Carbon Dioxide Mediates the Response to Temperature and Water Activity Levels in Aspergillus flavus during Infection of Maize Kernels
Abstract
:1. Introduction
2. Results
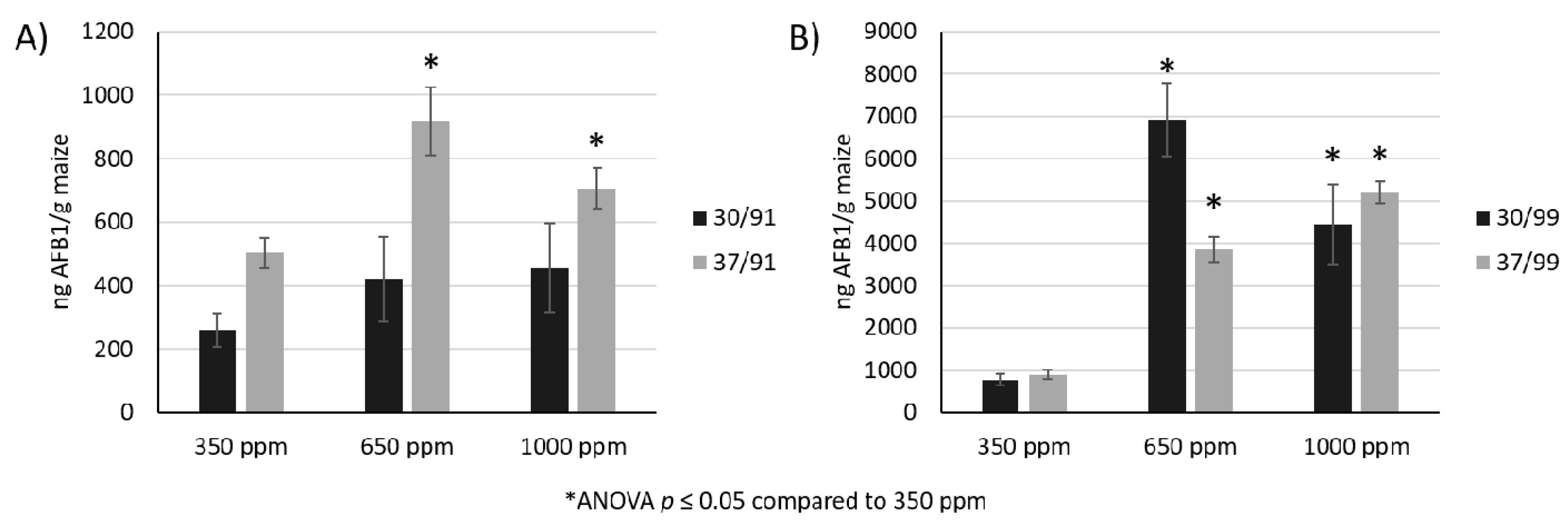
2.1. The Interaction of Water, Temperature, and CO2 Impact AFB1 Production in Maize Grain
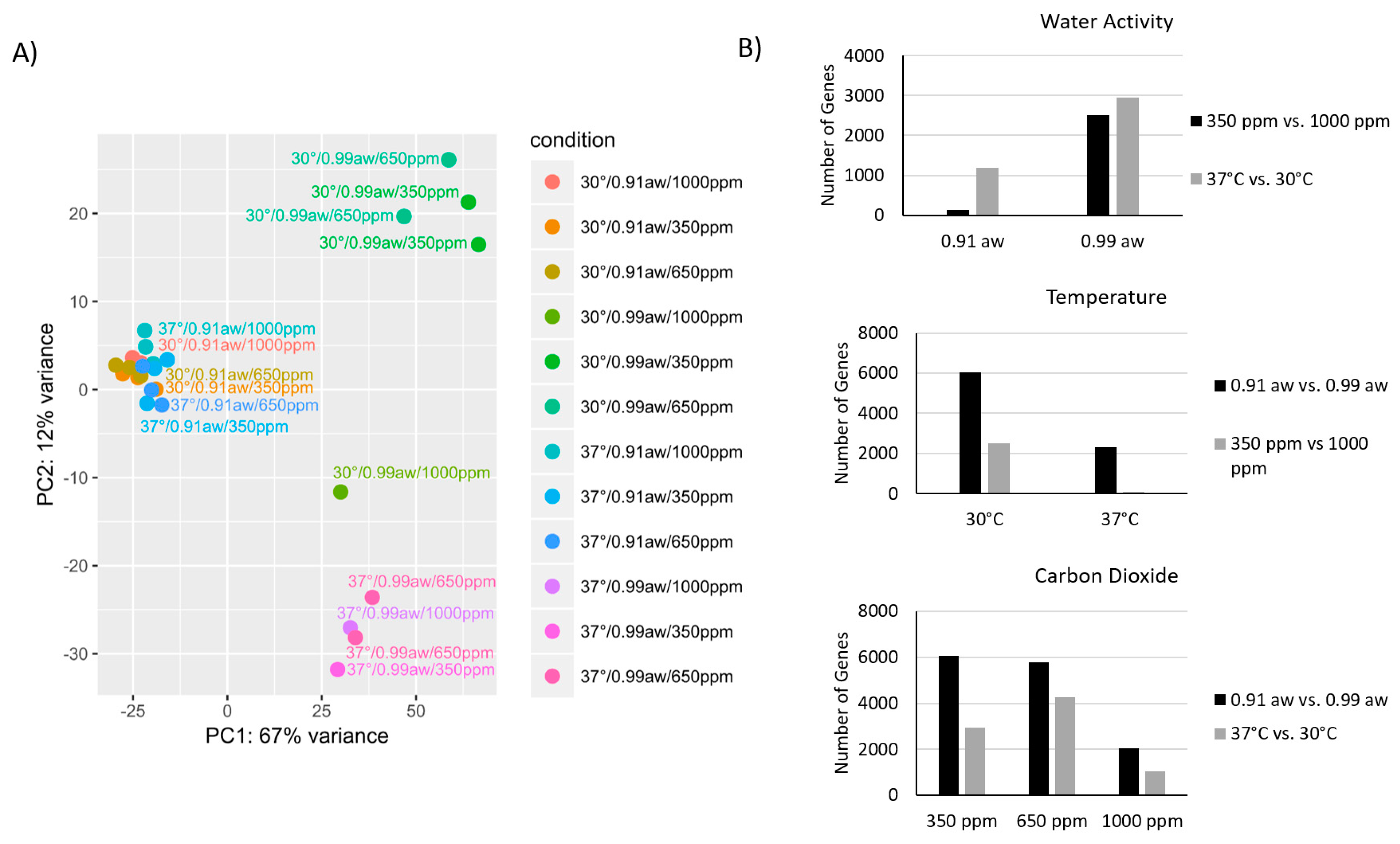
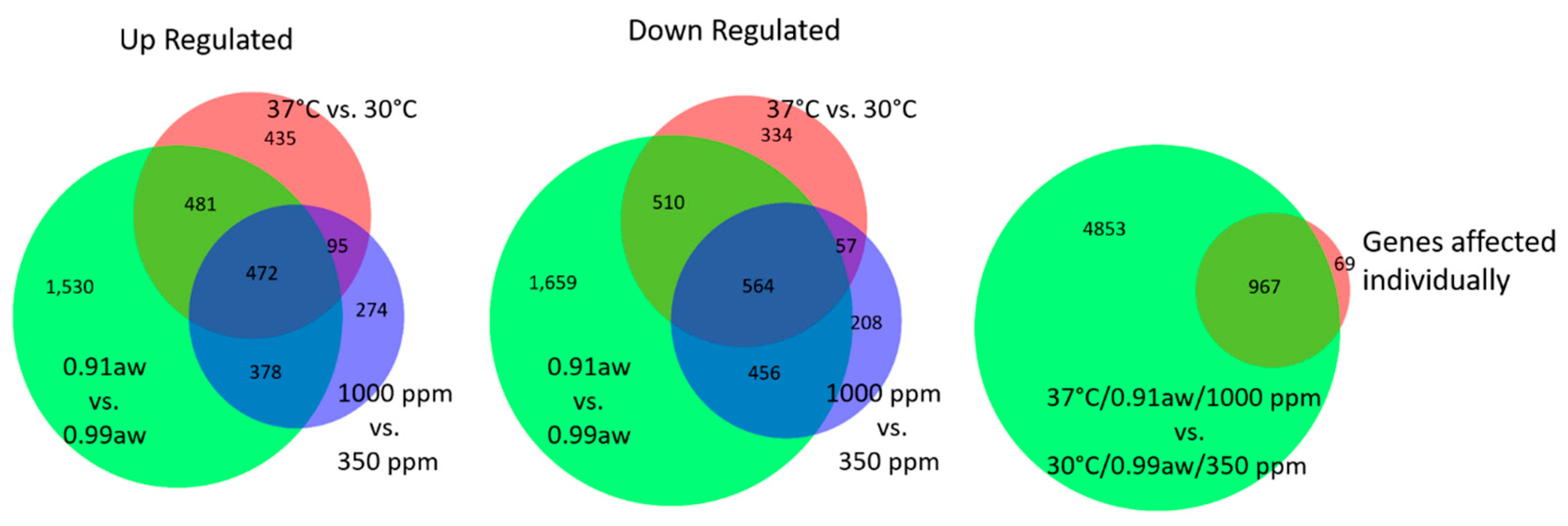
2.2. Effect of Three-Way Interacting CC Conditions on Gene Expression
2.3. Effect of Three-Way Interacting CC Conditions on Biological Processes
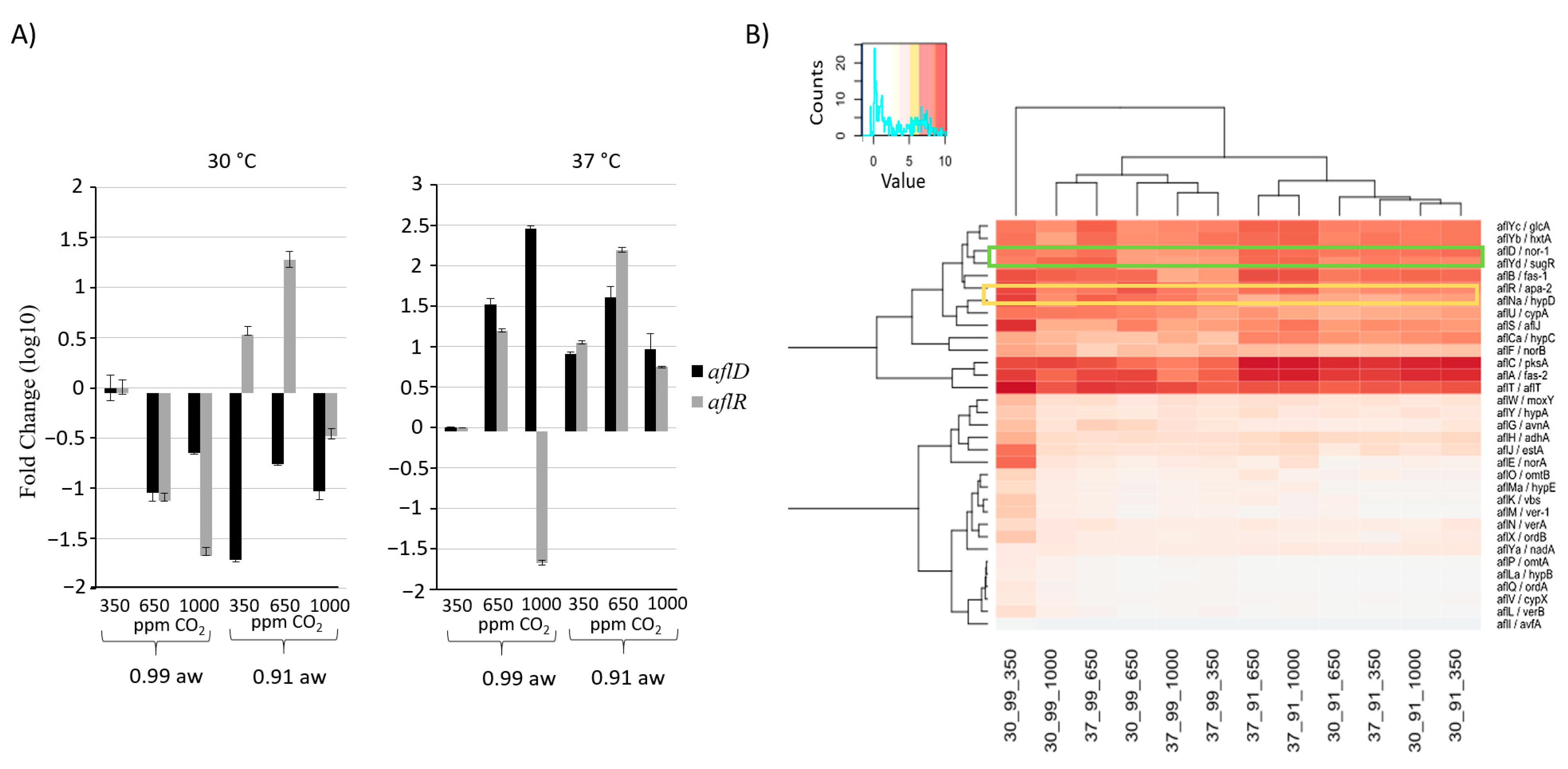
2.4. Effect of CO2 and Interactions with Other Abiotic Factors on Secondary Metabolite Gene Clusters
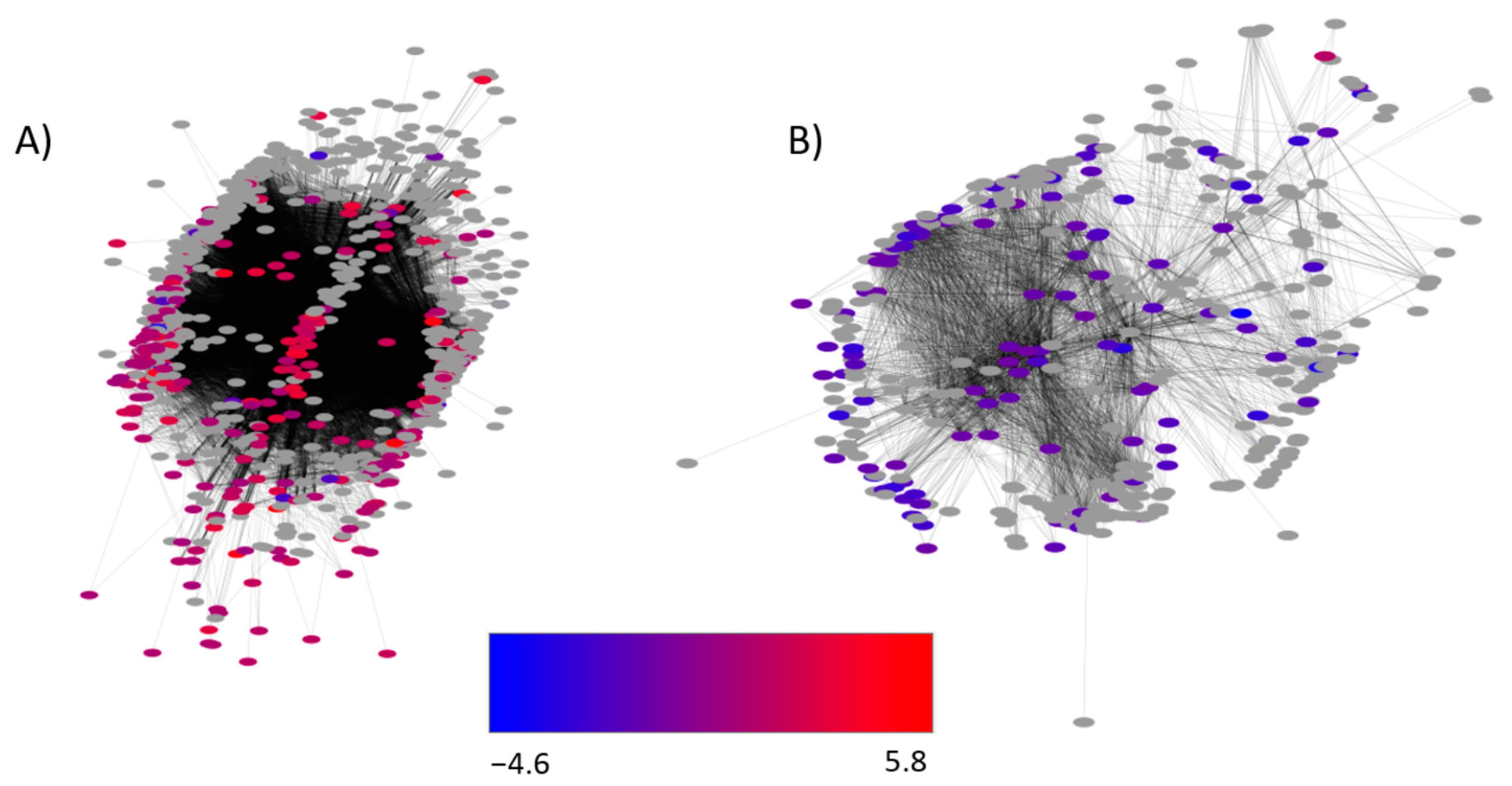
2.5. Identification of Gene Networks Affected by CO2 Levels
3. Discussion
4. Materials and Methods
4.1. Fungal Strain
4.2. Sample Preparation and Treatment
4.3. Aflatoxin Analysis
4.4. Total RNA Extraction
4.5. RNA Sequencing
4.6. RNA Isolation, cDNA Synthesis and Quantitative PCR
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vardon, P.; McLaughlin, C.; Nardinelli, C. Potential economic costs of mycotoxins in the United States. In Council for Agricultural Science and Technology (Cast). Mycotoxins: Risks in Plant, Animal, and Human Systems; Vol. Task Force Report No. 139; Council for Agricultural: Ames, IA, USA, 2003. [Google Scholar]
- Wu, F. Mycotoxin reduction in Bt corn: Potential economic, health, and regulatory impacts. Transgenic Res. 2006, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Rigo, K.; Varga, J.; Toth, B.; Teren, J.; Mesterhazy, A.; Kozakiewicz, Z. Evolutionary relationships within Aspergillus section flavi based on sequences of the intergenic transcribed spacer regions and the 5.8s rRNA gene. J. Gener. Appl. Microbiol. 2002, 48, 9–16. [Google Scholar] [CrossRef]
