Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication
Abstract
:1. Introduction
2. Results
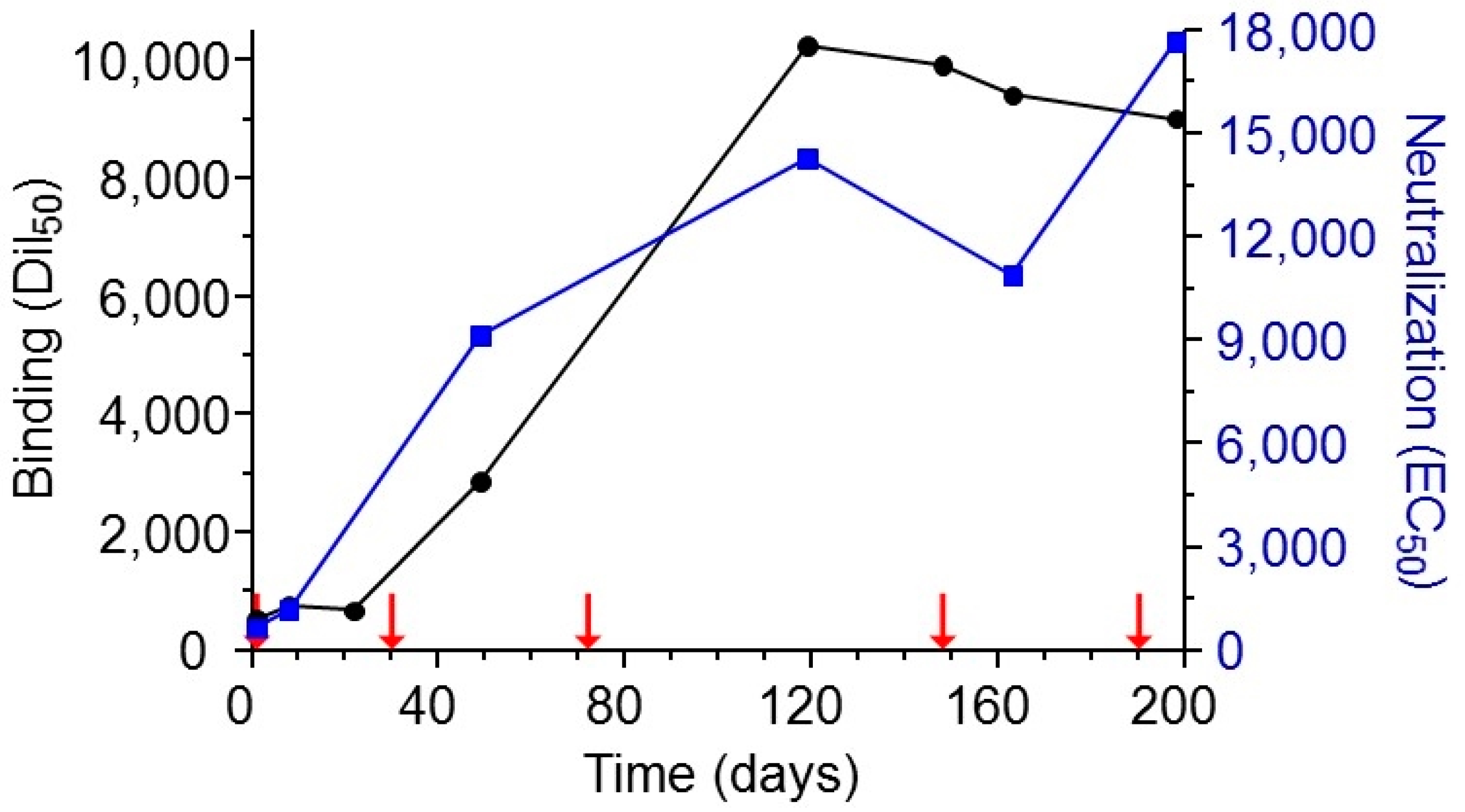
2.1. Immunization and Characterization of Elicited Antibodies
2.2. Isolation of Anti-Abrin Antibodies
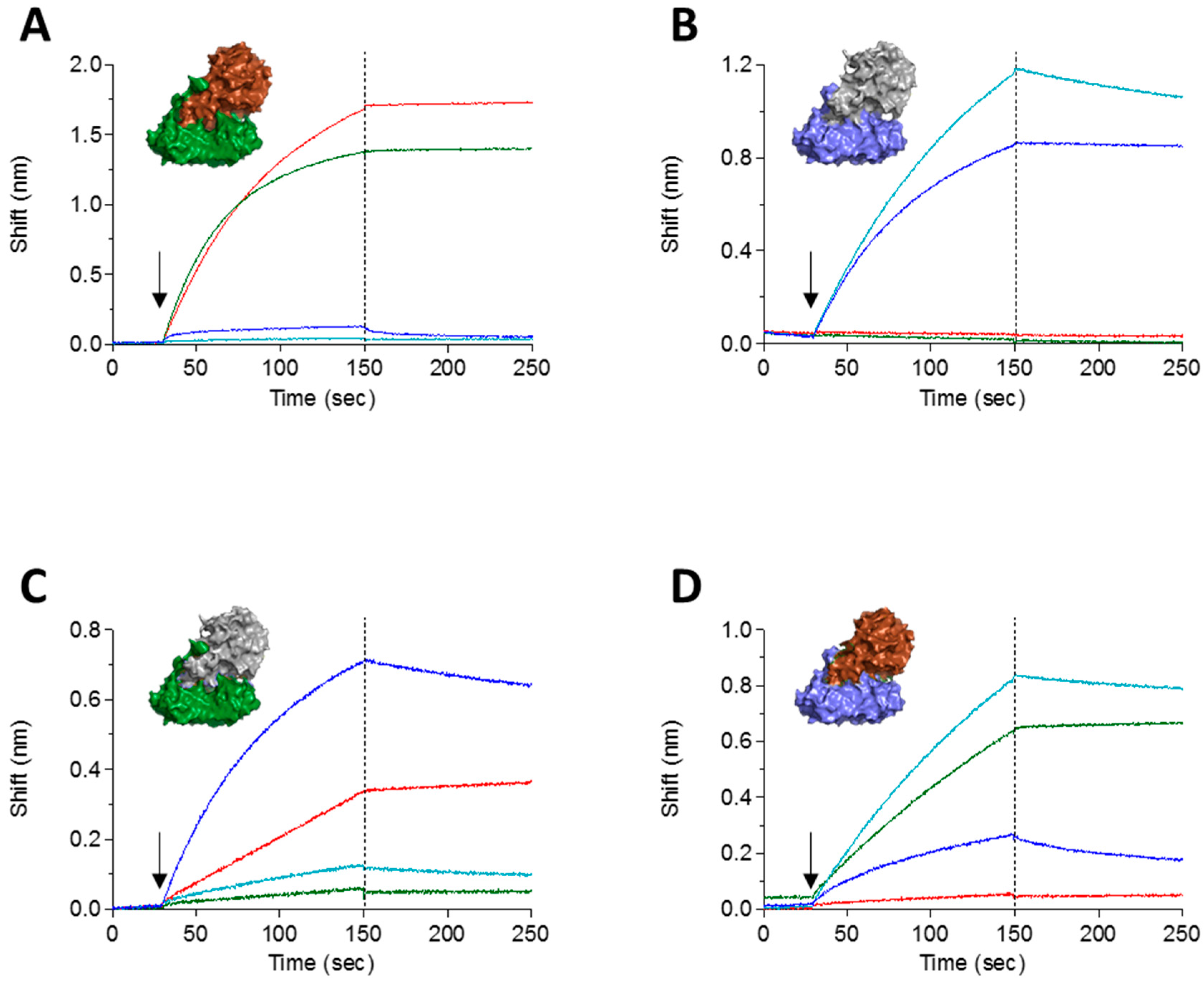
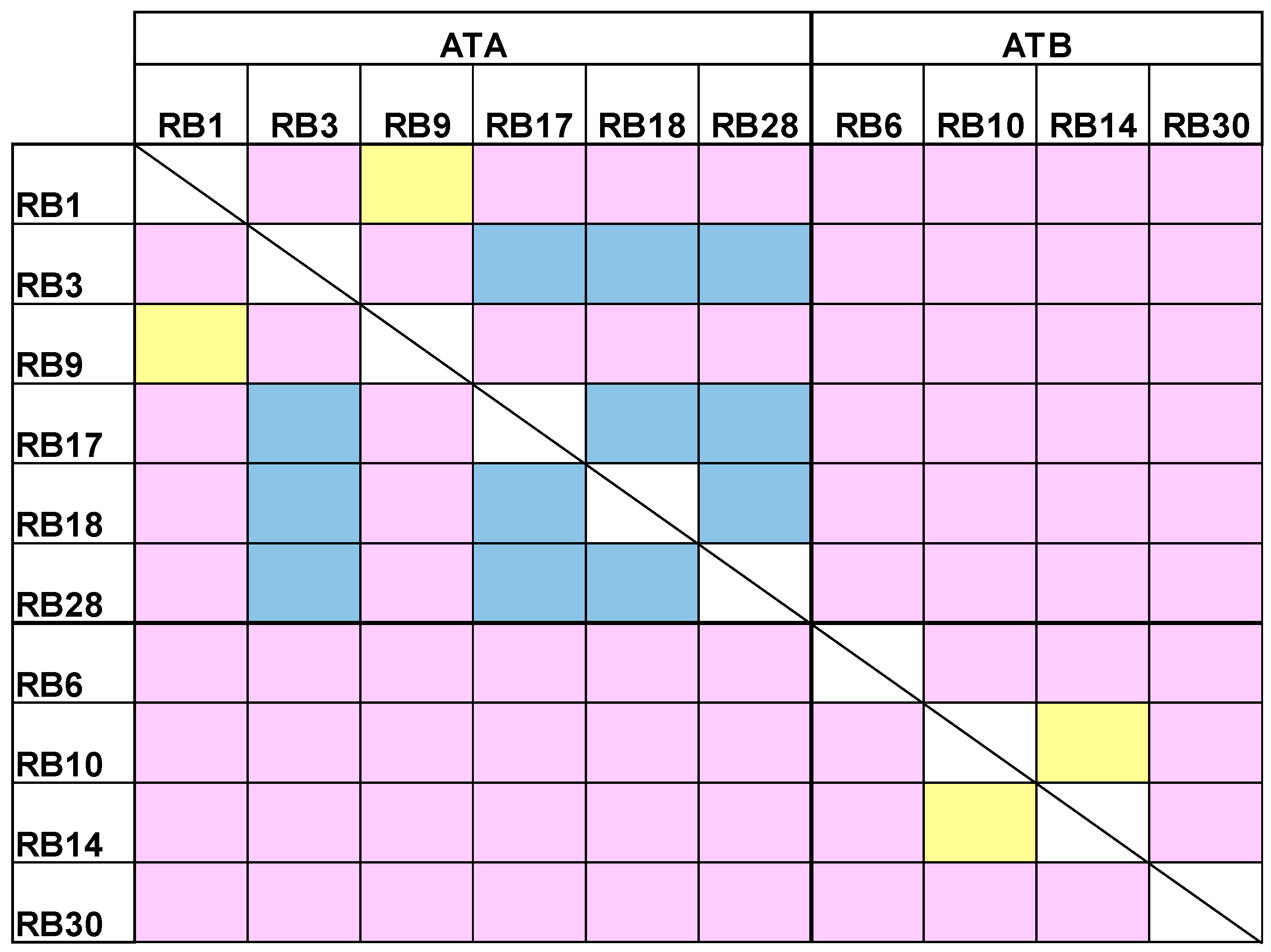
2.3. Epitope Binning
2.4. In Vitro Neutralization of Abrin Cytotoxicity
2.5. Affinity of the Anti-Abrin Antibodies
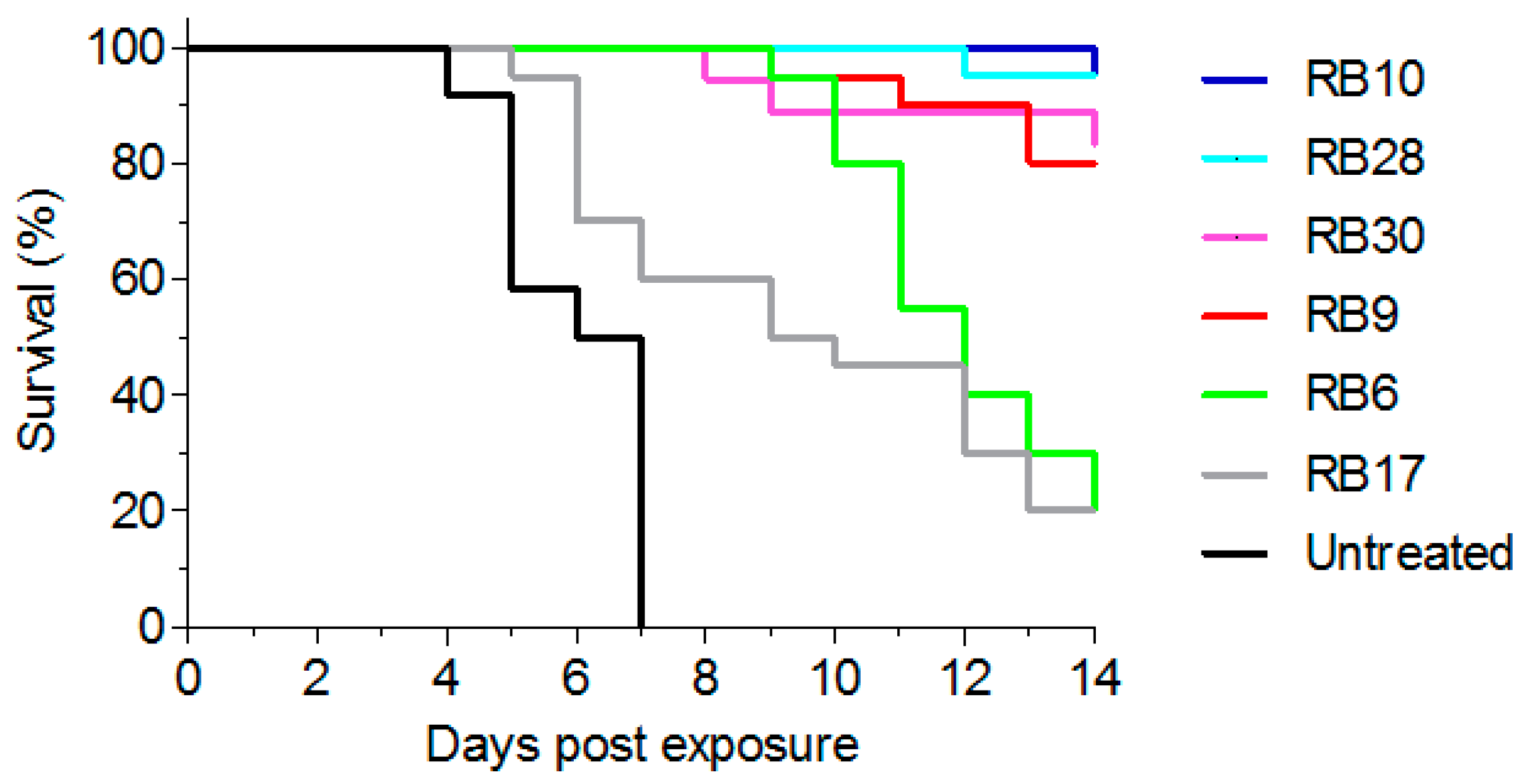
2.6. Post-Exposure Treatment of Abrin Intoxicated Mice
3. Discussion
4. Experimental Section
4.1. Animal Immunization
4.2. scFv Library Construction and Screening
4.3. Fingerprint and Nucleic Acid Analysis
4.4. ELISA
4.5. Production of Chimeric Antibodies
4.6. Affinity Measurements, Epitope Binning and Subunit Designation Studies
4.7. Abrin Neutralization in Vitro Assay
4.8. In Vivo Protection Assay
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsnes, S.; Kozlov, J.V. Ricin. Toxicon 2001, 39, 1723–1728. [Google Scholar] [CrossRef]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 s ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [PubMed]
