Contractile Response of Bovine Lateral Saphenous Vein to Ergotamine Tartrate Exposed to Different Concentrations of Molecularly Imprinted Polymer
Abstract
:1. Introduction
2. Results
2.1. Dose Response to ETA
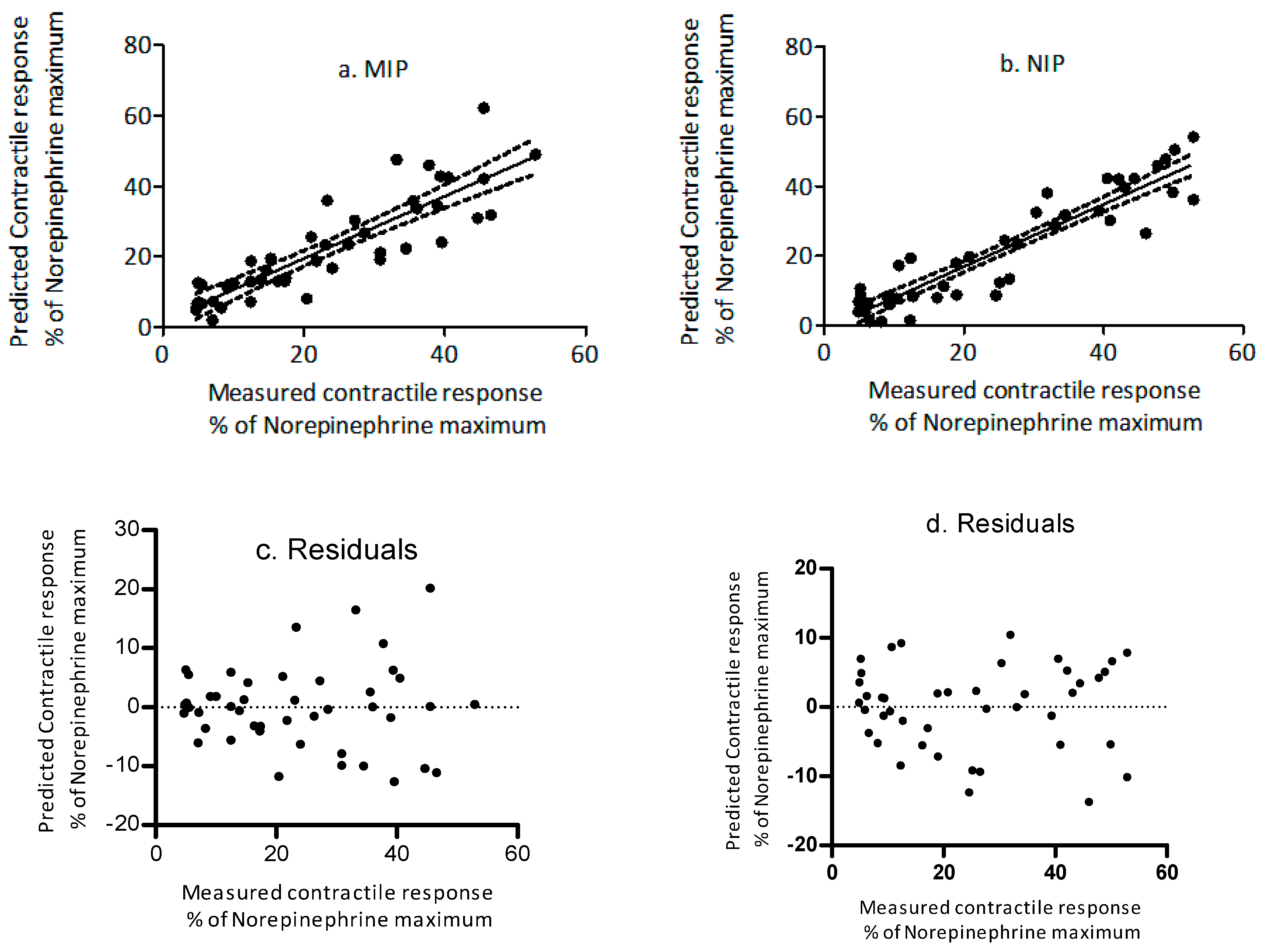
2.2. Validation Studies
2.3. Polymer Evaluation: Isothermal Adsorption Studies
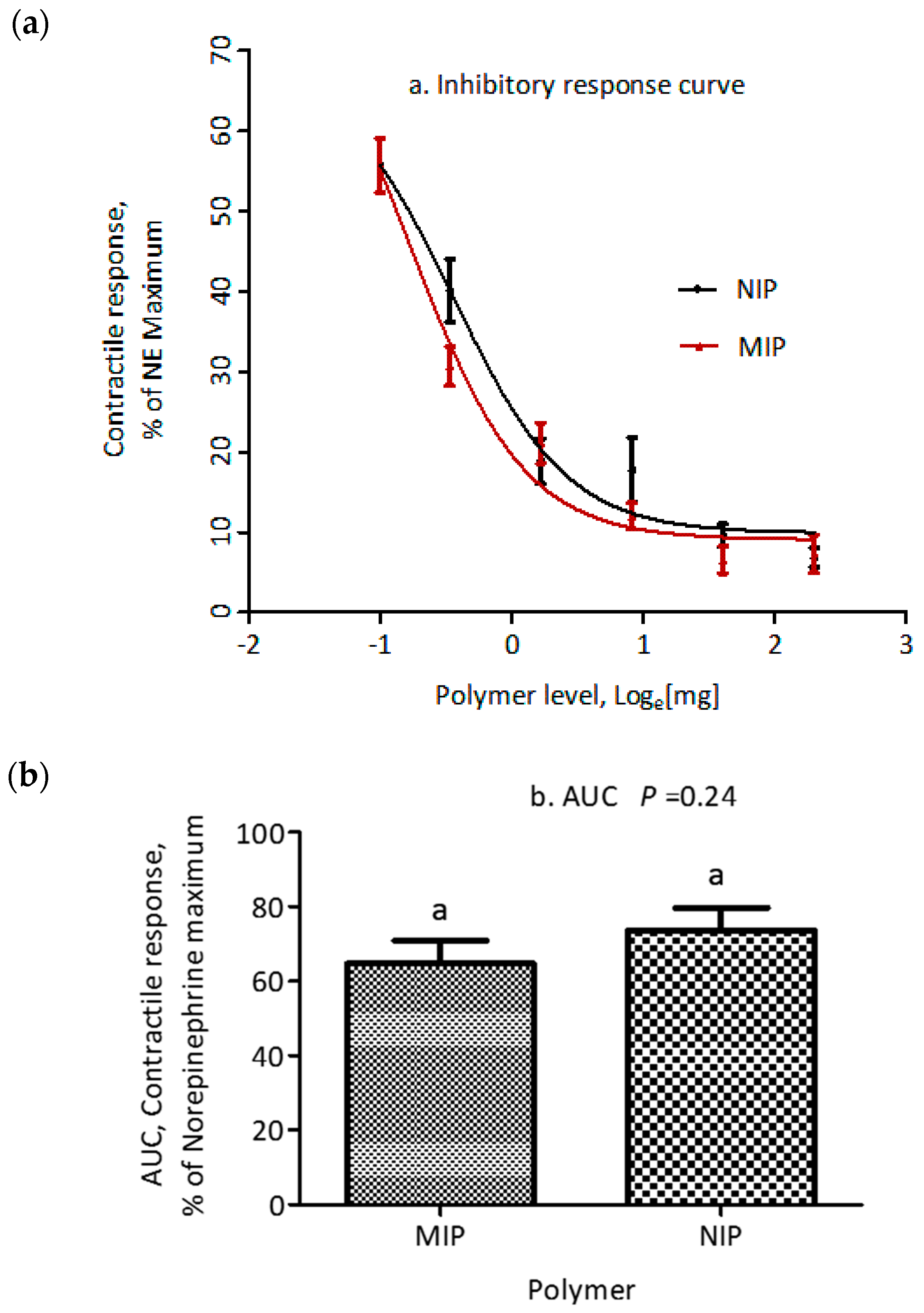
2.4. Polymer Evaluation: Myographic Studies
2.5. Biological Implications of In Vitro Parameters
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Tissues
5.2. Dose Response to ETA
5.3. Validation Studies
5.4. Polymer Evaluation: Isothermal Adsorption Studies
5.5. Myograph Experiments
5.6. HPLC Analysis of ETA
5.7. Data and Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berde, B.; Stürmer, E. Introduction to the pharmacology of ergot alkaloids and related compounds as a basis of their therapeutic application. In Ergot Alkaloids and Related Compounds; Springer: Berlin, Germany, 1978; pp. 1–28. [Google Scholar]
- Østergaard, J.R.; Mikkelsen, E.; Voldby, B. Effects of 5-hydroxytryptamine and ergotamine on human superficial temporal artery. Cephalalgia 1981, 1, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schweinitzer, E. In vitro studies on the duration of action of dihydroergotamine. Int. J. Clin. Pharmacol. Ther. Toxicol. 1980, 18, 88–91. [Google Scholar] [PubMed]
- Schöning, C.; Flieger, M.; Pertz, H. Complex interaction of ergovaline with 5-ht2a, 5-ht1b/1d, and alpha1 receptors in isolated arteries of rat and guinea pig. J. Anim. Sci. 2001, 79, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.; Bush, L.; Smith, D.; Shafer, W.; Smith, L.; Arrington, B.; Strickland, J. Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 2007, 85, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.; Harmon, D.; Strickland, J.; Bush, L.; Klotz, J. Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein. J. Anim. Sci. 2011, 89, 2944–2949. [Google Scholar] [CrossRef] [PubMed]