- Kumeda, Y.; Asao, T.; Takahashi, H.; Ichinoe, M. High prevalence of B and G aflatoxin-producing fungi in sugarcane field soil in Japan: Heteroduplex panel analysis identifies a new genotype within Aspergillus section flavi and Aspergillus nomius. FEMS Microbiol. Ecol. 2003, 45, 229–238. [Google Scholar] [CrossRef]
- Arias, R.S.; Dang, P.M.; Sobolev, V.S. RNAi-mediated control of aflatoxins in peanut: Method to analyze mycotoxin production and transgene expression in the peanut/Aspergillus pathosystem. J. Vis. Exp. JoVE 2015, e53398. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Rajasekaran, K.; Brown, R.L.; Luo, M.; Chen, Z.-Y.; Bhatnagar, D. Developing resistance to aflatoxin in maize and cottonseed. Toxins 2011, 3, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Menkir, A.; Chen, Z.Y.; Bhatnagar, D.; Yu, J.; Yao, H.; Cleveland, T.E. Breeding aflatoxin-resistant maize lines using recent advances in technologies—A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, V.; Magan, N. Environmental conditions affecting mycotoxins. In Mycotoxins in Food; Magan, N., Olsen, M., Eds.; Woodhead Publishing-Elsevier: Amsterdam, The Netherlands, 2004; pp. 174–189. [Google Scholar]
- Abdel-Hadi, A.; Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Lond. Interface 2012, 9, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, G.A.; Thompson, D.L.; Lillehoj, E.B.; Zuber, M.S.; Adkins, C.R. Effect of temperature on the preharvest infection of maize kernels by Aspergillus flavus. Phytopathology 1988, 78, 1376–1380. [Google Scholar] [CrossRef]
- Medina, A.; Schmidt-Heydt, M.; Rodriguez, A.; Parra, R.; Geisen, R.; Magan, N. Impacts of environmental stress on growth, secondary metabolite biosynthetic gene clusters and metabolite production of xerotolerant/xerophilic fungi. Curr. Genet. 