- Sabo, T.; Gal, Y.; Elhanany, E.; Sapoznikov, A.; Falach, R.; Mazor, O.; Kronman, C. Antibody treatment against pulmonary exposure to abrin confers significantly higher levels of protection than treatment against ricin intoxication. Toxicol. Lett. 2015, 237, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.R.; Anderson, G.P.; Zabetakis, D.; Walper, S.; Liu, J.L.; Bernstein, R.; Calm, A.; Carney, J.P.; O’Brien, T.W.; Walker, J.L.; et al. Llama-derived single domain antibodies specific for Abrus Agglutinin. Toxins 2011, 3, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Patfield, S.; Cheng, L.W.; Stanker, L.H.; Rasooly, R.; McKeon, T.A.; Zhang, Y.; Brandon, D.L. Detection of abrin holotoxin using novel monoclonal antibodies. Toxins 2017, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Karande, A.A. A monoclonal antibody to an abrin chimera recognizing a unique epitope on abrin a chain confers protection from abrin-induced lethality. Hum. Vaccines Immunother. 2016, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Yang, W.; Zhang, Y.; Zhang, Z.G.; Kong, T.; Li, D.N.; Tang, J.J.; Liu, L.; Liu, G.W.; Wang, Z. Preparation and identification of monoclonal antibody against abrin-a. J. Agric. Food Chem. 2011, 59, 9796–9799. [Google Scholar] [CrossRef] [PubMed]
- Surendranath, K.; Karande, A.A. A neutralizing antibody to the A chain of abrin inhibits abrin toxicity both in vitro and in vivo. Clin. Vaccine Immunol. 