- Egert, A.; Kim, D.H.; Schrick, F.N.; Harmon, D.; Klotz, J. Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. J. Anim. Sci. 2014, 92, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.; Brown, K.; Xue, Y.; Matthews, J.; Boling, J.; Burris, W.; Bush, L.; Strickland, J. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 2012, 90, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Spring, C.; Mevissen, M.; Reist, M.; Zulauf, M.; Steiner, A. Modification of spontaneous contractility of smooth muscle preparations from the bovine abomasal antrum by serotonin receptor agonists. J. Vet. Pharmacol. Ther. 2003, 26, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.T.; Mullikin, D.; Lefkowitz, R. Identification of alpha-adrenergic receptors in uterine smooth muscle membranes by [3h] dihydroergocryptine binding. J. Biol. Chem. 1976, 251, 6915–6923. [Google Scholar] [PubMed]
- Innes, I. Identification of the smooth muscle excitatory receptors for ergot alkaloids. Br. J. Pharmacol. 1962, 19, 120–128. [Google Scholar] [CrossRef]
- Ishii, Y.; Takayanagi, I. Effects of some ergot alkaloids on dopamine receptors of molluscan smooth muscle. J. Pharmacobiol. Dyn. 1982, 5, 748–750. [Google Scholar] [CrossRef]
- McLeay, L.M.; Smith, B.L. Effects of ergotamine and ergovaline on the electromyographic activity of smooth muscle of the reticulum and rumen of sheep. Am. J. Vet. Res. 2006, 67, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Pesqueira, A.; Harmon, D.; Branco, A.; Klotz, J. Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax. J. Anim. Sci. 2014, 92, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.; Tolkachev, O. The chemistry of peptide ergot alkaloids. Part 1 classification and chemistry of ergot peptides. Pharm. Chem. J. 2001, 35, 504–513. [Google Scholar] [CrossRef]