2015, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Mack, B.; Payne, G.; Bhatnagar, D. Use of functional genomics to assess the climate change impact on Aspergillus flavus and aflatoxin production. World Mycotoxin J. 2016, 9, 665–672. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin contamination of foods in southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Gilbert, M.K.; Mack, B.M.; GR, O.B.; Rodriguez, A.; Bhatnagar, D.; Payne, G.; Magan, N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. Int. J. Food Microbiol. 2017, 256, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Fedorova, N.D.; Burroughs, J.L.; Dolezal, A.L.; Bok, J.W.; Horowitz-Brown, S.; Woloshuk, C.P.; Yu, J.; Keller, N.P.; Payne, G.A. Beyond aflatoxin: Four distinct expression patterns and functional roles associated with Aspergillus flavus secondary metabolism gene clusters. Mol. Plant Pathol. 2010, 11, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Medina, Á.; Rodríguez, A.; Sultan, Y.; Magan, N. Climate change factors and Aspergillus flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015, 8, 171–179. [Google Scholar] [CrossRef]
- Hall, R.A.; De Sordi, L.; Maccallum, D.M.; Topal, H.; Eaton, R.; Bloor, J.W.; Robinson, G.K.; Levin, L.R.; Buck, J.; Wang, Y.; et al. CO2 acts as a signalling molecule in populations of the fungal pathogen Candida albicans. PLoS Pathog. 2010, 6, e1001193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romer, D.; Bollazzi, M.; Roces, F. Carbon dioxide sensing in an obligate insect-fungus symbiosis: CO2 preferences of leaf-cutting ants to rear their mutualistic fungus. PLoS ONE 2017, 12, e0174597. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Nakadai, T.; Usami, Y.; Satoh, M.; Shiyomi, M.; Oikawa, T. Effect of carbon dioxide concentration on microbial respiration in soil. Ecol. Res. 1991, 6, 227–232. [Google Scholar] [CrossRef]