2008, 15, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.C.; Cheng, L.W.; He, X.; Merrill, P.; Hodge, D.; Stanker, L.H. A monoclonal-monoclonal antibody based capture ELISA for abrin. Toxins 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, B.; Ma, H.; Carney, C.; Janda, K.D. Selection and characterization of human monoclonal antibodies against abrin by phage display. Bioorg. Med. Chem. Lett. 2007, 17, 5690–5692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, X.L.; Li, Y.S.; Pan, F.G.; Zhang, Y.Y.; Zhang, J.H.; Wang, X.R.; Ren, H.L.; Lu, S.Y.; Li, Z.H.; et al. Development of a monoclonal antibody-based sandwich-type enzyme-linked immunosorbent assay (ELISA) for detection of abrin in food samples. Food Chem. 2012, 135, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Noy-Porat, T.; Rosenfeld, R.; Ariel, N.; Epstein, E.; Alcalay, R.; Zvi, A.; Kronman, C.; Ordentlich, A.; Mazor, O. Isolation of anti-ricin protective antibodies exhibiting high affinity from immunized non-human primates. Toxins 2016, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Noy-Porat, T.; Alcalay, R.; Epstein, E.; Sabo, T.; Kronman, C.; Mazor, O. Extended therapeutic window for post-exposure treatment of ricin intoxication conferred by the use of high-affinity antibodies. Toxicon 2017, 127, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Lee, M.C.; Lee, T.C.; Lin, J.Y. Primary structure of three distinct isoabrins