- Maulding, H.; Zoglio, M. Physical chemistry of ergot alkaloids and derivatives i: Ionization constants of several medicinally active bases. J. Pharm. Sci. 1970, 59, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Petrzilka, T.; Rutschmann, J.; Hofmann, A.; Günthard, H.H. Über die stereochemie der lysergsäuren und der dihydro-lysergsäuren. 37. Mitteilung über mutterkornalkaloide. Helv. Chim. Acta 1954, 37, 2039–2057. [Google Scholar] [CrossRef]
- Pierri, L.; Pitman, I.; Rae, I.; Winkler, D.; Andrews, P. Conformational analysis of the ergot alkaloids ergotamine and ergotaminine. J. Med. Chem. 1982, 25, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Lampen, A.; Klaffke, H. Mutterkornalkaloide in lebensmitteln i. Zusammenfassende darstellung. J. Verbraucherschutz Lebensmittelsicherheit 2006, 1, 148–149. [Google Scholar] [CrossRef]
- Buchta, M.; Cvak, L. Ergot alkaloids and other metabolites of the genus claviceps. In Ergot, the Genus Claviceps; Kren, V., Cvak, L., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; Volume 6, pp. 173–200. [Google Scholar]
- Rutschmann, J.; Stadler, P. Chemical background. In Ergot Alkaloids and Related Compounds; Springer: Berlin, Germany, 1978; pp. 29–85. [Google Scholar]
- Bürk, G.; Höbel, W.; Richt, A. Ergot alkaloids in cereal products. Mol. Nutr. Food Res. 2006, 50, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M.; Lawrence Guillaume, A. Losses of ergot alkaloids during making of bread and pancakes. J. Agric. Food Chem. 1982, 30, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Dawson, K. The ability of mycosorb to bind toxins present in endophyte-infected tall fescue. In Biotechnology in the Feed Industry: Proceeding from Alltech’s 16th Annual Symposium; Lyons, T.P., Jacques, K.A., Eds.; Nottingham Press University: Nottingham, UK, 2000; pp. 409–422. [Google Scholar]