- Gunterus, A.; Roze, L.V.; Beaudry, R.; Linz, J.E. Ethylene inhibits aflatoxin biosynthesis in Aspergillus parasiticus grown on peanuts. Food Microbiol. 2007, 24, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Ehrlich, K.C.; Fujii, I. Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins (Basel) 2009, 1, 74–99. [Google Scholar] [CrossRef] [PubMed]
- Gerhards, N.; Neubauer, L.; Tudzynski, P.; Li, S.M. Biosynthetic pathways of ergot alkaloids. Toxins (Basel) 2014, 6, 3281–3295. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Uka, V.; Han, Z.; Buyst, D.; Harris-Coward, P.Y.; Ehrlich, K.C.; Wei, Q.; Bhatnagar, D.; Dowd, P.F.; Martens, S.L.; et al. An Aspergillus flavus secondary metabolic gene cluster containing a hybrid PKS-NRPS is necessary for synthesis of the 2-pyridones, leporins. Fungal Genet. Biol. 2015, 81, 88–97. [Google Scholar] [CrossRef] [PubMed]
- TePaske, M.R.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Leporin A: An antiinsectan n-alkoxypyridone from the sclerotia of Aspergillus leporis. Tetrahedron Lett. 1991, 32, 5687–5690. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, L.; Mondie, B.; Mitchell, S.S.; Castelhano, A.L.; Cai, W.; Bergenhem, N. Leporin B: A novel hexokinase II gene inducing agent from an unidentified fungus. Bioorg. Med. Chem. Lett. 2003, 13, 1433–1435. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2012, 3, 439. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Nierman, W.C.; Wortman, J.R.; Pritchard, B.L.; Brown, D.; Dean, R.A.; Bhatnagar, D.; Cleveland, T.E.; Machida, M.; Yu, J. Whole genome comparison of Aspergillus flavus and A. oryzae. Med. Mycol. 2006, 44, S9–S11. [Google Scholar] [CrossRef]