determined by cDNA sequencing. Conservation and significance. J. Mol. Biol. 1993, 229, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tahirov, T.H.; Lu, T.H.; Liaw, Y.C.; Chen, Y.L.; Lin, J.Y. Crystal structure of abrin-a at 2.14 A. J. Mol. Biol. 1995, 250, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Pappenheimer, A.M., Jr.; Meren, R. Lectins from abrus precatorius and ricinus communis. II. Hybrid toxins and their interaction with chain-specific antibodies. J. Immunol. 1974, 113, 842–847. [Google Scholar] [PubMed]
- Audette, G.F.; Vandonselaar, M.; Delbaere, L.T. The 2.2 a resolution structure of the O(H) blood-group-specific lectin I from ulex europaeus. J. Mol. Biol. 2000, 304, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Bujak, E.; Matasci, M.; Neri, D.; Wulhfard, S. Reformatting of scFv antibodies into the scFv-Fc format and their downstream purification. Methods Mol. Biol. 2014, 1131, 315–334. [Google Scholar] [PubMed]
- Rosenfeld, R.; Marcus, H.; Ben-Arie, E.; Lachmi, B.E.; Mechaly, A.; Reuveny, S.; Gat, O.; Mazor, O.; Ordentlich, A. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal Bacillus Anthracis infection. PLoS ONE 2009, 4, e6351. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.; Cohen, H.; Cohen, O.; Mazor, O. A biolayer interferometry-based assay for rapid and highly sensitive detection of biowarfare agents. Anal. Biochem. 