- Akay, V.; Dawson, K.A.; Ely, D.G.; Aaron, D.K. Evaluation of a carbohydrate-based adsorbent for controlling intoxication associated with endophyte-infected pasture grasses. In Biotechnology in the Feed Industry, 2003 Proceedings (19th Annual); Lyons, T.P., Jacques, K.A., Eds.; Alltech Nottingham Univ. Press: Nottingham, UK, 2003; pp. 267–274. [Google Scholar]
- Karsten, H.; Klaus, M. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar]
- Lian, Z.; Liang, Z.; Wang, J. Selective extraction and concentration of mebendazole in seawater samples using molecularly imprinted polymer as sorbent. Mar. Pollut. Bull. 2015, 91, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sellergren, B.; Andersson, L.I. Application of imprinted synthetic polymers in binding assay development. Methods 2000, 22, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Cormack, P.A.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterisation. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sellergren, B. Imprinted chiral stationary phases in high-performance liquid chromatography. J. Chromatogr. A 2001, 906, 227–252. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Rampey, A.M.; Rushton, G.T.; Chen, Y.; Shimizu, K.D. Characterization of the heterogeneous binding site affinity distributions in molecularly imprinted polymers. J. Chromatogr. B 2004, 804, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, H.; Zhang, L.; Chen, X.; Qiao, X. The biomimetic immunoassay based on molecularly imprinted polymer: A comprehensive review of recent progress and future prospects. J. Food Sci. 2011, 76, R69–R75. [Google Scholar] [CrossRef] [PubMed]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. Mip sensors-the electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- Rottinghaus, G.E.; Garner, G.B.; Cornell, C.N.; Ellis, J.L. Hplc method for quantitating ergovaline in endophyte-infested tall fescue: Seasonal variation of ergovaline levels in stems with leaf sheaths, leaf blades, and seed heads. J. Agric. Food Chem. 1991, 39, 112–115. [Google Scholar] [CrossRef]
- Thom, E.R.; Popay, A.J.; Hume, D.E.; Fletcher, L.R. Evaluating the performance of endophytes in farm systems to improve farmer outcomes—A review. Crop Pasture Sci. 2013, 63, 927–943. [Google Scholar] [CrossRef]
- Yates, I.; Porter, J. Bacterial bioluminescence as a bioassay for mycotoxins. Appl. Environ. Microbiol. 1982, 44, 1072–1075. [Google Scholar] [PubMed]
- Krska, R.; Crews, C. Significance, chemistry and determination of ergot alkaloids: A review. Food Addit. Contam. 2008, 25, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.; Bush, L.; Smith, D.; Shafer, W.; Smith, L.; Vevoda, A. Assessment of vasoconstrictive potential of D-lysergic acid using an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 2006, 84, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Abney, L.; Strickland, J.; Linnabary, R. Vasoconstriction in bovine vasculature induced by the tall fescue alkaloid lysergamide. J. Anim. Sci. 1993, 71, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Kudupoje, M.K.; Vanzant, E.S.; Yiannikouris, A.; Dawson, K.A.; McLeod, K.R. Characterization of novel polymers for alkaloid adsorption. J. Dairy Sci. 2015, 98 (Suppl. 2), 675. [Google Scholar]
- Kudupoje, M.B. Molecularly Imprinted Polymers Synthesized as Adsorbents for Ergot Alkaloids: Characterization and In Vitro and Ex Vivo Assessment of Effects on Ergot Alkaloid Bioavailability. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2017. [Google Scholar]