- Nierman, W.C.; Yu, J.; Fedorova-Abrams, N.D.; Losada, L.; Cleveland, T.E.; Bhatnagar, D.; Bennett, J.W.; Dean, R.; Payne, G.A. Genome sequence of Aspergillus flavus NRRL 3357, A strain that causes aflatoxin contamination of food and feed. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Mack, B.M. Comparison of expression of secondary metabolite biosynthesis cluster genes in Aspergillus flavus, A. parasiticus, and A. oryzae. Toxins 2014, 6, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Linz, J.E.; Wee, J.; Roze, L.V. Aspergillus parasiticus SU-1 genome sequence, predicted chromosome structure, and comparative gene expression under aflatoxin-inducing conditions: Evidence that differential expression contributes to species phenotype. Eukaryot. Cell 2014, 13, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.; Atehnkeng, J.; Sikora, R.A.; Bandyopadhyay, R.; Cotty, P.J. Molecular characterization of atoxigenic strains for biological control of aflatoxins in Nigeria. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, A.; Carter, D.; Magan, N. Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B(1) production during storage of peanuts under different water activity levels. J. Appl. Microbiol. 2010, 109, 1914–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Heydt, M.; Abdel-Hadi, A.; Magan, N.; Geisen, R. Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 2009, 135, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Heydt, M.; Rufer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010, 26, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Heydt, M.; Magan, N.; Geisen, R. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol. Lett. 2008, 284, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mohale, S.; Medina, A.; Rodriguez, A.; Sulyok, M.; Magan, N. Mycotoxigenic fungi and mycotoxins associated with stored maize from different regions of Lesotho. Mycotoxin Res. 2013, 29, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 2010, 26, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Watanabe, C.K. GMAP: A genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn—A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priebe, S.; Kreisel, C.; Horn, F.; Guthke, R.; Linde, J. FungiFun2: A comprehensive online resource for systematic analysis of gene lists from fungal species. Bioinformatics 2015, 31, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Morishima, K. Blastkoala and ghostkoala: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| 30 °C | 37 °C | ||
|---|---|---|---|
| 0.91 aw | 350 ppm | 3.37 × 107 | 2.13 × 107 |
| 650 ppm | 2.64 × 107 | 3.00 × 106 | |
| 1000 ppm | 3.04 × 107 | 6.84 × 106 | |
| 0.99 aw | 350 ppm | 1.04 × 107 | 8.01 × 105 |
| 650 ppm | 4.69 × 106 | 2.24 × 106 | |
| 1000 ppm | 6.22 × 105 | 1.03 × 106 |
| Carbon Dioxide | Temperature | ||
| (30 °C/0.99 aw/350 ppm vs. 30 °C/0.99 aw/1000 ppm) | (37 °C/0.99 aw/350 ppm vs. 30 °C/0.99 aw/350 ppm) | ||
| KEGG Category | p-Value | KEGG Category | p-Value |
| Glycolysis/Gluconeogenesis | 0.003 | Glycolysis/Gluconeogenesis | 0.000 |
| Purine metabolism | 0.008 | Starch and sucrose metabolism | 0.002 |
| Cysteine and methionine metabolism | 0.009 | Methane metabolism | 0.017 |
| Fructose and mannose metabolism | 0.011 | Riboflavin metabolism | 0.024 |
| Pyrimidine metabolism | 0.025 | Glutathione metabolism | 0.024 |
| Folate biosynthesis | 0.030 | Glycosphingolipid biosynthesis - globo series | 0.025 |
| Carbon fixation in photosynthetic organisms | 0.041 | Fructose and mannose metabolism | 0.029 |
| Inositol phosphate metabolism | 0.049 | Pentose phosphate pathway | 0.043 |
| Water Activity | Combined | ||
| (30 °C/0.91 aw/350 ppm vs. 30 °C/0.99 aw/350 ppm) | (37 °C/0.91 aw/1000 ppm vs. 30 °C/0.99 aw/350 ppm) | ||
| KEGG Category | p-Value | KEGG Category | p-Value |
| Riboflavin metabolism | 0.001 | Glycolysis/Gluconeogenesis | 0.000 |