2016, 506, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.; Alcalay, R.; Sabo, T.; Noy-Porat, T.; Epstein, E.; Kronman, C.; Mazor, O. Rapid assessment of antibody-induced ricin neutralization by employing a novel functional cell-based assay. J. Immunol. Methods 2015, 424, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Ciani, M.; Girbes, T.; Liu, W.Y.; Van Damme, E.J.; Peumans, W.J.; Stirpe, F. Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett. 2004, 563, 219–222. [Google Scholar] [CrossRef]
- Falach, R.; Sapoznikov, A.; Gal, Y.; Israeli, O.; Leitner, M.; Seliger, N.; Ehrlich, S.; Kronman, C.; Sabo, T. Quantitative profiling of the in vivo enzymatic activity of ricin reveals disparate depurination of different pulmonary cell types. Toxicol. Lett. 2016, 258, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, O.; Sugendran, K.; Vijayaraghavan, R. Oxidative stress associated hepatic and renal toxicity induced by ricin in mice. Toxicon 2003, 41, 333–338. [Google Scholar] [CrossRef]
- Chi, X.S.; Landt, Y.; Crimmins, D.L.; Dieckgraefe, B.K.; Ladenson, J.H. Isolation and characterization of rabbit single chain antibodies to human Reg Iα protein. J. Immunol. Methods 2002, 266, 197–207. [Google Scholar] [CrossRef]