- Dyer, D.C. Evidence that ergovaline acts on serotonin receptors. Life Sci. 1993, 53, PL223–PL228. [Google Scholar] [CrossRef]
- Görnemann, T.; Jähnichen, S.; Schurad, B.; Latté, K.P.; Horowski, R.; Tack, J.; Flieger, M.; Pertz, H.H. Pharmacological properties of a wide array of ergolines at functional alpha1-adrenoceptor subtypes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 376, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Horemans, F.; Weustenraed, A.; Spivak, D.; Cleij, T. Towards water compatible mips for sensing in aqueous media. J. Mol. Recognit. 2012, 25, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Andrae, K.; Merkel, S.; Durmaz, V.; Fackeldey, K.; Köppen, R.; Weber, M. Investigation of the ergopeptide epimerization process. Computation 2014, 2, 102–111. [Google Scholar] [CrossRef]
- Hafner, M.; Sulyok, M.; Schuhmacher, R.; Crews, C.; Krska, R. Stability and epimerisation behaviour of ergot alkaloids in various solvents. World Mycotoxin J. 2008, 1, 67–78. [Google Scholar] [CrossRef]
- Crews, C. Analysis of ergot alkaloids. Toxins 2015, 7, 2024–2050. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.F.; Neves, J.S.; Couto, G.C.; Cotias, A.C.; Pão, C.R.; Olsen, P.C.; de Carvalho, K.I.M.; Anjos-Valotta, E.A.; Faria, R.X.; Costa, J.C. Jm25-1, a lidocaine analog combining airway relaxant and antiinflammatory propertiesimplications for new bronchospasm therapy. Anesthesiol. J. Am. Soc. Anesthesiol. 2016, 124, 109–120. [Google Scholar]
- Wolff, A.R.; Isringhausen, C.; Widmer, J.; Coons, A.; Loewen, G.; Ogilvie, B.W.; Buckley, D.B. Comparison of Ki and IC50 Values for Prototypical Inhibitors of the Major Drug Uptake Transporters; Drug Metabolism Reviews; Taylor & Francis Ltd.: Abingdon, UK, 2015; pp. 261–262. [Google Scholar]