| Inositol phosphate metabolism | 0.002 | Methane metabolism | 0.002 |
| Glyoxylate and dicarboxylate metabolism | 0.011 | Riboflavin metabolism | 0.005 |
| Purine metabolism | 0.012 | Inositol phosphate metabolism | 0.009 |
| Methane metabolism | 0.019 | Taurine and hypotaurine metabolism | 0.014 |
| Starch and sucrose metabolism | 0.020 | Fructose and mannose metabolism | 0.014 |
| Glycolysis/Gluconeogenesis | 0.023 | Starch and sucrose metabolism | 0.015 |
| Cyanoamino acid metabolism | 0.031 | Cyanoamino acid metabolism | 0.017 |
| Fructose and mannose metabolism | 0.039 | Purine metabolism | 0.019 |
| Pyrimidine metabolism | 0.022 | ||
| Ether lipid metabolism | 0.049 | ||
| Effect of Carbon Dioxide | Effect of Water | Effect of Temperature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| At 30 °C/0.99 aw: | At 30 °C, 0.91 aw vs. 0.99 aw: | At 0.99 aw, 37 °C vs. 30 °C: | |||||||
| # | A. flavus 3357 | Name | SM* Product | 650 ppm/350 ppm | 1000 ppm/350 ppm | 350 ppm | 1000 ppm | 350 ppm | 1000 ppm |
| 5 | AFLA_006170 | polyketide synthetase (PksP) | naphthopyrone | - | 3.37 | 5.01 | 1.96 | 1.86 | - |
| 10 | AFLA_016140 | scytalone dehydratase (Arp1) (conidial pigment biosynthesis) | conidial pigment 1,8-dihydroxynaphthalene-melanin | - | - | −3.54 | −2.21 | - | - |
| 15 | AFLA_045490 | dimethylallyl tryptophan synthase, putative | aflatrem, ATM2 | 2.50 | - | - | - | - | - |
| 19 | AFLA_060680 | dimethylallyl tryptophan synthase | Unknown | −3.38 | −4.09 | −4.17 | - | - | 2.68 |
| 20 | AFLA_062860 | polyketide synthase (PkfA) | 3-(2,4-dihydroxy-6-methylbenzyl)-O rsellinaldehyde | 1.35 | - | - | −1.87 | 2.42 | - |
| 21 | AFLA_064240 | nonribosomal peptide synthase (wykN) | WYK peptidase inhibitor | - | 2.23 | - | −2.14 | 1.31 | −2.19 |
| 23 | AFLA_066840 | hybrid NRPS/PKS enzyme | Leporins | 1.59 | 2.45 | - | −3.27 | - | −3.40 |