- Hofer, T.; Tangkeangsirisin, W.; Kennedy, M.G.; Mage, R.G.; Raiker, S.J.; Venkatesh, K.; Lee, H.; Giger, R.J.; Rader, C. Chimeric rabbit/human Fab and IgG specific for members of the Nogo-66 receptor family selected for species cross-reactivity with an improved phage display vector. J. Immunol. Methods 2007, 318, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Im, S.Y.; Kim, K.S.; Yun, C.O.; Kim, J.H.; Yi, K.S.; Chung, J. Generation of a rabbit V(H) domain antibody polyspecific to c-Met and adenoviral knob protein. Biochem. Biophys. Res. Commun. 2006, 339, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kodangattil, S.; Huard, C.; Ross, C.; Li, J.; Gao, H.; Mascioni, A.; Hodawadekar, S.; Naik, S.; Min-debartolo, J.; Visintin, A.; et al. The functional repertoire of rabbit antibodies and antibody discovery via next-generation sequencing. MAbs 2014, 6, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Barbas, C.F., 3rd; Schleef, R.R. Recombinant rabbit Fab with binding activity to type-1 plasminogen activator inhibitor derived from a phage-display library against human alpha-granules. Gene 1996, 172, 295–298. [Google Scholar] [CrossRef]
- Lavinder, J.J.; Hoi, K.H.; Reddy, S.T.; Wine, Y.; Georgiou, G. Systematic characterization and comparative analysis of the rabbit immunoglobulin repertoire. PLoS ONE 2014, 9, e101322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cockburn, W.; Kilpatrick, J.B.; Whitelam, G.C. High affinity scFvs from a single rabbit immunized with multiple haptens. Biochem. Biophys. Res. Commun. 