- Lenain, P.; Diana Di Mavungu, J.; Dubruel, P.; Robbens, J.; De Saeger, S. Development of suspension polymerized molecularly imprinted beads with metergoline as template and application in a solid-phase extraction procedure toward ergot alkaloids. Anal. Chem. 2012, 84, 10411–10418. [Google Scholar] [CrossRef] [PubMed]
- Briejer, M.; Mathis, C.; Schuurkes, J. 5-ht receptor types in the rat ileum longitudinal muscle: Focus on 5-ht2 receptors mediating contraction. Neurogastroenterol. Motil. 1997, 9, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Galligan, J.J. 5-ht1a and 5-ht4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 266, G230–G238. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.R.; Aiken, G.E.; Klotz, J.L. Ergot alkaloid induced blood vessel dysfunction contributes to fescue toxicosis. Forage Grazinglands 2009, 7. [Google Scholar] [CrossRef]
- Millan, M.J.; Marin, P.; Bockaert, J.; la Cour, C.M. Signaling at g-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.; Brady, A.E.; Nickols, H.H.; Wang, Q.; Limbird, L.E. Membrane trafficking of g protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 559–609. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.; Kirch, B.; Aiken, G.; Bush, L.; Strickland, J. Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro. J. Anim. Sci. 2009, 87, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, I. A note on the cause of tall fescue lameness in cattle. Aust. Vet. J. 1949, 25, 27–28. [Google Scholar] [CrossRef]
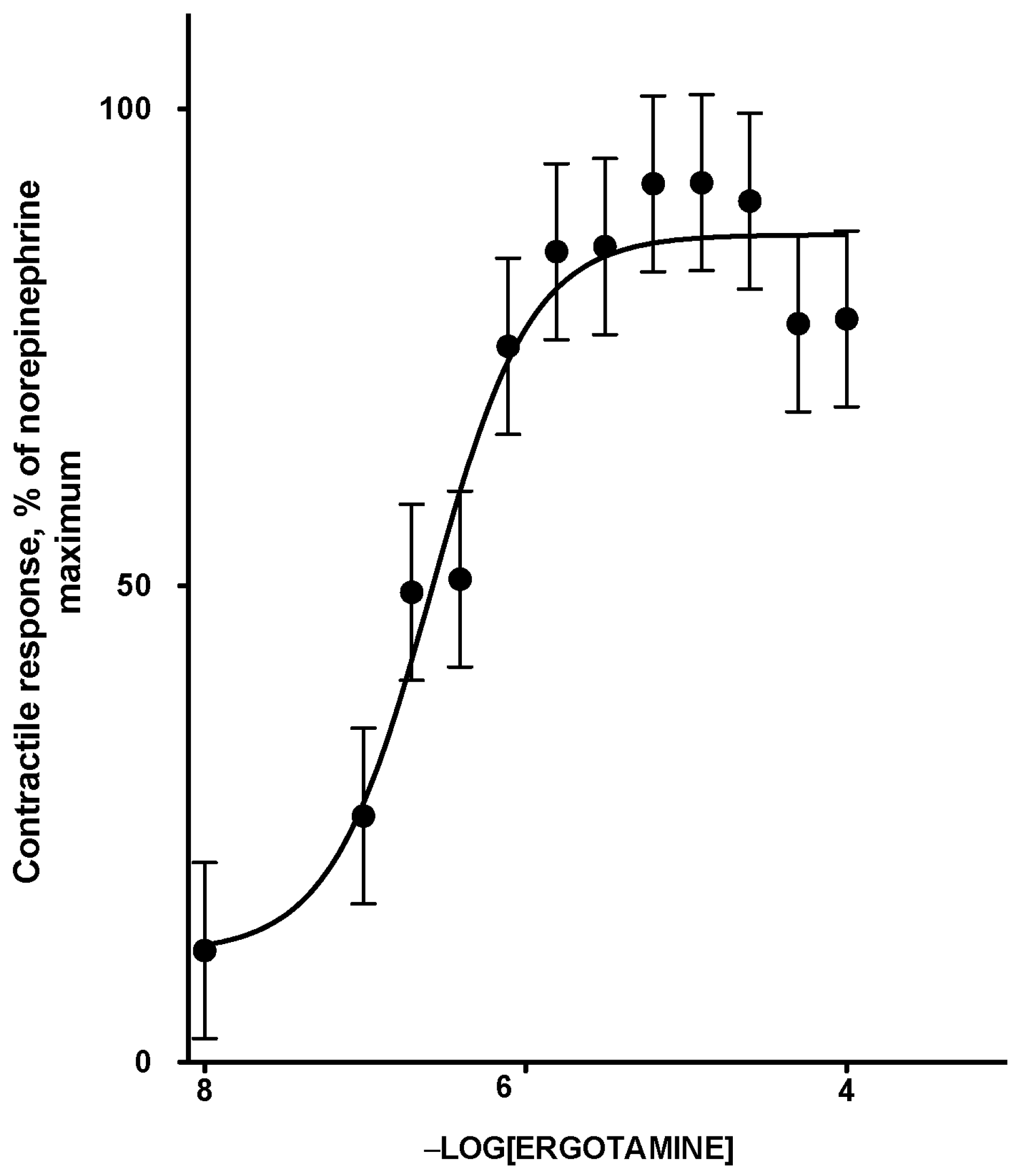
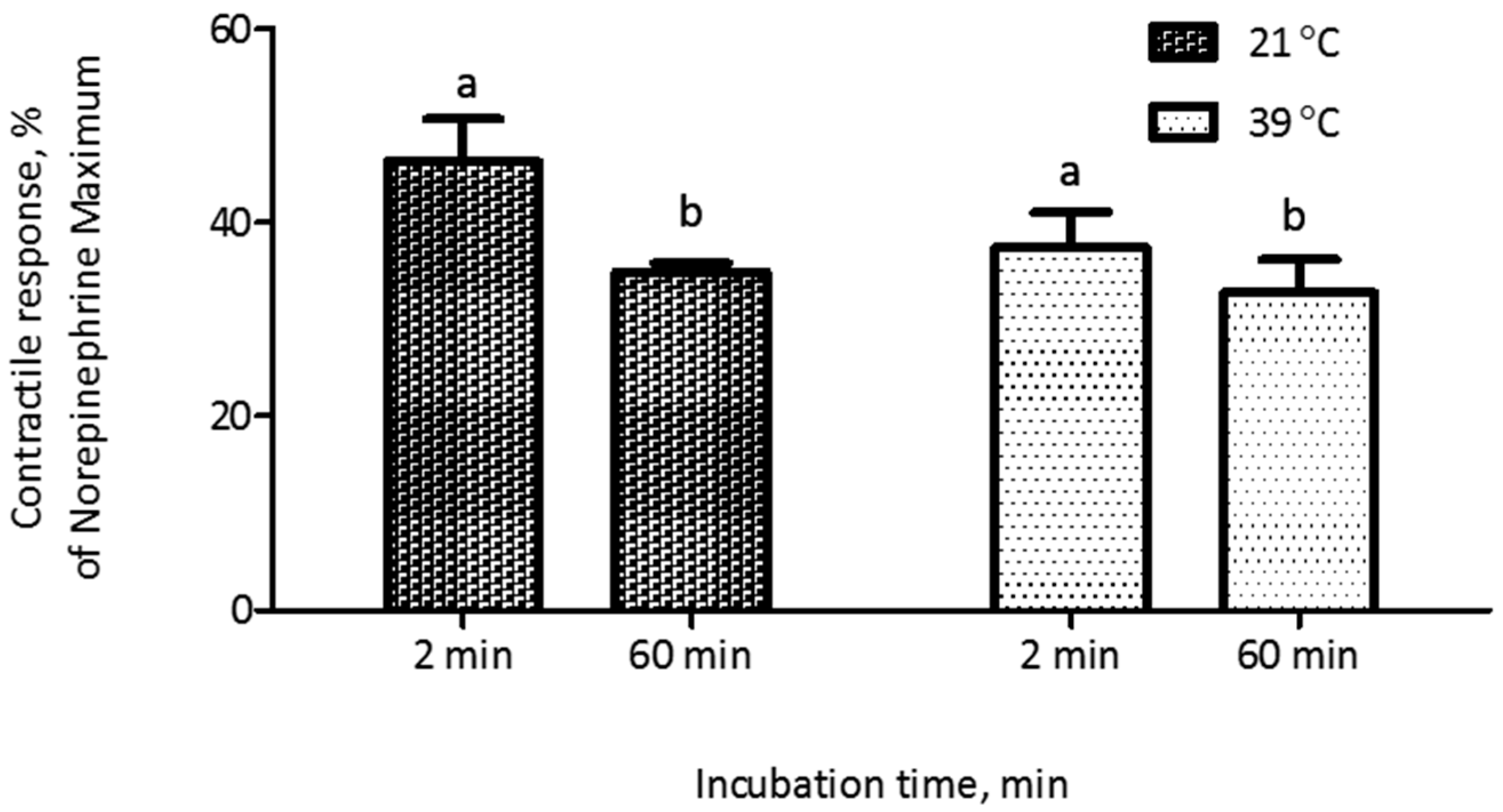
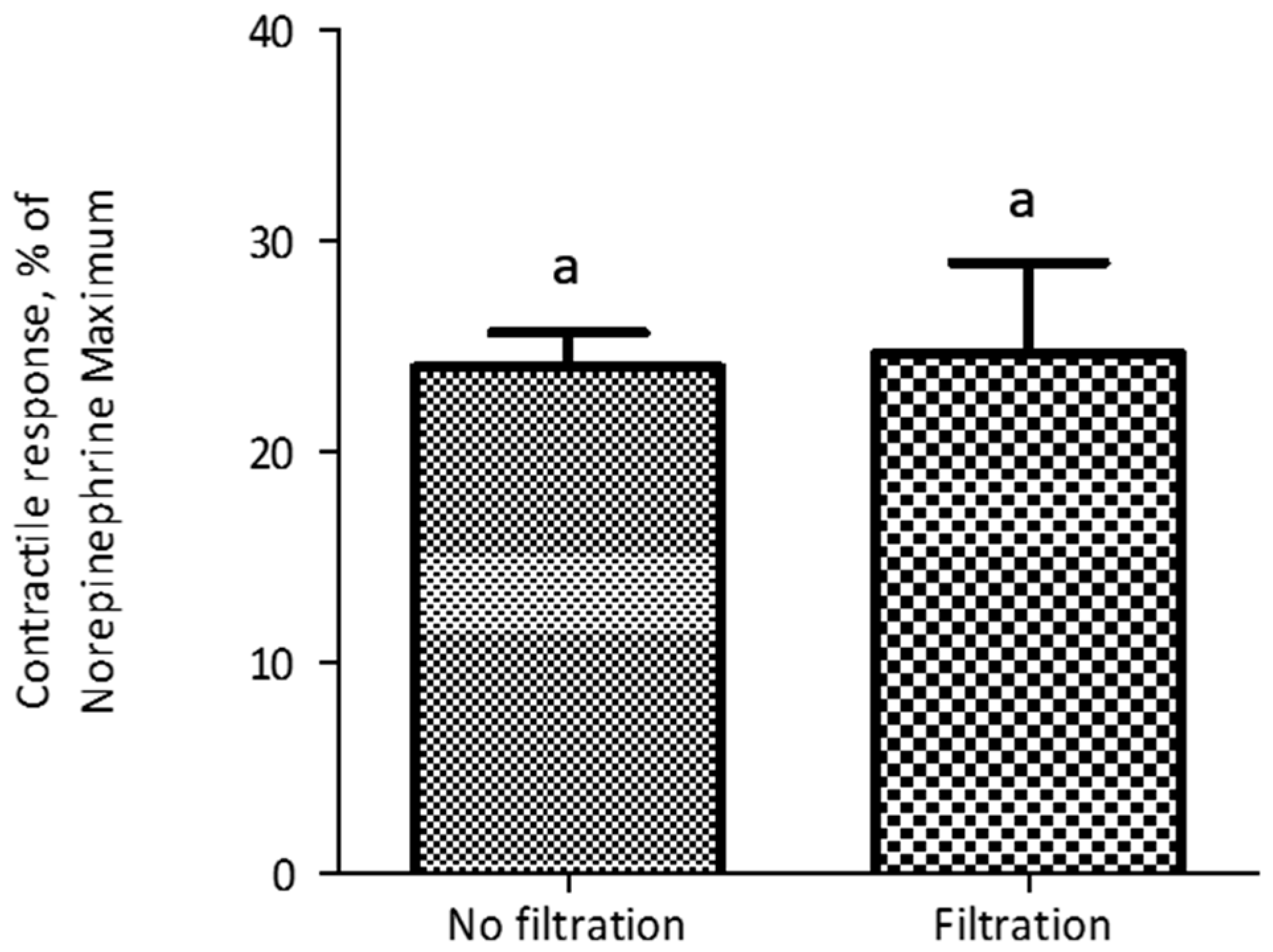
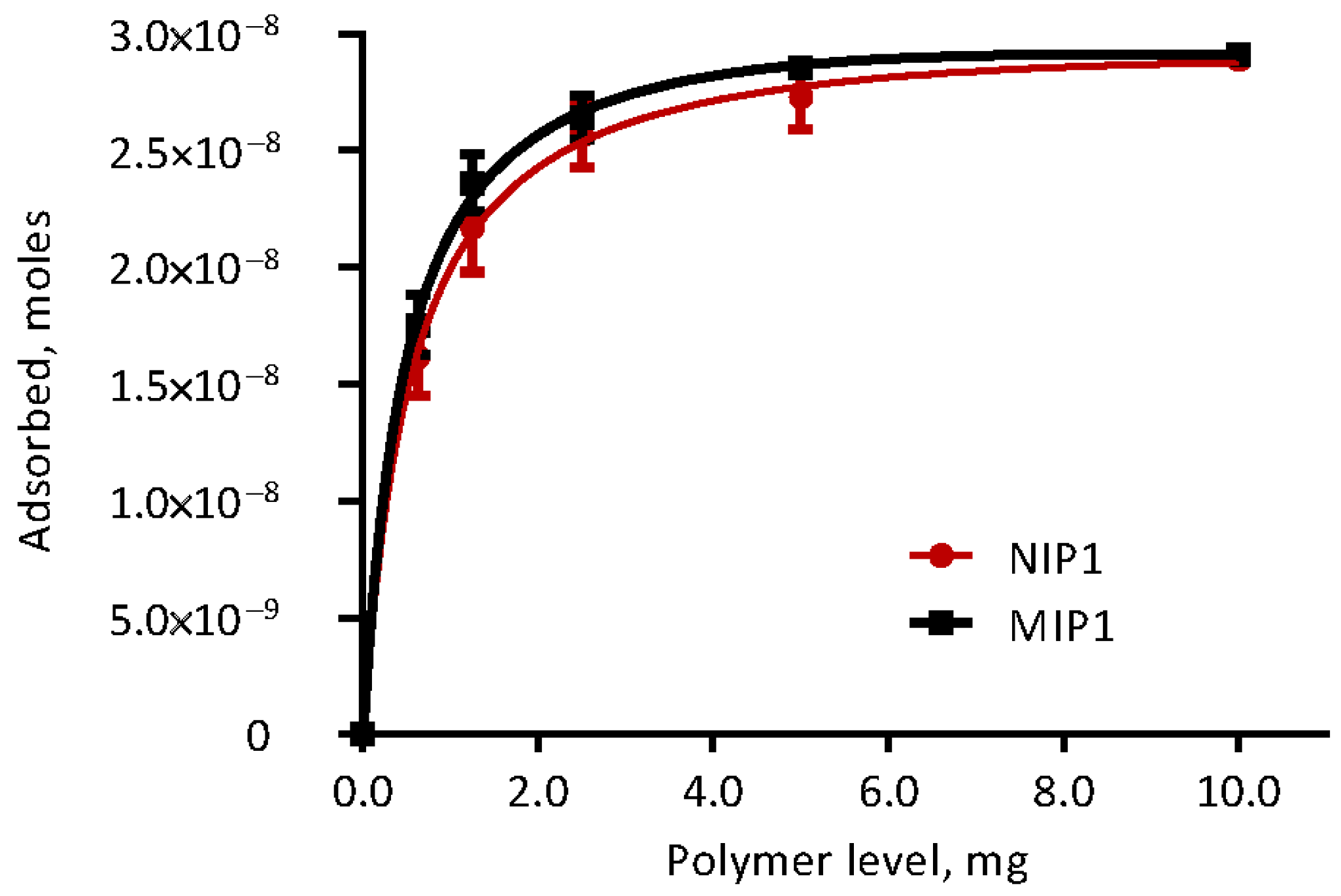
| Dose Response Parameters 1 | Mean | 95% Confidence Interval |
|---|---|---|
| Minimum contractile response, % | 5.7 | −13.9 to 25.4 |
| Maximum contractile response, % | 88.5 | 80.8 to 96.1 |
| Log EC50, −Log [ETA] | 6.66 | 6.32 to 7.01 |
| Goodness of fit | ||
| Degrees of freedom | 9 | |