| 35 | AFLA_101700 | NRPS enzyme (lnaA) | piperazines | - | 2.15 | - | −1.94 | 2.66 | - |
| 36 | AFLA_104210 | PKS-like enzyme, putative | dihydrocurvularin | - | - | −3.04 | - | −2.94 | - |
| 39 | AFLA_108550 | polyketide synthase | monodictylphenone | - | - | - | - | - | - |
| 41 | AFLA_114820 | polyketide synthase (fluP) (pksL2) | 6-MSAi | - | 1.55 | - | −2.17 | - | - |
| 44 | AFLA_116890 | polyketide synthase (PkiA) | 6-hydroxy-7-methyl-3-nonylisoquinoline-5,8-dione | - | −2.52 | −3.49 | - | - | - |
| 54 | AFLA_139410 | polyketide synthase (aflC/pksA/pksL1) | aflatoxin | - | 1.20 | 0.95 | - | - | −2.05 |
| 55 | AFLA_139490 | hybrid PKS/NRPS enzyme | cyclopiazonic acid | - | - | −2.75 | −5.39 | - | −3.09 |
| AFLA_125760 | Class 2 Terpene Cyclase | Unknown | 1.99 | 4.65 | 3.98 | - | 3.48 | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, M.K.; Medina, A.; Mack, B.M.; Lebar, M.D.; Rodríguez, A.; Bhatnagar, D.; Magan, N.; Obrian, G.; Payne, G. Carbon Dioxide Mediates the Response to Temperature and Water Activity Levels in Aspergillus flavus during Infection of Maize Kernels. Toxins 2018, 10, 5. https://doi.org/10.3390/toxins10010005
Gilbert MK, Medina A, Mack BM, Lebar MD, Rodríguez A, Bhatnagar D, Magan N, Obrian G, Payne G. Carbon Dioxide Mediates the Response to Temperature and Water Activity Levels in Aspergillus flavus during Infection of Maize Kernels. Toxins. 2018; 10(1):5. https://doi.org/10.3390/toxins10010005
Chicago/Turabian StyleGilbert, Matthew K., Angel Medina, Brian M. Mack, Matthew D. Lebar, Alicia Rodríguez, Deepak Bhatnagar, Naresh Magan, Gregory Obrian, and Gary Payne. 2018. "Carbon Dioxide Mediates the Response to Temperature and Water Activity Levels in Aspergillus flavus during Infection of Maize Kernels" Toxins 10, no. 1: 5. https://doi.org/10.3390/toxins10010005