2000, 268, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Rader, C.; Barbas, C.F., 3rd. Phage display of combinatorial antibody libraries. Curr. Opin. Biotechnol. 1997, 8, 503–508. [Google Scholar] [CrossRef]
- Rader, C.; Ritter, G.; Nathan, S.; Elia, M.; Gout, I.; Jungbluth, A.A.; Cohen, L.S.; Welt, S.; Old, L.J.; Barbas, C.F., 3rd. The rabbit antibody repertoire as a novel source for the generation of therapeutic human antibodies. J. Biol. Chem. 2000, 275, 13668–13676. [Google Scholar] [CrossRef] [PubMed]
- Ridder, R.; Gram, H. Generation of rabbit immune libraries. In Antibody Engineering; Kontermann, R., Dubel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Azriel-Rosenfeld, R.; Valensi, M.; Benhar, I. A human synthetic combinatorial library of arrayable single-chain antibodies based on shuffling in vivo formed cdrs into general framework regions. J. Mol. Biol. 2004, 335, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Luker, G.D.; Pica, C.M.; Song, J.; Luker, K.E.; Piwnica-Worms, D. Imaging 26s proteasome activity and inhibition in living mice. Nat. Med. 2003, 9, 969–973. [Google Scholar] [CrossRef] [PubMed]
