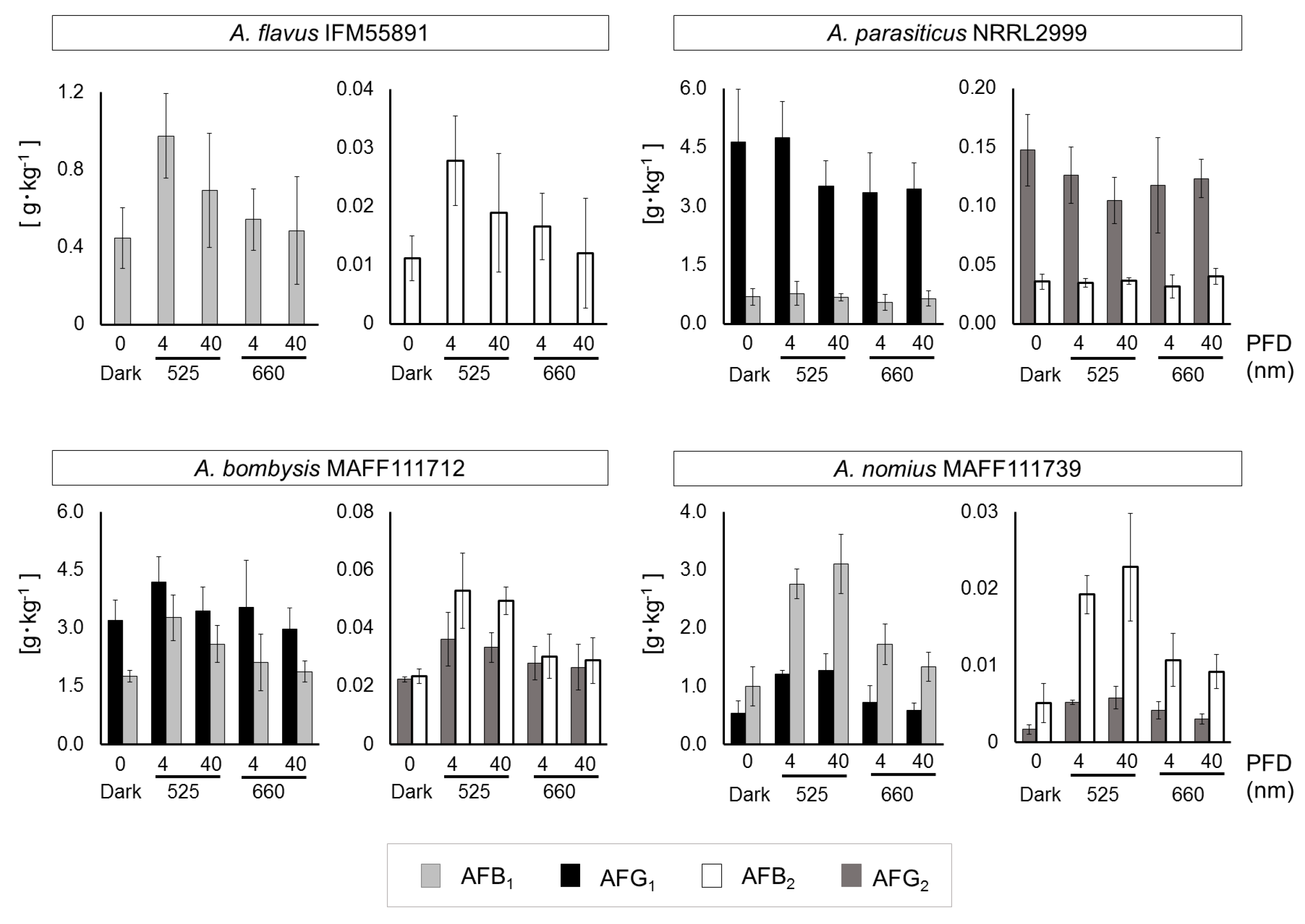
2.2. Measurement of Aflatoxins in Liquid Cultures
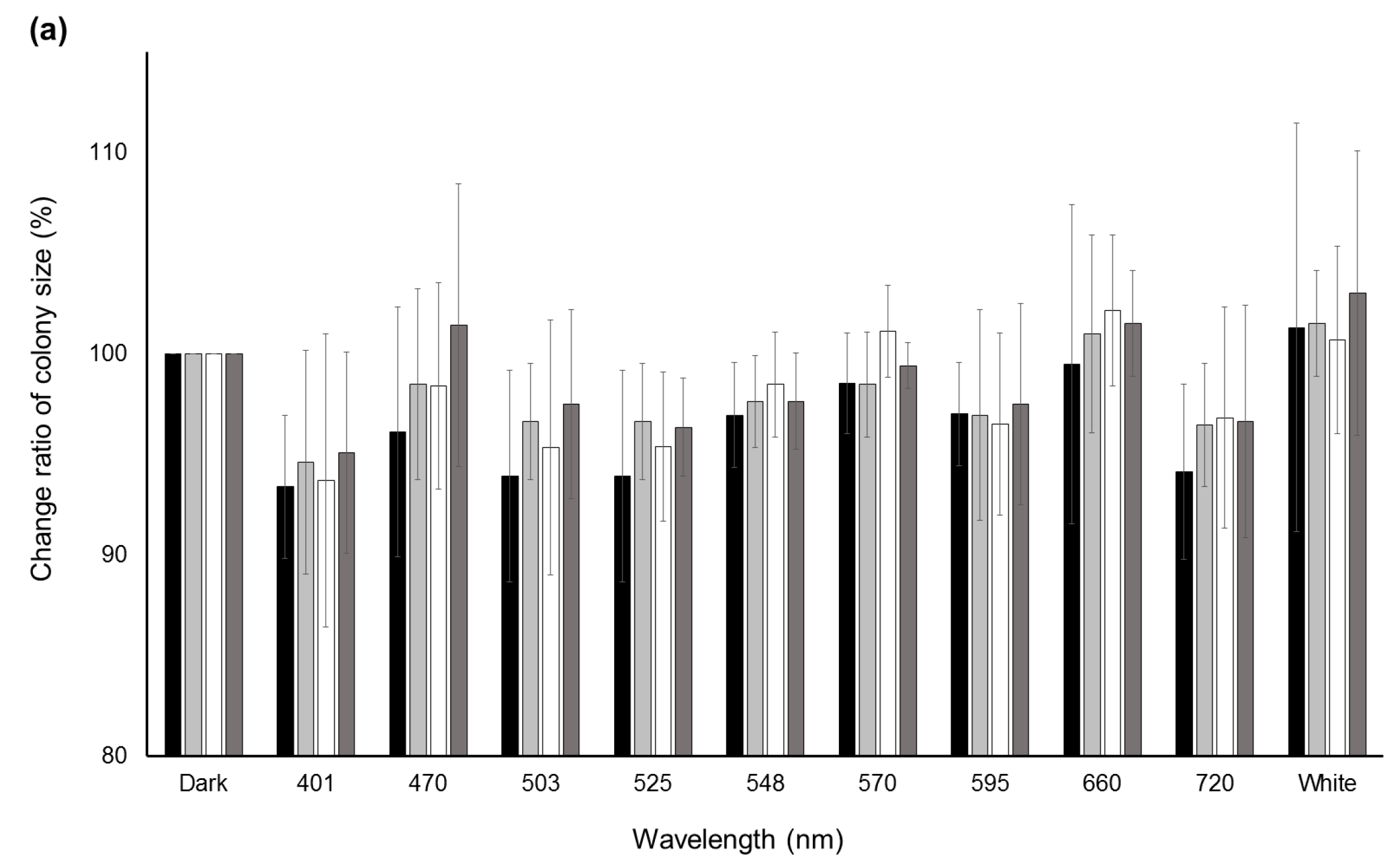
2.3. Exposure to Different Irradiation Intensity Levels Results in Varied Aflatoxin Synthesis
3. Discussion
3.1. Blue-Light Irradiation Facilitates Conidial Formation under Low-Intensity Conditions
3.2. Fungal Plate Cultures Undergoing Light Irradiation Provide an Improved Detection Technique for Aflatoxin Synthesis
3.3. Low-Intensity Bluish-Green-Light Irradiation Is More Effective in Increasing AF Synthesis than High-Intensity Irradiation or Other Wavelength Conditions, Although This Is Not Universal among Fungi
4. Conclusions
5. Materials and Methods
5.1. Fungal Species
5.2. Culture Conditions and Apparatus
5.3. Light Conditions and Measurement of Fluorescence Intensity Levels
5.4. High-Performance Liquid Chromatography
5.5. Statistical Analyses
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Wu, F. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim. Feed Sci. Technol. 2007, 137, 363–374. [Google Scholar] [CrossRef]
- Gibb, H.; Devleesschauwer, B.; Bolger, P.M.; Wu, F.; Ezendam, J.; Cliff, J.; Zeilmaker, M.; Verger, P.; Pitt, J.; Baines, J.; et al. World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: A data synthesis. F1000Res 2015, 4, 1393. [Google Scholar] [CrossRef] [PubMed]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Iwahashi, Y. Phytotoxicity evaluation of type B trichothecenes using a Chlamydomonas reinhardtii model system. Toxins (Basel) 2014, 6, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kihara, J.; Moriwaki, A.; Tanaka, N.; Ueno, M.; Arase, S. Characterization of the BLR1 gene encoding a putative blue-light regulator in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol. Lett. 2007, 266, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, A.; Katsube, H.; Ueno, M.; Arase, S.; Kihara, J. Cloning and characterization of the BLR2, the homologue of the blue-light regulator of Neurospora crassa WC-2, in the phytopathogenic fungus Bipolaris oryzae. Curr. Microbiol. 