| R square | 0.95 |
| MIP | NIP | p-Value | |
|---|---|---|---|
| Bmax (Moles) 1 | 3.27 × 10−8 ± 2.391 × 10−9 | 3.15 × 10−8 ± 3.877 × 10−9 | 0.78 |
| Kd (mg of polymer) 2 | 0.51 ± 0.108 | 0.57 ± 0.193 | 0.80 |
| NS (Moles mg−1) 3 | −2.06 × 10−10 ± 2.361 × 10−10 | −9.63 × 10−11 ± 3.776 × 10−10 | 0.81 |
| R2 | 0.927 | 0.835 |
| Contractile Response | Polymers | |||
|---|---|---|---|---|
| MIP | NIP | SEM | p-Value | |
| At maximum inhibition, % | 9.1 | 9.2 | 1.79 | 0.96 |
| At minimum inhibition, % | 88.0 | 70.0 | 10.92 | 0.26 |
| IC50, mg of polymer | 0.51 | 0.73 | 0.105 | 0.16 |
| 95% CI | |||||
|---|---|---|---|---|---|
| Regression Equation | R2 | p-Value | Slope | y-Intercept | |
| MIP | Y = 0.98 ± 0.07(x) + 0.15 ± 2.07 | 0.82 | <0.01 | 0.85 to 1.12 | −4.01 to 4.32 |
| NIP | Y = 0.92 ± 0.05(x) − 1.35 ± 1.75 | 0.87 | <0.01 | 0.81 to 1.03 | −4.87 to 2.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudupoje, M.B.; Klotz, J.L.; Yiannikouris, A.; Dawson, K.A.; McLeod, K.R.; Vanzant, E.S. Contractile Response of Bovine Lateral Saphenous Vein to Ergotamine Tartrate Exposed to Different Concentrations of Molecularly Imprinted Polymer. Toxins 2018, 10, 58. https://doi.org/10.3390/toxins10020058
Kudupoje MB, Klotz JL, Yiannikouris A, Dawson KA, McLeod KR, Vanzant ES. Contractile Response of Bovine Lateral Saphenous Vein to Ergotamine Tartrate Exposed to Different Concentrations of Molecularly Imprinted Polymer. Toxins. 2018; 10(2):58. https://doi.org/10.3390/toxins10020058
Chicago/Turabian StyleKudupoje, Manoj B., James L. Klotz, Alexandros Yiannikouris, Karl A. Dawson, Kyle R. McLeod, and Eric S. Vanzant. 2018. "Contractile Response of Bovine Lateral Saphenous Vein to Ergotamine Tartrate Exposed to Different Concentrations of Molecularly Imprinted Polymer" Toxins 10, no. 2: 58. https://doi.org/10.3390/toxins10020058