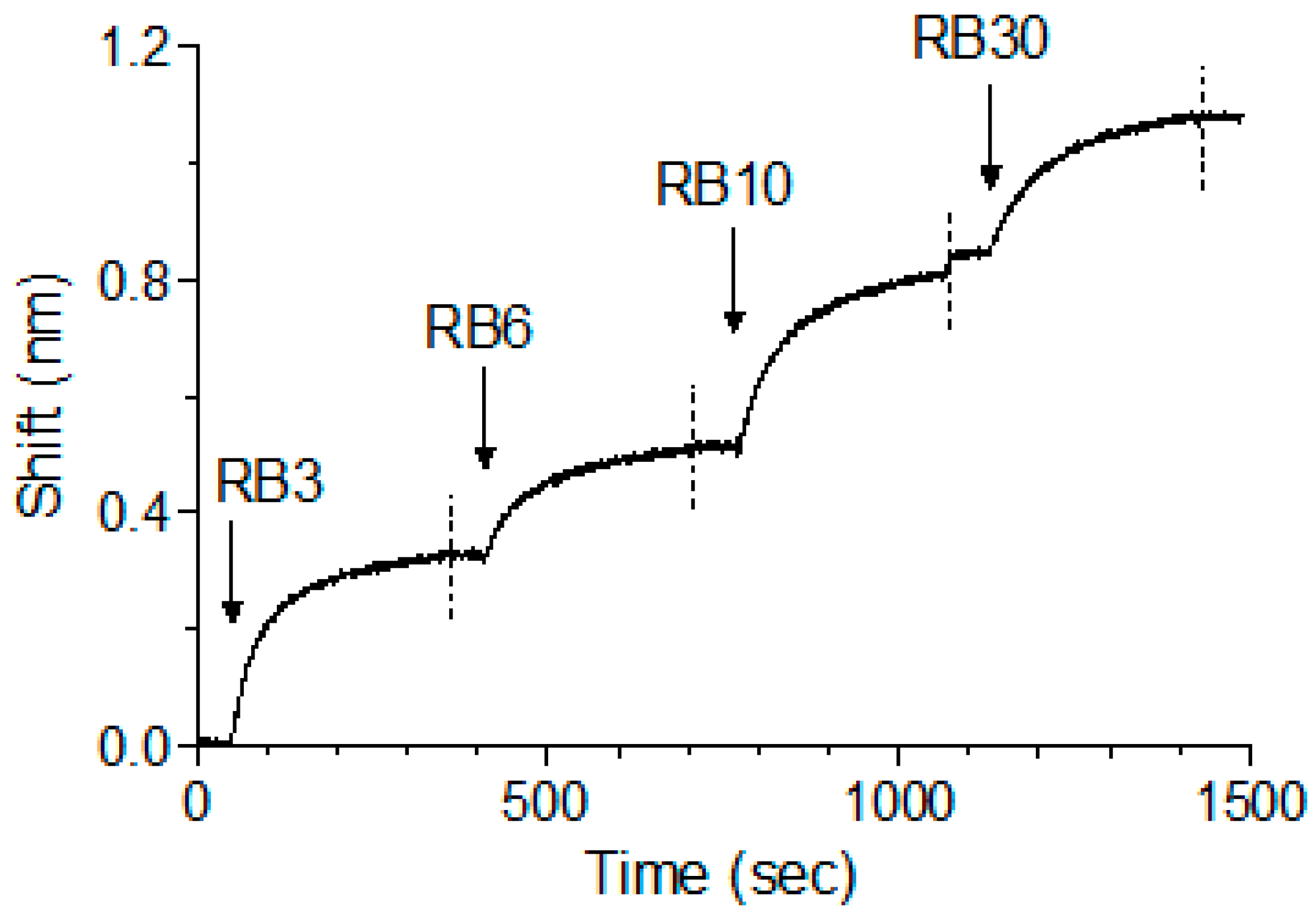
| Antibody | Binding a | ED50 (ng/mL) b | kon (1/Ms) | koff (1/s) | KD (nM) |
|---|---|---|---|---|---|
| RB1 | ATA | 5520 | 4.4 × 105 | 4.5 × 10−5 | 0.1 |
| RB3 | ATA | >100,000 | 1.2 × 105 | 6.9 × 10−5 | 0.6 |
| RB6 | ATB | 5350 | 3.8 × 105 | 2.1 × 10−5 | 0.06 |
| RB9 | ATA | 11,000 | 2.1 × 105 | 3.1 × 10−4 | 1.5 |
| RB10 | ATB | 3800 | 2.2 × 105 | 5.9 × 10−5 | 0.3 |
| RB14 | ATB | 6520 | 2.2 × 105 | 1.5 × 10−4 | 0.7 |
| RB17 | ATA | >100,000 | 1.1 × 105 | 4.9 × 10−5 | 0.5 |
| RB18 | ATA | >100,000 | 1.4 × 104 | 1.6 × 10−5 | 0.3 |
| RB28 | ATA | 11,600 | 2.3 × 105 | 2.2 × 10−5 | 0.2 |
| RB30 | ATB | 710 | 4.4 × 105 | 7.8 × 10−6 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechaly, A.; Alcalay, R.; Noy-Porat, T.; Epstein, E.; Gal, Y.; Mazor, O. Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication. Toxins 2018, 10, 80. https://doi.org/10.3390/toxins10020080
Mechaly A, Alcalay R, Noy-Porat T, Epstein E, Gal Y, Mazor O. Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication. Toxins. 2018; 10(2):80. https://doi.org/10.3390/toxins10020080
Chicago/Turabian StyleMechaly, Adva, Ron Alcalay, Tal Noy-Porat, Eyal Epstein, Yoav Gal, and Ohad Mazor. 2018. "Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication" Toxins 10, no. 2: 80. https://doi.org/10.3390/toxins10020080