2008, 56, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ballario, P.; Vittorioso, P.; Magrelli, A.; Talora, C.; Cabibbo, A.; Macino, G. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 1996, 15, 1650–1657. [Google Scholar] [PubMed]
- Schwerdtfeger, C.; Linden, H. Localization and light-dependent phosphorylation of white collar 1 and 2, the two central components of blue light signaling in Neurospora crassa. Eur. J. Biochem. 2000, 267, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Casas-Flores, S.; Rios-Momberg, M.; Bibbins, M.; Ponce-Noyola, P.; Herrera-Estrella, A. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 2004, 150, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Proietto, M.; Bianchi, M.M.; Ballario, P.; Brenna, A. Epigenetic and Posttranslational Modifications in Light Signal Transduction and the Circadian Clock in Neurospora crassa. Int. J. Mol. Sci. 2015, 16, 15347–15383. [Google Scholar] [CrossRef] [PubMed]
- Hedtke, M.; Rauscher, S.; Röhrig, J.; Rodríguez-Romero, J.; Yu, Z.; Fischer, R. Light-dependent gene activation in Aspergillus nidulans is strictly dependent on phytochrome and involves the interplay of phytochrome and white collar-regulated histone H3 acetylation. Mol. Microbiol. 2015, 97, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Armant, O.; Fischer, R. Fungi use the SakA (HogA) pathway for phytochrome-dependent light signalling. Nat. Microbiol. 2016, 1, 16019. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.K.; Ringelberg, C.S.; Loros, J.J.; Dunlap, J.C. The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. MBio 2013, 4, e00142-13. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, R.; Nakahama, T.; Higuchi, Y.; Kitamoto, K. Light represses conidiation in koji mold Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2007, 71, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Rüfer, C.; Raupp, F.; Bruchmann, A.; Perrone, G.; Geisen, R. Influence of light on food relevant fungi with emphasis on ochratoxin producing species. Int. J. Food Microbiol. 2011, 145, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.K.; Strub, C.; Montet, D.; Durand, N.; Alter, P.; Meile, J.C.; Schorr Galindo, S.; Fontana, A. Effect of different light wavelengths on the growth and ochratoxin A production in Aspergillus carbonarius and Aspergillus westerdijkiae. Fungal Biol. 2016, 120, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Miller, J.D.; Groopman, J.D. Mycotoxin control in low- and middle-income countries. In IARC Working Group Reports; Wild, C.P., Miller, J.D., Groopman, J.D., Eds.; International Agency for Research on Cancer: Lyon, France, 2015; Volume 9, pp. 1–54. ISBN 978-92-832-2510-2. [Google Scholar]
- Umesha, S.; Manukumar, H.M.; Chandrasekhar, B.; Shivakumara, P.; Shiva Kumar, J.; Raghava, S.; Avinash, P.; Shirin, M.; Bharathi, T.R.; Rajini, S.B.; et al. Aflatoxins and food pathogens: Impact of biologically active aflatoxins and their control strategies. J. Sci. Food Agric. 2017, 97, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Cepeda, A.; Prognon, P.; Mahuzier, G.; Blais, J. Cyclodextrins as modifiers of the luminescence characteristics of aflatoxins. Anal. Chim. Acta 1991, 255, 343–350. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwahashi, Y. Addition of Carbon to the Culture Medium Improves the Detection Efficiency of Aflatoxin Synthetic Fungi. Toxins (Basel) 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; Appell, M.; Lippolis, V.; Visconti, A.; Catucci, L.; Pascale, M. Use of cyclodextrins as modifiers of fluorescence in the detection of mycotoxins. Food Addit. Contam. 2008, 25, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Yabe, K.; Nakamura, H.; Ando, Y.; Terakado, N.; Nakajima, H.; Hamasaki, T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl. Environ. Microbiol. 1988, 54, 2096–2100. [Google Scholar] [PubMed]
- Ramírez-Galicia, G.; Garduño-Juárez, R.; Gabriela Vargas, M. Effect of water molecules on the fluorescence enhancement of Aflatoxin B1 mediated by Aflatoxin B1:beta-cyclodextrin complexes. A theoretical study. Photochem. Photobiol. Sci. 2007, 6, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; Dall’Asta, C.; Restivo, F.M. Development of a simple and high-throughput method for detecting aflatoxins production in culture media. Lett. Appl. Microbiol. 2012, 55, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liang, L.; Ran, F.; Liu, Y.; Li, Z.; Lan, H.; Gao, P.; Zhuang, Z.; Zhang, F.; Nie, X.; et al. The DmtA methyltransferase contributes to Aspergillus flavus conidiation, sclerotial production, aflatoxin biosynthesis and virulence. Sci. Rep. 2016, 6, 23259. [Google Scholar] [CrossRef] [PubMed]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 9 August 2009).
- Ministry of Agriculture, Forestry and Fisheries. Available online: http://www.maff.go.jp/j/seisan/boeki/beibaku_anzen/kabikabidokukensa_surveillance/pdf/110816-3af.pdf (accessed on 8 June 2016).
| 401 | 470 | 503 | 525 | 548 | 570 | 595 | 660 | 720 | White | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | **** | **** | **** | 1 ++** | ++** | **** | **** | **** | **** | **** |
| B | 2 − − −* | **** | **** | **** | **** | **** | − *** | − *** | − − − * | **** |
| C | 3 **** | *+** | ++*+ | ++** | *+*+ | *+** | **** | **** | **** | ++** |
| D | ++++ | ++++ | ++++ | ++++ | ++++ | *+*+ | ++++ | **** | *+++ | ++++ |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T. Light-Irradiation Wavelength and Intensity Changes Influence Aflatoxin Synthesis in Fungi. Toxins 2018, 10, 31. https://doi.org/10.3390/toxins10010031
Suzuki T. Light-Irradiation Wavelength and Intensity Changes Influence Aflatoxin Synthesis in Fungi. Toxins. 2018; 10(1):31. https://doi.org/10.3390/toxins10010031
Chicago/Turabian StyleSuzuki, Tadahiro. 2018. "Light-Irradiation Wavelength and Intensity Changes Influence Aflatoxin Synthesis in Fungi" Toxins 10, no. 1: 31. https://doi.org/10.3390/toxins10010031




